Validation of the Malay Self-Report Quick Inventory of Depressive Symptomatology in a Malaysian Sample
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Procedure
2.2. Measures
Study Instruments
2.3. Data Analysis and Estimated Sample Size
3. Results
3.1. Sample Characteristics
3.2. Discriminative Validity
3.3. Construct Validity
3.4. Reliability Analysis
3.5. Concurrent Validity
4. Discussion
4.1. Strengths and Limitations
4.2. Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease (GBD) Compare. 2019. Available online: https://vizhub.healthdata.org/gbd-compare/ (accessed on 20 January 2022).
- Santomauro, D.F.; Herrera, A.M.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Ferrari, A.; Somerville, A.; Baxter, A.; Norman, R.; Patten, S.; Vos, T.; Whiteford, H. Global variation in the prevalence and incidence of major depressive disorder: A systematic review of the epidemiological literature. Psychol. Med. 2013, 43, 471–481. [Google Scholar] [CrossRef]
- Zimmerman, M. Using Scales to Monitor Symptoms and Treat Depression (Measurement Based Care). 2016. Available online: https://www.uptodate.com/contents/using-scales-to-monitor-symptoms-and-treat-depression-measurement-based-care (accessed on 20 January 2022).
- Rush, A.J.; Trivedi, M.H.; Ibrahim, H.M.; Carmody, T.J.; Arnow, B.; Klein, D.N.; Markowitz, J.C.; Ninan, P.T.; Kornstein, S.; Manber, R. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol. Psychiatry 2003, 54, 573–583. [Google Scholar] [CrossRef]
- Cameron, I.; Cardy, A.; Crawford, J. Assessing the Validity of the PHQ-9, HADS, BDI-II and QIDS-SR16 in Measuring of Depression in a UK Sample of Primary Care Patients with a Diagnosis of Depression; Healthcare Improvement Scotland: Edinburgh, UK, 2011. [Google Scholar]
- Sung, S.C.; Low, C.C.H.; Fung, D.S.S.; Chan, Y.H. Screening for major and minor depression in a multiethnic sample of A sian primary care patients: A comparison of the nine-item Patient Health Questionnaire (PHQ-9) and the 16-item Quick Inventory of Depressive Symptomatology–Self-Report (QIDS-SR16). Asia-Pac. Psychiatry 2013, 5, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. Hospital Anxiety and Depression Scale (HADS); Mapi Research Trust: Lyon, France, 1983. [Google Scholar]
- Reilly, T.J.; MacGillivray, S.A.; Reid, I.C.; Cameron, I.M. Psychometric properties of the 16-item Quick Inventory of Depressive Symptomatology: A systematic review and meta-analysis. J. Psychiatr. Res. 2015, 60, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.J. Self-Report Quick Inventory of Depressive Symptomatology (QIDS-SR16); Mapi Research Trust: Lyon, France, 2003. [Google Scholar]
- Yusof, Y. Ethnic Groups of Malaysia. World Atlas. 2019. Available online: https://www.worldatlas.com/articles/ethnic-groups-of-malaysia.html (accessed on 20 January 2022).
- David, M.K.; Dealwis, C.; Hei, K.C. Language Policy and Language Use in Multilingual Malaysia. In Pursuit of Societal Harmony: Reviewing the Experiences and Approaches in Officially Monolingual and Officially Multilingual Countries; SUN MeDia: Bloemfontein, South Africa, 2017; p. 83. [Google Scholar]
- The World Bank in Malaysia; The World Bank: Washington, DC, USA, 2021; Available online: https://data.worldbank.org/country/malaysia (accessed on 20 January 2022).
- The World Bank in Indonesia; The World Bank: Washington, DC, USA, 2017; Available online: https://data.worldbank.org/country/indonesia?view=chart (accessed on 20 January 2022).
- Ali, G.-C.; Ryan, G.; De Silva, M.J. Validated screening tools for common mental disorders in low and middle income countries: A systematic review. PLoS ONE 2016, 11, e0156939. [Google Scholar] [CrossRef]
- Sherina, M.; Arroll, B.; Goodyear-Smith, F. Criterion validity of the PHQ-9 (Malay version) in a primary care clinic in Malaysia. Med. J. Malays. 2012, 67, 309–315. [Google Scholar]
- Chai, Y.C.; Mahadevan, R.; Ng, C.G.; Chan, L.F.; Md Dai, F. Caregiver depression: The contributing role of depression in patients, stigma, social support and religiosity. Int. J. Soc. Psychiatry 2018, 64, 578–588. [Google Scholar] [CrossRef]
- Loo, J.L.; Kamal, N.A.M.; Goon, J.A.; Damanhuri, H.A.; Tan, J.A.C.; Murad, N.A.A.; Shah, S.A.; Sulaiman, S.A.; Fazry, S.; Sharip, S. The role of oxidative stress in suicidal behaviour among bipolar patients: A cross-sectional study in a Malaysian sample. Front. Psychiatry 2021, 12, 698–911. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; Text Revision (DSM-IV-TR); American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- World Health Organization. Process of Translation and Adaptation of Instruments; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Mukhtar, F.; Bakar, A.K.A.; Junus, M.M.; Awaludin, A.; Aziz, S.A.; Midin, M.; Razak, M.F.A.; Ibrahim, N.; Teng, A.K.; Kaur, J. A preliminary study on the specificity and sensitivity values and inter-rater reliability of mini international neuropsychiatric interview (MINI) in Malaysia. ASEAN J. Psychiatry 2012, 13, 157–164. [Google Scholar]
- Hayes, A.F.; Krippendorff, K. Answering the call for a standard reliability measure for coding data. Commun. Methods Meas. 2007, 1, 77–89. [Google Scholar] [CrossRef]
- Williams, B.; Onsman, A.; Brown, T. Exploratory factor analysis: A five-step guide for novices. Australas. J. Paramed. 2010, 8. [Google Scholar] [CrossRef] [Green Version]
- Tabachnick, B.G.; Fidell, L.S.; Ullman, J.B. Using Multivariate Statistics; Pearson: Boston, MA, USA, 2007; Volume 5. [Google Scholar]
- Beavers, A.S.; Lounsbury, J.W.; Richards, J.K.; Huck, S.W.; Skolits, G.J.; Esquivel, S.L. Practical considerations for using exploratory factor analysis in educational research. Pract. Assess. Res. Eval. 2013, 18, 6. [Google Scholar]
- Tavakol, M.; Dennick, R. Making sense of Cronbach’s alpha. Int. J. Med. Educ. 2011, 2, 53. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Carley, S.; Harrison, M. An introduction to power and sample size estimation. Emerg. Med. J. EMJ 2003, 20, 453. [Google Scholar] [CrossRef] [PubMed]
- Hajian-Tilaki, K. Sample size estimation in diagnostic test studies of biomedical informatics. J. Biomed. Inform. 2014, 48, 193–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, C. A review of depression research in malaysia. Med. J. Malays. 2014, 69, 42–45. [Google Scholar]
- McIntyre, R.S.; Cha, D.S.; Soczynska, J.K.; Woldeyohannes, H.O.; Gallaugher, L.A.; Kudlow, P.; Alsuwaidan, M.; Baskaran, A. Cognitive deficits and functional outcomes in major depressive disorder: Determinants, substrates, and treatment interventions. Depress. Anxiety 2013, 30, 515–527. [Google Scholar] [CrossRef]
- Samuels, P. Advice on Exploratory Factor Analysis. 2017. Available online: http://www.open-access.bcu.ac.uk/6076/1/__staff_shares_storage%20500mb_Library_ID112668_Stats%20Advisory_New%20Statistics%20Workshops_18ExploratoryFactorAnalysis_ExploratoryFactorAnalysis4.pdf (accessed on 20 January 2022).
- Zailinawati, A.; Ariff, K.; Nurjahan, M.; Teng, C. Epidemiology of insomnia in Malaysian adults: A community-based survey in 4 urban areas. Asia Pac. J. Public Health 2008, 20, 224–233. [Google Scholar] [CrossRef]
- Zailinawati, A.-H.; Mazza, D.; Teng, C.L. Prevalence of insomnia and its impact on daily function amongst Malaysian primary care patients. Asia Pac. Fam. Med. 2012, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, M.H.; Rush, A.; Ibrahim, H.; Carmody, T.; Biggs, M.; Suppes, T.; Crismon, M.; Shores-Wilson, K.; Toprac, M.; Dennehy, E. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: A psychometric evaluation. Psychol. Med. 2004, 34, 73–82. [Google Scholar] [PubMed]
- Doraiswamy, P.M.; Bernstein, I.; Rush, A.; Kyutoku, Y.; Carmody, T.; Macleod, L.; Venkatraman, S.; Burks, M.; Stegman, D.; Witte, B. Diagnostic utility of the Quick Inventory of Depressive Symptomatology (QIDS-C16 and QIDS-SR16) in the elderly. Acta Psychiatr. Scand. 2010, 122, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, I.H.; Rush, A.J.; Stegman, D.; Macleod, L.; Witte, B.; Trivedi, M.H. A comparison of the QIDS-C16, QIDS-SR16, and the MADRS in an adult outpatient clinical sample. CNS Spectr. 2010, 15, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Cameron, I.M.; Crawford, J.R.; Cardy, A.H.; du Toit, S.W.; Lawton, K.; Hay, S.; Mitchell, K.; Sharma, S.; Shivaprasad, S.; Winning, S. Psychometric properties of the Quick Inventory of Depressive Symptomatology (QIDS-SR) in UK primary care. J. Psychiatr. Res. 2013, 47, 592–598. [Google Scholar] [CrossRef]
- Liu, J.; Xiang, Y.-T.; Lei, H.; Wang, Q.; Wang, G.; Ungvari, G.S.; Morris, D.W.; Zhu, X.-Z.; Lai, K.Y.; Zhong, B.-L. Guidance on the conversion of the Chinese versions of the Quick Inventory of Depressive Symptomatology-Self-Report (C-QIDS-SR) and the Montgomery–Asberg Scale (C-MADRS) in Chinese patients with major depression. J. Affect. Disord. 2014, 152, 530–533. [Google Scholar] [CrossRef]
- Lamoureux, B.E.; Linardatos, E.; Fresco, D.M.; Bartko, D.; Logue, E.; Milo, L. Using the QIDS-SR16 to identify major depressive disorder in primary care medical patients. Behav. Ther. 2010, 41, 423–431. [Google Scholar] [CrossRef]
- Cusin, C.; Yang, H.; Yeung, A.; Fava, M. Rating scales for depression. In Handbook of Clinical Rating Scales and Assessment in Psychiatry and Mental Health; Humana Press: Totowa, NJ, USA, 2009; pp. 7–35. [Google Scholar]
- Sheehan, D.; Lecrubier, Y.; Sheehan, K.H.; Janavs, J.; Weiller, E.; Keskiner, A.; Schinka, J.; Knapp, E.; Sheehan, M.; Dunbar, G. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur. Psychiatry 1997, 12, 232–241. [Google Scholar] [CrossRef]
- American Psychiatry Association. The Structured Clinical Interview for DSM-5. Available online: https://www.appi.org/products/structured-clinical-interview-for-dsm-5-scid-5 (accessed on 20 January 2022).
- Hershenberg, R.; McDonald, W.M.; Crowell, A.; Riva-Posse, P.; Craighead, W.E.; Mayberg, H.S.; Dunlop, B.W. Concordance between clinician-rated and patient reported outcome measures of depressive symptoms in treatment resistant depression. J. Affect. Disord. 2020, 266, 22–29. [Google Scholar] [CrossRef]
| Characteristic | Patients with a Current MDE # | Patients with Lifetime MDE # in Remission | Healthy Participants | Total |
|---|---|---|---|---|
| Female (%) | 64.1 | 62.4 | 61.1 | 62.7 |
| Age (mean ± SD) | 44.9 ± 16.3 | 46.2 ± 17.4 | 35.4 ± 10.6 | 43.6 ± 16.3 |
| Ethnicity (%) | ||||
| Malay | 53.8 | 61.5 | 38.9 | 54.2 |
| Chinese | 34.6 | 32.5 | 42.6 | 35.3 |
| Indian | 11.5 | 5.1 | 3.7 | 7.2 |
| Others | 0 | 0.9 | 14.8 | 3.3 |
| Marital Status (%) | ||||
| Married | 48.0 | 52.9 | 49.1 | 50.3 |
| Educational level (%) | ||||
| <10 years | 6.3 | 8.6 | 3.7 | 6.3 |
| >10 years | 50 | 36.2 | 16.7 | 33.8 |
| Higher education | 43.8 | 55.2 | 79.6 | 60.0 |
| Employment (%) | ||||
| Unemployed or part time | 18.0 | 14.7 | 7.4 | 13.4 |
| Fulltime employment | 44.0 | 47.1 | 75.9 | 55.2 |
| Retired/pensioner | 10.0 | 16.2 | 1.9 | 9.9 |
| Housewife | 14.0 | 7.4 | 7.4 | 9.3 |
| Students | 14.0 | 14.7 | 7.4 | 12.2 |
| QIDS-SR16 * (Malay version) score (%) | ||||
| 0–5 | 1.0 | 58.0 | 51.9 | 35.4 |
| 6–10 | 15.4 | 23.5 | 42.6 | 24.2 |
| 11–15 | 41.3 | 12.6 | 3.7 | 21.7 |
| 16–20 | 23.1 | 5.9 | 1.9 | 11.6 |
| >20 | 19.2 | 0 | 0 | 7.2 |
| MINI 6.0 Classification | n (%) |
QIDS-SR16 Score Mean (SD) | t-Value | p-Value * |
|---|---|---|---|---|
| Healthy | 54 (20.9) | 5.80 (2.90) | ||
| Patients with current MDE | 104 (36.1) | 15.29 (5.01) | 15.053 | <0.001 |
| Patients with lifetime MDE | 119 (43.0) | 5.84 (4.94) | 0.073 | 0.942 |
| QIDS-SR16 Items/Domains | Factor Loading |
|---|---|
| Sad mood (item no. 5) | 0.775 |
| Reduced concentration (item no. 10) | 0.758 |
| Reduced interest (item no. 13) | 0.736 |
| Self-criticism (item no. 11) | 0.676 |
| Suicide ideation (item no. 12) | 0.664 |
| Psychomotor agitation/retardation (items no. 15–16) | 0.663 |
| Reduced energy/fatigue (item no. 14) | 0.625 |
| Appetite/weight changes (items no. 6–9) | 0.613 |
| Sleep disturbances (items no. 1–4) | 0.538 |
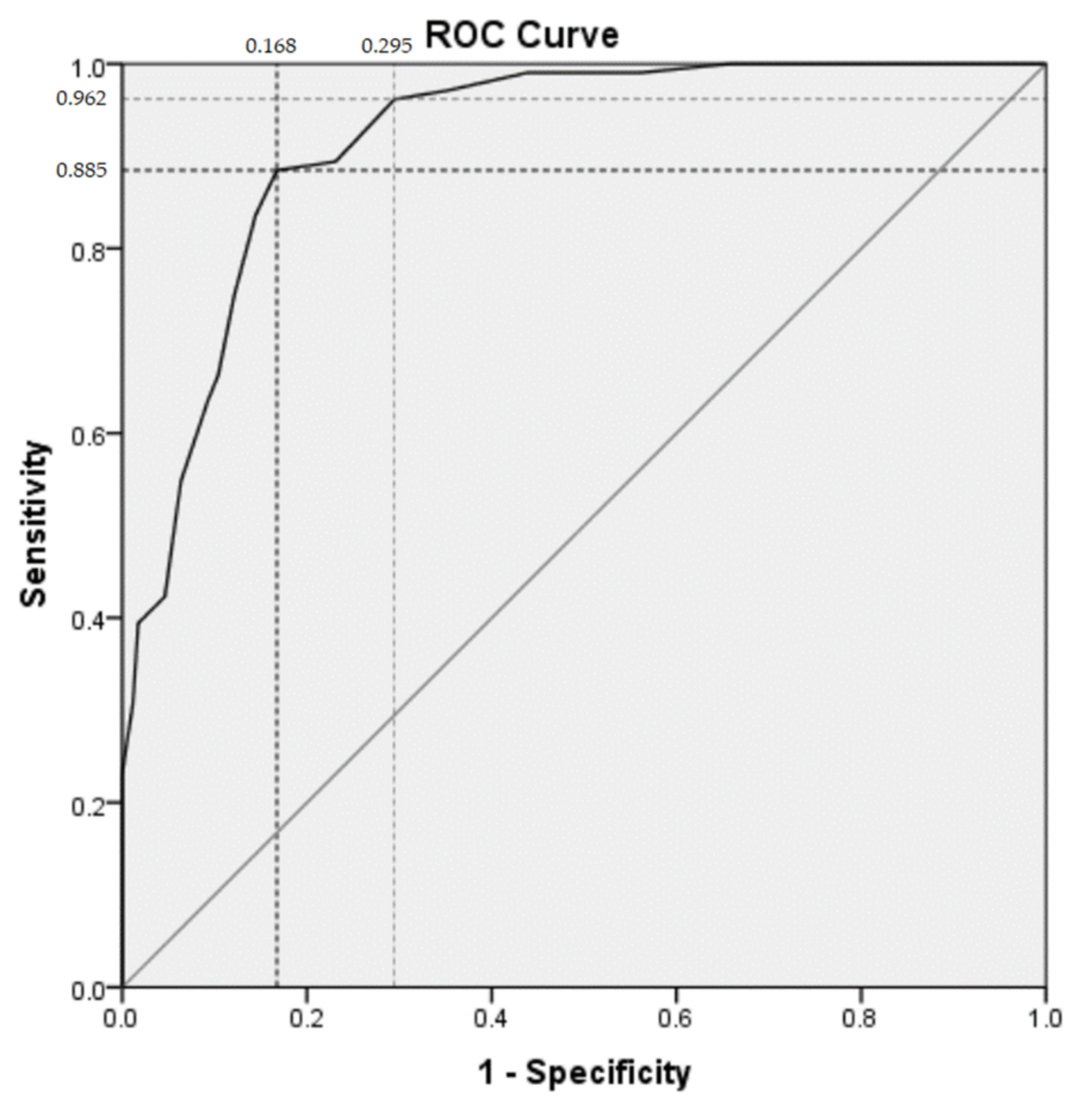
| Cutoff Score (Points) | Number of Subjects | Sensitivity (%) | Specificity (%) | Positive Likelihood Ratio | Negative Likelihood Ratio | Positive Predictive Value (%) | Negative Predictive Value (%) |
|---|---|---|---|---|---|---|---|
| ≥9 | 121 | 88.5 | 83.2 | 5.3 | 0.14 | 76.0 | 92.3 |
| ≥7 | 151 | 96.2 | 70.5 | 3.3 | 0.05 | 66.2 | 96.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, L.F.; Eu, C.L.; Tong, S.F.; Chin, S.J.; Sharip, S.; Chai, Y.C.; Loo, J.L.; Mohamad Kamal, N.A.; Goon, J.A.; Mahadevan, R.; et al. Validation of the Malay Self-Report Quick Inventory of Depressive Symptomatology in a Malaysian Sample. Int. J. Environ. Res. Public Health 2022, 19, 2801. https://doi.org/10.3390/ijerph19052801
Chan LF, Eu CL, Tong SF, Chin SJ, Sharip S, Chai YC, Loo JL, Mohamad Kamal NA, Goon JA, Mahadevan R, et al. Validation of the Malay Self-Report Quick Inventory of Depressive Symptomatology in a Malaysian Sample. International Journal of Environmental Research and Public Health. 2022; 19(5):2801. https://doi.org/10.3390/ijerph19052801
Chicago/Turabian StyleChan, Lai Fong, Choon Leng Eu, Seng Fah Tong, Song Jie Chin, Shalisah Sharip, Yee Chin Chai, Jiann Lin Loo, Nurul Ain Mohamad Kamal, Jo Aan Goon, Raynuha Mahadevan, and et al. 2022. "Validation of the Malay Self-Report Quick Inventory of Depressive Symptomatology in a Malaysian Sample" International Journal of Environmental Research and Public Health 19, no. 5: 2801. https://doi.org/10.3390/ijerph19052801
APA StyleChan, L. F., Eu, C. L., Tong, S. F., Chin, S. J., Sharip, S., Chai, Y. C., Loo, J. L., Mohamad Kamal, N. A., Goon, J. A., Mahadevan, R., Liu, C. Y., Yeoh, C. N., & Mohd Daud, T. I. (2022). Validation of the Malay Self-Report Quick Inventory of Depressive Symptomatology in a Malaysian Sample. International Journal of Environmental Research and Public Health, 19(5), 2801. https://doi.org/10.3390/ijerph19052801






