Estimation of Target Hazard Quotients and Potential Health Risks for Toxic Metals and Other Trace Elements by Consumption of Female Fish Gonads and Testicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Analysis of Female Fish Gonads, Testicles, and Muscles
2.3. Analysis Quality Assessment
2.4. Calculation of Micronutrient Uptake
2.5. Risk Assessment to Human Health
- Target Hazard Quotient (THQ) (Equation (2)) [9,20].where: EF—exposure frequency to trace elements (365 days/year); ED—exposure duration (70 years); MS—food ingestion rate, 34.8 g/day [17]; C—concentration of trace element in fish muscles and female gonads and testicles (mg/kg); RfD—oral reference dose of trace element (mg/kg BW/day) (Zn = 0.3; Ni = 0.02; Fe = 0.7; Mn = 0.14; Cr = 0.003; Cu = 0.04; Li = 0.02; Pb = 0.0035; Cd = 0.001; Hg = 0.0001) [3,9]; BW—reference body weight of 70 kg; AT—averaged exposure time e to non-carcinogenic trace elements (365 days × 70 years).
- Provisional Tolerable Weekly Intake (PTWI) (Equation (4))
2.6. Statistical Analyses
3. Results
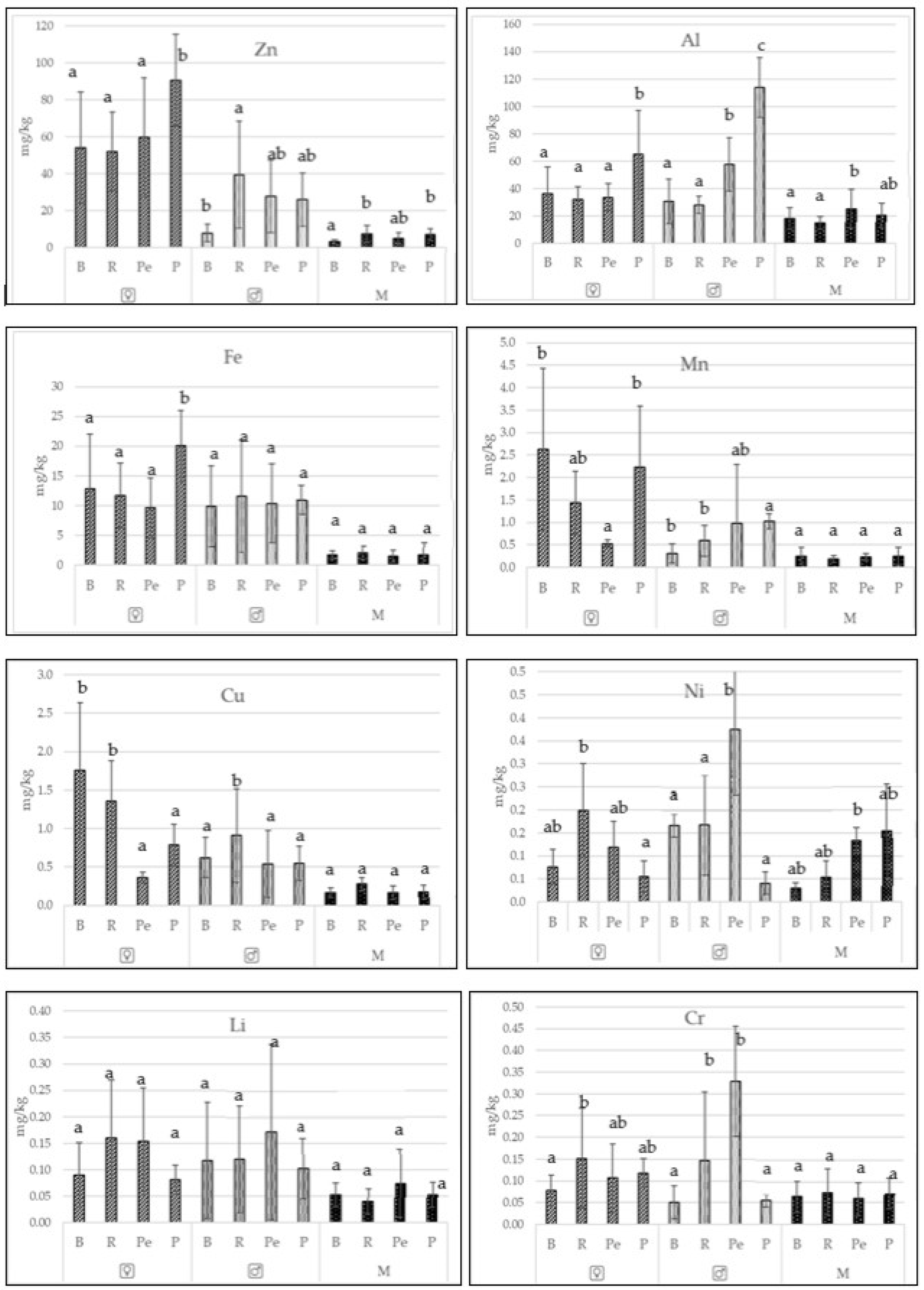
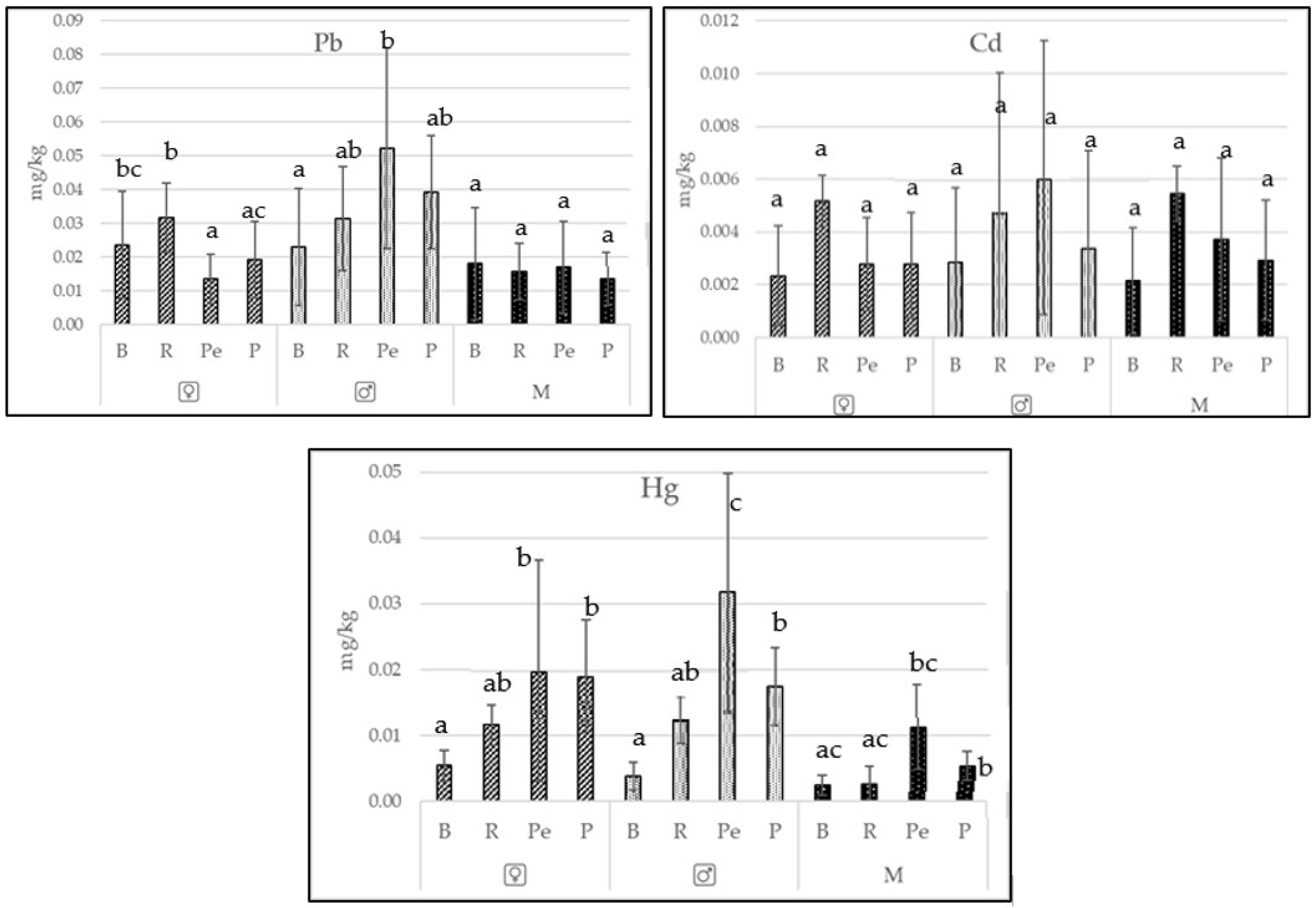
3.1. Contents of Essential Trace Elements and Toxic Metals in Freshwater Fishes
3.2. Human Health Risk
4. Discussion
Human Health Risk
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maleki, A.; Azadi, N.A.; Mansouri, B.; Majnoni, F.; Rezaei, Z.; Gharibi, F. Health risk assessment of trace elements in two fish species of Sanandaj Gheshlagh Reservoir, Iran. Toxicol. Environ. Health Sci. 2015, 7, 43–49. [Google Scholar] [CrossRef]
- Varol, M.; Kaya, G.K.; Alp, S.A.; Sünbül, M.R. Trace metal levels in rainbow trout (Oncorhynchus mykiss) cultured in net cages in a reservoir and evaluation of human health risks from consumption. Biol. Trace Elem. Res. 2018, 184, 268–278. [Google Scholar] [CrossRef]
- Ravanbakhsh, M.; Javid, A.Z.; Hadi, M.; Fard, N.J.H. Heavy metals risk assessment in fish species (Johnius Belangerii (C) and Cynoglossus Arel) in Musa Estuary, Persian Gulf. Environ. Res. 2020, 188, 109560. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.-D.; Morton, J.D.; Dawson, C.O. Effect of processing conditions on trace elements in fish roe from six commercial New Zealand fish species. J. Agric. Food Chem. 2008, 56, 4846–4853. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Abib, B.; Tawfik, S.; Shafik, N.; Khattab, A.R. Caviar and fish roe substitutes: Current status of their nutritive value, bio-chemical diversity, authenticity and quality control methods with future perspectives. Trends Food Sci. Tech. 2021, 110, 405–417. [Google Scholar] [CrossRef]
- Vasconi, M.; Tirloni, E.; Stella, S.; Coppola, C.; Lopez, A.; Bellagamba, F.; Bernardi, C.; Moretti, V.M. Comparison of chemical composition and safety issues in fish roe products: Application of chemometrics to chemical data. Foods 2020, 9, 540. [Google Scholar] [CrossRef]
- Jiang, H.; Qin, D.; Chen, Z.; Tang, S.; Bai, S.; Mou, Z. Heavy metal levels in fish from Heilongjiang River and potential health risk assessment. Bull. Environ. Contam. Toxicol. 2016, 97, 536–542. [Google Scholar] [CrossRef]
- Griboff, J.; Wunderlin, D.A.; Monferran, M.V. Metals, As and Se determination by inductively coupled plasma-mass spectrometry (ICP-MS) in edible fish collected from three eutrophic reservoirs their consumption represents a risk for human health? Microchem. J. 2017, 130, 236–244. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (USEPA). Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories, Volume II. Risk Assessment and Fish Consumption Limits; EPA 823-B-00-008; USEPA: Washington, DC, USA, 2000.
- Varol, M.; Sünbül, M.R. Comparison of heavy metal levels of farmed and escaped farmed rainbow trout and health risk assessment associated with their consumption. Environ. Sci. Pollut. Res. 2017, 24, 23114–23124. [Google Scholar] [CrossRef]
- Daniszewski, P.; Konieczny, R. Heavy Metal Content in Water of Miedwie Lake (North-West Poland). Int. Lett. Chem. Phys. Astron. 2013, 15, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Bojakowska, G.; Sokołowska, G. Geochemical classes of water sediment purity. Przeg. Geolog. 1998, 46, 49–54. (In Polish) [Google Scholar]
- MacDonald, D.D.; Ingersoll, C.G.; Berger, T.A. Development and Evaluation of Consensus-Based Sediment Quality Guidelines for Freshwater Systems. Arch. Environ. Contam. Toxicol. 2000, 39, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Bakierowska, A.; Bursztynowicz, M.; Bykowszczenko, N.; Chałupińska, J.; Gajdecki, A.; Jurkowska, K.; Kordas, A.; Landsberg-Uczciwek, M.; Michalska, M.; Miluch, A.; et al. The State of the Environment in the West Pomeranian Voivodeship 2018 Report. Voivodship Inspectorate of Environmental Protection in Szczecin. 2018. Available online: https://wios.szczecin.pl/chapter_16003.asp (accessed on 24 July 2021). (In Polish).
- Czerniejewski, P.; Czerniawski, R. Ryby Słodkowodne i Morskie Polski [Freshwater and Marine Fishes of Poland]; Wyd. Nauk. Frel.: Warsaw, Poland, 2016; p. 335. (In Polish) [Google Scholar]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ihthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Statistical Yearbook of Agriculture. 2020; p. 322. Available online: https://stat.gov.pl/en/topics/statistical-yearbooks/statistical-yearbooks/statistical-yearbook-of-agriculture-2020,6,15.html (accessed on 17 September 2021). (In Polish)
- Jarosz, M. Human Nutrition Recommendations for Polish Population; IZZ: Warsaw, Poland, 2017. (In Polish) [Google Scholar]
- Copat, C.; Arena, G.; Fiore, M.; Ledda, C.; Fallico, R.; Sciacca, S.; Ferrante, M. Heavy metals concentrations in fish and shellfish from eastern Mediterranean Sea: Consumption advisories. Food Chem. Toxicol. 2013, 53, 33–37. [Google Scholar] [CrossRef] [PubMed]
- EPA/540/1-89/002; Risk Assessment Guidance for Superfund. Volume I: Human Health Evaluation Manual (Part A). Environmental Protection Agency: Washington, DC, USA, 1989.
- USEPA. USEPA Regional Screening Level (RSL) Summary Table: November 2011. (HI); USEPA: Washington, DC, USA, 2011.
- Copat, C.; Conti, G.O.; Fallico, R.; Sciacca, S.; Ferrante, M. Chapter 49—Heavy metals in fish from the Mediterranean Sea: Potential impact on diet. In The Mediterranean Diet; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 547–562. ISBN 9780124078499. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA 2012, 10, 2985. [Google Scholar]
- Ulusoy, S.; Mol, S. Mercury intake via consumption of imported Atlantic mackerel (Scomber scombrus) in Istanbul. Int. J. Agric. Food Sci. 2020, 4, 165–172. [Google Scholar] [CrossRef]
- USEPA. Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites; Office of Solid Waste and Emergency Response: Washington, DC, USA, 2001.
- Shirai, N.; Higuchi, T.; Suzuki, H. Analysis of lipid classes and the fatty acid composition of the salted fish roe food products, Ikura, Tarako, Tobiko and Kazunoko. Food Chem. 2006, 94, 61–67. [Google Scholar] [CrossRef]
- Lima, R.G., Jr.; Araújo, F.G.; Maia, M.F.; da Silveira Braz Pinto, A.S. Evaluation of heavy metals in fish of Sepetiba and Ilha Grande Bays, Rio de Janeiro, Brazil. Environ. Res. 2002, 89, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Olmedo, P.; Hernández, A.F.; Pla, A.; Femia, P.; Navas-Acien, A.; Gil, F. Determination of essential elements (copper, manganese, selenium and zinc) in fish and shellfish samples. Risk and nutritional assessment and mercury-selenium balance. Food Chem. Toxicol. 2013, 62, 299–307. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Das, D.; Dhara, A.; Chakraborty, S.B. Enzymatic, non-enzymatatic antioxidant levels and heat shock protein expression as indicators of metal induced toxicity and reproductive modulation in female indian major carp Cirrhinus cirrhosus. Bull. Environ. Contam. Toxicol. 2020, 104, 235–244. [Google Scholar] [CrossRef]
- Calza, C.; Anjos, M.J.; Castro, C.R.; Barroso, R.; Araújo, F.G.; Lopes, R.T. Evaluation of heavy metals levels in the Paraíba do Sul River by SRTXRF in muscle, gonads and gills of Geophagus brasiliensis. Radiat. Phys. Chem. 2004, 71, 787–788. [Google Scholar] [CrossRef]
- Topuz, K.O.; Yerlikaya, P.; Yatmaz, H.A.; Kaya, A.; Alp, A.C.; Kiliç, M. Comparison of essential trace element profiles of rainbow trout fish (Oncorhynchus mykiss) meat and egg. Anim. Sci. Sci. Pap. Ser. D 2017, 60, 316–322. [Google Scholar]
- Mortula, M.; Bard, S.M.; Walsh, M.E.; Gagnon, G.A. Aluminum toxicity and ecological risk assessment of dried alum residual into surface water disposal. Can. J. Civ. Eng. 2008, 36, 127–136. [Google Scholar] [CrossRef]
- Sapozhnikova, Y.; Zubcov, N.; Hungerford, S.; Roy, L.A.; Boicenco, N.; Zubcov, E.; Schlenk, D. Evaluation of pesticides and metals in fish of the Dniester River, Moldova. Chemosphere 2005, 60, 196–205. [Google Scholar] [CrossRef]
- Niemiec, M.; Kuboń, M.; Komorowska, M.; Kuzminova, N.; Sikora, J.; Szeląg-Sikora, A. Content of Cd, Cu, Cr, Fe Mn, Ni and Pb in water and selected organs of blotched Picarel Spicara maena and Mezgit Merlangius euxmus L. from Karantinna Bay and Balaklava Bay in the region of Sevastopol Middle Pomeranian. Annu. Set Environ. Prot. 2019, 21, 201–216. [Google Scholar]
- Gárriz, Á.; Del Fresno, P.S.; Carriquiriborde, P.; Miranda, L.A. Effects of heavy metals identified in Chascomús shallow lake on the endocrine-reproductive axis of pejerrey fish (Odontesthes bonariensis). Gen. Comp. Endocrinol. 2019, 273, 152–162. [Google Scholar] [CrossRef]
- Lin, S.; Taylor, A.A.; Ji, Z.; Chang, C.H.; Kinsinger, N.M.; Ueng, W.; Walker, S.L.; Nel, A.E.; Zhaoxia, J. Understanding the transformation, speciation, and hazard potential of copper particles in a model septic tank system using zebrafish to monitor the effluent. ACS Nano 2015, 9, 2038–2048. [Google Scholar] [CrossRef] [Green Version]
- Türkmen, M.; Türkmen, A.; Tepe, Y. Comparison of metals in tissues of fish from paradeniz lagoon in the coastal area of northern East Mediterranean. Bull. Environ. Contam. Toxicol. 2011, 87, 381. [Google Scholar] [CrossRef]
- Franco-Fuentes, E.; Moity, N.; Ramírez-González, J.; Andrade-Vera, S.; Hardisson, A.; Gonzalez-Weller, D.; Paz, S.; Rubio, C.; Gutiérrez, A.J. Metals in commercial fish in the Galapagos marine reserve: Contribution to food security and toxic risk assessment. J. Environ. Manag. 2021, 286, 112188. [Google Scholar] [CrossRef]
- Jesus, I.S.; De Da Silva Medeiros, R.L.; Cestari, M.M.; De Almeida Bezerra, M.; De Mello Affonso, P.R.A. Analysis of metal contamination and bioindicator potential of predatory fish species along Contas River basin in northeastern Brazil. Bull. Environ. Contam. Toxicol. 2014, 92, 551–556. [Google Scholar] [CrossRef]
- Lima, M.W.; Pereira, W.V.S.; Souza, E.S.; Teixeira, R.A.; da Conceição Palheta, D.; Faial, K.C.F.; Costa, H.F.; Fernandes, A.R. Bioaccumulation and human health risks of potentially toxic elements in fish species from the southeastern Carajás Mineral Province, Brazil. Environ. Res. 2022, 204, 112024. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, T.; Gashkina, N. Distribution and bioaccumulation of heavy metals (Hg, Cd, and Pb) in fish: Influence of the aquatic environment and climate. Environ. Res. Lett. 2020, 15, 115013. [Google Scholar] [CrossRef]
- Majnoni, F.; Rezaei, M.; Mansouri, B.; Hamidian, A.H. Metal concentrations in tissues of common carp, Cyprinus carpio, and silver carp, Hypophthalmichthys molitrix from the Zarivar Wetland in Western Iran. Arch. Pol. Fish. 2013, 21, 11–18. [Google Scholar] [CrossRef]
- Peakall, D.; Burger, J. Methodologies for assessing exposure to metals: Speciation, bioavailability of metals, and ecological host factors. Ecotoxicol. Environ. Saf. 2003, 56, 110–121. [Google Scholar] [CrossRef]
- Nowosad, J.; Kucharczyk, D.; Szmyt, M.; Łuczynska, J.; Tamás, M.; Horváth, L. Changes in cadmium concentration in muscles, ovaries, and eggs of Silver European Eel (Anguilla anguilla) during maturation under controlled conditions. Animals 2021, 11, 1027. [Google Scholar] [CrossRef]
- Wirth, M.; Kirschbaum, F.; Gessner, J.; Krüger, A.; Patriche, N.; Billard, R. Chemical and biochemical composition of caviar from different sturgeon species and origins. Nahrung 2000, 44, 233–237. [Google Scholar] [CrossRef]
- Has-Schön, E.; Bogut, I.; Strelec, I. Heavy metal profile in five fish species included in human diet, domiciled in the end flow of River Neretva (Croatia). Arch. Environ. Contam. Toxicol. 2006, 50, 545–551. [Google Scholar] [CrossRef]
- Anandkumar, A.; Nagarajan, R.; Prabakaran, K.; Bing, C.H.; Rajaram, R. Human health risk assessment and bioaccumulation of trace metals in fish species collected from the Miri coast, Sarawak, Borneo. Mar. Pollut. Bull. 2018, 133, 655–663. [Google Scholar] [CrossRef]
- Morcillo, P.; Esteban, M.A.; Cuesta, A. Mercury and its toxic effects on fish. AIMS Environ. Sci. 2017, 4, 386–402. [Google Scholar] [CrossRef]
- Bronzi, P.; Chebanov, M.; Michaels, J.T.; Wei, Q.; Rosenthal, H.; Gessner, J. Sturgeon meat and caviar production: Global update 2017. J. Appl. Ichthyol. 2019, 35, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Sicuro, B. The future of caviar production on the light of social changes: A new dawn for caviar? Rev. Aquac. 2019, 11, 204–219. [Google Scholar] [CrossRef] [Green Version]
- European Commission (EC). Commission Regulation No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs (Text with EEA relevance). 2006. Available online: http://faolex.fao.org/docs/pdf/eur68134.pdf (accessed on 24 October 2021).
- Mansouri, B.; Maleki, A.; Davari, B.; Karimi, J.; Momeneh, V. Estimation of target hazard quotients for heavy metals intake through the consumption of fish from Sirvan River in Kermanshah Province, Iran. J. Adv. Environ. Health Res. 2015, 3, 235–241. [Google Scholar] [CrossRef]
- Esfahani, N.B.; Jafari, M.; Moravejolahkami, A.R. Heavy metals concentration and target hazard quotients assessment through the consumption of fish muscle Ctenopharyngodon Idella (Cyprinidae) from markets in Ahvaz province, Iran. Nutr. Food Sci. 2020, 50, 529–537. [Google Scholar] [CrossRef]
- Mukherjee, J.; Saha, N.C.; Karan, S. Bioaccumulation pattern of heavy metals in fish tissues and associated health hazards in human population. Environ. Sci. Pollut. Res. 2021, 1–15. [Google Scholar] [CrossRef]
- Rezaei, H.; Zarei, A.; Kamarehie, B.; Jafari, A.; Fakhri, Y.; Bidarpoor, F.; Karami, M.A.; Farhang, M.; Ghaderpoori, M.; Sadeghi, H.; et al. Levels, distributions and health risk assessment of lead, cadmium and arsenic found in drinking groundwater of Dehgolan’s Villages, Iran. Toxicol. Environ. Health Sci. 2019, 11, 54–62. [Google Scholar] [CrossRef]
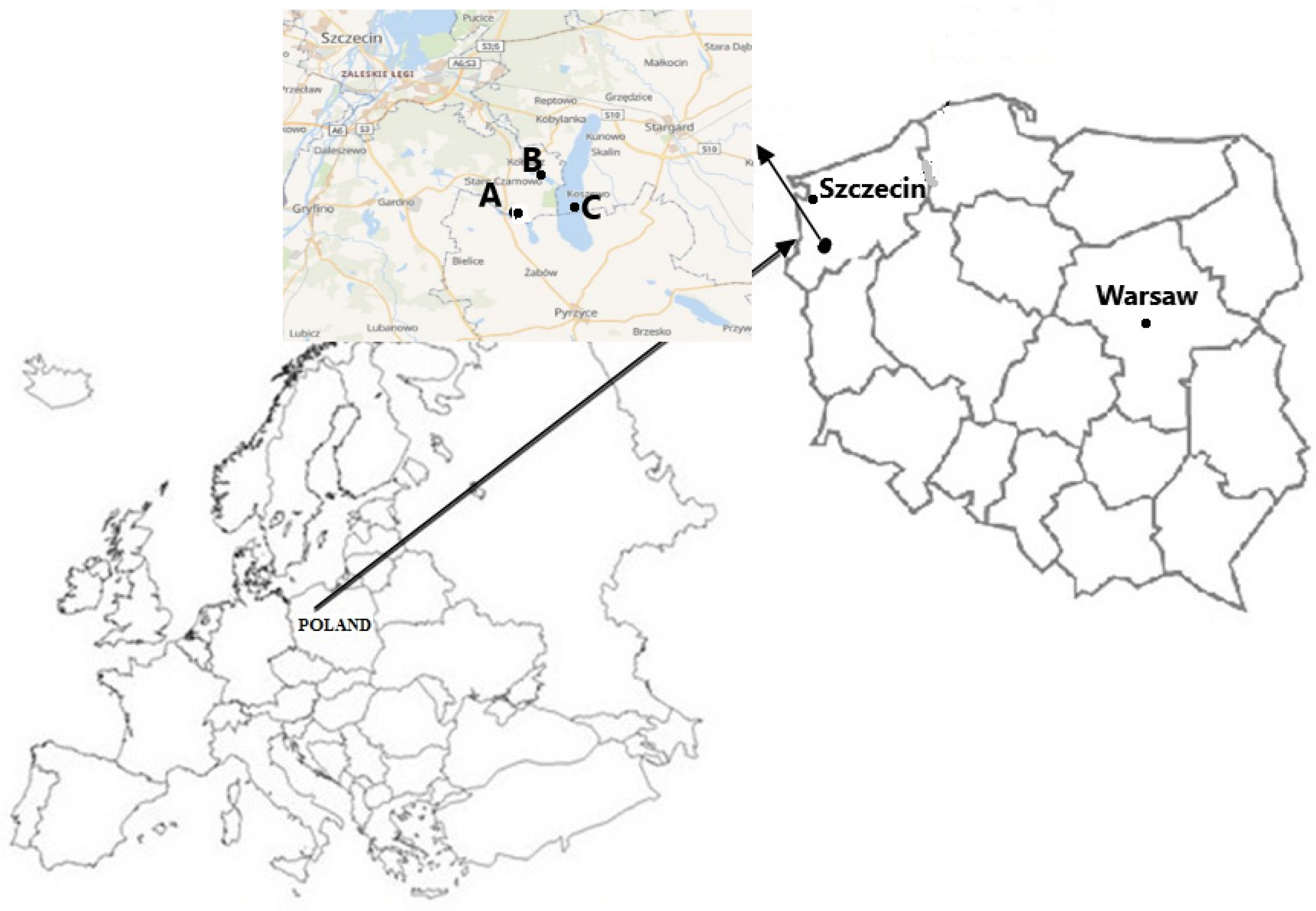
| Species | n | Fish Weight (g) | Fish Length (cm) | Gonad Weight (g) | Fulton’s Condition Factor | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | SD | x | SD | x | SD | x | SD | ||
| (Min–Max) | (Min–Max) | (Min–Max) | (Min–Max) | ||||||
| Bream (Abramis brama) | 120 | 654.45 | 285.53 | 37.20 | 5.50 | 24.51 | 37.06 | 1.20 | 0.15 |
| (170.1–1360.6) | (25.3–49.0) | (1.4–230.7) | (0.88–1.51) | ||||||
| Roach (Rutilus rutilus) | 120 | 147.33 | 48.94 | 22.81 | 2.41 | 7.94 | 10.22 | 1.20 | 0.17 |
| (5.52–350.4) | (17–30) | (0.5–53.5) | (0.75–1.78) | ||||||
| Perch (Perca fluviatilis) | 120 | 157.47 | 35.48 | 23.18 | 1.67 | 2.67 | 6.97 | 1.25 | 0.13 |
| (94.2–240.4) | (20.1–26.5) | (0.2–40.5) | (1.05–1.62) | ||||||
| Pike (Esox lucius) | 90 | 2811.39 | 10621.40 | 49.47 | 6.22 | 40.54 | 36.07 | 0.72 | 0.09 |
| (211.2–60,000.4) | (31.5–72.5) | (0.2–40.5) | (0.52–0.98) | ||||||
| Species | Zn | Al | Fe | Mn | Cu | Ni | Li | Cr | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F + T | M | F + T | M | F + T | M | F + T | M | F + T | M | F + T | M | F + T | M | F + T | M | ||
| Bream (Abramis brama) | x | 31.0 | 1.2 | 33.38 | 7.94 | 11.39 | 0.69 | 1.47 | 0.19 | 1.19 | 0.06 | 0.121 | 0.026 | 0.104 | 0.023 | 0.064 | 0.037 |
| SD | 31.7 | 1.2 | 18.01 | 7.94 | 8.08 | 0.69 | 1.73 | 0.19 | 0.86 | 0.06 | 0.191 | 0.026 | 0.088 | 0.023 | 0.039 | 0.037 | |
| min | 2.5 | 0.5 | 5.26 | 2.98 | 0.60 | 0.44 | 0.11 | 0.06 | 0.20 | 0.02 | 0.002 | 0.000 | 0.007 | 0.016 | 0.002 | 0.005 | |
| max | 106.6 | 5.2 | 67.57 | 34.46 | 26.48 | 3.26 | 6.70 | 1.07 | 3.02 | 0.31 | 0.937 | 0.116 | 0.428 | 0.103 | 0.133 | 0.150 | |
| Roach (Rutilus rutilus) | x | 46.2 | 7.3 | 30.14 | 14.94 | 11.65 | 2.04 | 1.04 | 0.18 | 1.15 | 0.28 | 0.184 | 0.054 | 0.142 | 0.041 | 0.149 | 0.076 |
| SD | 25.7 | 4.6 | 8.26 | 4.61 | 7.48 | 1.12 | 0.70 | 0.08 | 0.60 | 0.09 | 0.193 | 0.052 | 0.161 | 0.038 | 0.135 | 0.054 | |
| min | 5.2 | 2.0 | 16.09 | 3.56 | 0.16 | 0.23 | 0.15 | 0.03 | 0.21 | 0.10 | 0.003 | 0.002 | 0.019 | 0.010 | 0.031 | 0.002 | |
| max | 113.1 | 28.9 | 48.97 | 23.76 | 27.82 | 6.41 | 3.47 | 0.40 | 2.80 | 0.48 | 0.638 | 0.235 | 0.868 | 0.228 | 0.681 | 0.296 | |
| Perch (Perca fluviatilis) | x | 46.7 | 4.9 | 43.36 | 25.22 | 9.92 | 1.45 | 0.71 | 0.23 | 0.43 | 0.16 | 0.223 | 0.134 | 0.161 | 0.074 | 0.197 | 0.059 |
| SD | 31.7 | 3.1 | 18.57 | 14.28 | 5.63 | 1.01 | 0.84 | 0.08 | 0.29 | 0.09 | 0.419 | 0.227 | 0.129 | 0.064 | 0.350 | 0.039 | |
| min | 6.1 | 1.1 | 20.86 | 8.55 | 2.47 | 0.25 | 0.25 | 0.11 | 0.19 | 0.03 | 0.008 | 0.010 | 0.046 | 0.012 | 0.001 | 0.009 | |
| max | 154.1 | 11.9 | 85.77 | 63.17 | 23.40 | 4.98 | 4.44 | 0.44 | 1.67 | 0.37 | 2.081 | 1.185 | 0.592 | 0.216 | 1.726 | 0.183 | |
| Pike (Esox lucius) | x | 58.2 | 7.0 | 89.42 | 20.48 | 15.49 | 1.76 | 1.63 | 0.25 | 0.67 | 0.17 | 0.048 | 0.103 | 0.092 | 0.053 | 0.086 | 0.072 |
| SD | 68.3 | 3.1 | 36.90 | 8.73 | 6.43 | 1.95 | 1.13 | 0.20 | 0.27 | 0.09 | 0.046 | 0.153 | 0.045 | 0.023 | 0.041 | 0.036 | |
| min | 11.9 | 3.5 | 19.07 | 7.19 | 7.45 | 0.01 | 0.31 | 0.06 | 0.24 | 0.07 | 0.003 | 0.005 | 0.053 | 0.016 | 0.036 | 0.033 | |
| max | 344.4 | 15.8 | 166.08 | 56.87 | 30.71 | 10.96 | 3.82 | 0.97 | 1.52 | 0.47 | 0.192 | 0.734 | 0.246 | 0.096 | 0.163 | 0.173 | |
| Species | Pb | Cd | Hg | ||||
|---|---|---|---|---|---|---|---|
| F + T | M | F + T | M | F + T | M | ||
| Bream | x | 0.023 | 0.017 | 0.003 | 0.002 | 0.005 | 0.001 |
| SD | 0.016 | 0.017 | 0.002 | 0.002 | 0.002 | 0.001 | |
| min | 0.003 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | |
| max | 0.063 | 0.060 | 0.011 | 0.009 | 0.010 | 0.006 | |
| Roach | x | 0.032 | 0.016 | 0.005 | 0.005 | 0.012 | 0.003 |
| SD | 0.013 | 0.008 | 0.009 | 0.010 | 0.003 | 0.003 | |
| min | 0.004 | 0.006 | 0.001 | 0.001 | 0.007 | 0.001 | |
| max | 0.071 | 0.044 | 0.056 | 0.055 | 0.018 | 0.020 | |
| Perch | x | 0.029 | 0.017 | 0.004 | 0.004 | 0.025 | 0.011 |
| SD | 0.027 | 0.014 | 0.004 | 0.003 | 0.027 | 0.006 | |
| min | 0.004 | 0.005 | 0.001 | 0.000 | 0.002 | 0.001 | |
| max | 0.103 | 0.073 | 0.019 | 0.017 | 0.097 | 0.028 | |
| Pike | x | 0.029 | 0.014 | 0.003 | 0.003 | 0.018 | 0.005 |
| SD | 0.017 | 0.008 | 0.003 | 0.002 | 0.007 | 0.002 | |
| min | 0.007 | 0.003 | 0.000 | 0.001 | 0.005 | 0.001 | |
| max | 0.068 | 0.033 | 0.015 | 0.009 | 0.032 | 0.010 | |
| Spring | Summer | Autumn | Winter | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female Gonads | Testicles | Female Gonads | Testicles | Female Gonads | Testicles | Female Gonads | Testicles | |||||||||
| x | SD | x | SD | x | SD | x | SD | x | SD | x | SD | x | SD | x | SD | |
| ZINC mg/kg | ||||||||||||||||
| Bream | 28.92 A | 9.62 | 6.73 W | 3.18 | 99.42 B | 9.14 | 7.07 W | 2.53 | 69.18 B | 17.08 | 9.92 W | 7.00 | n.s. | n.s. | n.s. | n.s. |
| Roach | 48.75 A | 18.09 | 19.26 W | 11.85 | 70.77 A | 40.26 | 37.98 Y | 23.20 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Perch | 36.52 A | 12.29 | 42.61 W | 26.97 | 43.30 A | 15.22 | 58.43 Y | 27.81 | 88.07 B | 8.23 | n.s. | n.s. | 65.11 B | 10.73 | 12.85 Y | 6.33 |
| Pike | 97.16 A | 18.03 | 40.53 W | 11.26 | 215.21 B | 132.86 | n.s. | n.s. | 46.98 C | 12.33 | 16.01 Y | 3.51 | n.s. | n.s. | n.s. | n.s. |
| MEAN | 44.07 | 24.26 | 26.21 | 22.04 | 86.34 | 75.31 | 41.34 | 29.80 | 61.23 | 20.55 | 13.57 | 5.84 | 65.11 | 10.73 | 12.85 | 6.33 |
| ALUMINUM mg/kg | ||||||||||||||||
| Bream | 25.1 B | 12.8 | 31.8 Y | 11.6 | 49.0 A | 3.9 | 23.1 Y | 1.3 | 46.2 A | 24.8 | 32.9 Y | 25.2 | n.s. | n.s. | n.s. | n.s. |
| Roach | 32.2 A | 9.2 | 57.8 Y | 24.1 | 35.1 A | 10.9 | 57.2 Y | 14.9 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Perch | 33.2 A | 11.8 | 28.5 Y | 6.5 | 31.4 A | 9.2 | 29.1 Y | 7.1 | 29.9 A | 4.8 | n.s. | n.s. | 31.9 A | 10.1 | 26.3 | 5.5 |
| Pike | 25.1 B | 6.3 | 106.2 | 18.6 | 77.1 A | 55.1 | n.s. | n.s. | 74.1 A | 18.1 | 119.1 | 23.3 | n.s. | n.s. | n.s. | n.s. |
| MEAN | 29.42 | 11.17 | 51.63 | 33.78 | 41.84 | 24.95 | 37.28 | 17.43 | 57.40 | 25.76 | 84.65 | 49.49 | 31.89 | 10.06 | 26.31 | 5.45 |
| IRON mg/kg | ||||||||||||||||
| Bream | 5.5 B | 4.1 | 6.1 W | 6.1 | 24.8 A | 1.5 | 17.8 Y | 7.3 | 18.0 B | 6.4 | 11.6 Y | 2.9 | n.s. | n.s. | n.s. | n.s. |
| Roach | 11.9 A | 5.6 | 11.5 W | 8.1 | 7.4 A | 3.5 | 9.1 W | 4.9 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Perch | 14.1 A | 7.4 | 16.4 W | 8.9 | 11.4 A | 4.5 | 14.3 W | 8.5 | 14.1 A | 1.4 | n.s. | n.s. | 7.7 A | 1.5 | 2.1 Y | 1.1 |
| Pike | 18.7 A | 8.1 | 11.3 W | 2.6 | 16.2 A | 4.1 | n.s. | n.s. | 21.8 A | 5.7 | 10.8 W | 2.4 | n.s. | n.s. | n.s. | n.s. |
| MEAN | 11.18 | 7.17 | 11.13 | 7.71 | 12.46 | 6.95 | 13.23 | 7.55 | 19.23 | 6.02 | 11.09 | 2.59 | 7.71 | 1.51 | 2.05 | 1.12 |
| MANGANESE mg/kg | ||||||||||||||||
| Bream | 2.1 A | 1.1 | 0.3 W | 0.1 | 0.9 A | 0.01 | 0.2 W | 0.06 | 4.4 B | 1.7 | 0.4 W | 0.3 | n.s. | n.s. | n.s. | n.s. |
| Roach | 0.6 A | 0.07 | 1.1 W | 1.6 | 0.5 A | 0.08 | 0.9 W | 0.9 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Perch | 1.2 A | 0.4 | 0.6 W | 0.3 | 1.2 A | 0.5 | 0.9 W | 0.3 | 1.9 A | 0.7 | n.s. | n.s. | 1.9 A | 0.9 | 0.2 W | 0.06 |
| Pike | 0.4 A | 0.07 | 1.1 W | 0.2 | 1.1 A | 0.7 | n.s. | n.s. | 3.22 B | 0.5 | 1.0 W | 0.2 | n.s. | n.s. | n.s. | n.s. |
| MEAN | 1.19 | 0.92 | 0.71 | 0.80 | 0.86 | 0.47 | 0.72 | 0.59 | 3.37 | 1.35 | 0.76 | 0.36 | 1.91 | 0.95 | 0.23 | 0.06 |
| COPPER mg/kg | ||||||||||||||||
| Bream | 1.14 A | 0.68 | 0.49 W | 0.17 | 2.39 B | 0.71 | 0.73 W | 0.15 | 2.38 B | 0.51 | 0.76 W | 0.35 | n.s. | n.s. | n.s. | n.s. |
| Roach | 0.39 A | 0.06 | 0.65 W | 0.52 | 0.33 A | 0.07 | 0.41 W | 0.33 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Perch | 1.22 A | 0.43 | 0.84 W | 0.39 | 1.32 A | 0.79 | 1.37 W | 0.77 | 1.17 A | 0.04 | n.s. | n.s. | 1.67A | 0.29 | 0.47 Y | 0.18 |
| Pike | 0.53 A | 0.11 | 0.69 W | 0.20 | 0.97 A | 0.53 | n.s. | n.s. | 0.81 A | 0.13 | 0.46 W | 0.19 | n.s. | n.s. | n.s. | n.s. |
| MEAN | 0.88 | 0.57 | 0.66 | 0.34 | 1.05 | 0.88 | 0.92 | 0.70 | 1.39 | 0.79 | 0.58 | 0.30 | 1.67 | 0.29 | 0.47 | 0.18 |
| NICKEL mg/kg | ||||||||||||||||
| Bream | 0.063 A | 0.053 | 0.075 Y | 0.048 | 0.041 A | 0.003 | 0.011 Y | 0.014 | 0.113 A | 0.115 | 0.380 Y | 0.365 | n.s. | n.s. | n.s. | n.s. |
| Roach | 0.108 A | 0.062 | 0.476 Y | 0.794 | 0.129 A | 0.052 | 0.252 Y | 0.449 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Perch | 0.412 B | 0.142 | 0.319 Y | 0.208 | 0.173 A | 0.164 | 0.119 Y | 0.104 | 0.025 A | 0.02 | n.s. | n.s. | 0.03 A | 0.031 | 0.02 Y | 0.006 |
| Pike | 0.054 A | 0.041 | 0.074 Y | 0.064 | 0.075 A | 0.052 | n.s. | n.s. | 0.048 A | 0.045 | 0.018 Y | 0.008 | n.s. | n.s. | n.s. | n.s. |
| MEAN | 0.175 | 0.176 | 0.225 | 0.391 | 0.124 | 0.107 | 0.142 | 0.265 | 0.066 | 0.078 | 0.163 | 0.285 | 0.032 | 0.031 | 0.021 | 0.006 |
| LITHIUM mg/kg | ||||||||||||||||
| Bream | 0.11 A | 0.05 | 0.17 W | 0.12 | 0.10 A | 0.08 | n.s. | n.s. | 0.05 A | 0.05 | 0.04 W | 0.02 | n.s. | n.s. | n.s. | n.s. |
| Roach | 0.17 A | 0.13 | 0.19 W | 0.20 | 0.14 A | 0.06 | 0.14 W | 0.13 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Perch | 0.33 B | 0.25 | 0.21 W | 0.14 | 0.12 A | 0.09 | 0.07 Y | 0.06 | 0.06 A | 0.02 | n.s. | n.s. | 0.03 A | 0.01 | 0.06 Y | 0.02 |
| Pike | 0.10 A | 0.04 | 0.08 W | 0.03 | 0.10 A | 0.04 | n.s. | n.s. | 0.07 A | 0.01 | 0.12 W | 0.07 | n.s. | n.s. | n.s. | n.s. |
| MEAN | 0.190 | 0.174 | 0.167 | 0.135 | 0.120 | 0.072 | 0.103 | 0.098 | 0.062 | 0.031 | 0.085 | 0.066 | 0.035 | 0.014 | 0.056 | 0.022 |
| CHROMIUM mg/kg | ||||||||||||||||
| Bream | 0.07 A | 0.04 | 0.07 Y | 0.04 | 0.10 A | 0.02 | 0.01 Y | 0.01 | 0.08 A | 0.03 | 0.04 Y | 0.02 | n.s. | n.s. | n.s. | n.s. |
| Roach | 0.09 A | 0.03 | 0.41 Y | 0.65 | 0.12 A | 0.11 | 0.23 Y | 0.37 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Perch | 0.19 A | 0.14 | 0.17 Y | 0.14 | 0.18 A | 0.14 | 0.18 Y | 0.23 | 0.09 A | 0.01 | n.s. | n.s. | 0.10 A | 0.01 | 0.08 Y | 0.02 |
| Pike | 0.08 A | 0.01 | 0.06 Y | 0.01 | 0.12 A | 0.05 | n.s. | n.s. | 0.13 A | 0.02 | 0.05 Y | 0.01 | n.s. | n.s. | n.s. | n.s. |
| MEAN | 0.110 | 0.093 | 0.164 | 0.316 | 0.138 | 0.106 | 0.164 | 0.263 | 0.108 | 0.034 | 0.048 | 0.018 | 0.099 | 0.012 | 0.080 | 0.020 |
| LEAD mg/kg | ||||||||||||||||
| Bream | 0.03 A | 0.02 | 0.03 Y | 0.02 | 0.01 A | 0.01 | 0.01 Y | 0.01 | 0.02 A | 0.01 | 0.03 Y | 0.02 | n.s. | n.s. | n.s. | n.s. |
| Roach | 0.01 A | 0.01 | 0.07 Y | 0.02 | 0.02 A | 0.01 | 0.03 W | 0.02 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Perch | 0.03 A | 0.01 | 0.03 Y | 0.02 | 0.04 A | 0.01 | 0.04 Y | 0.01 | 0.03 A | 0.01 Y | n.s. | n.s. | 0.03 A | 0.00 | 0.02 Y | 0.00 |
| Pike | 0.01 A | 0.01 | 0.04 | 0.02 | 0.01 A | 0.01 | n.s. | n.s. | 0.02 A | 0.01 | 0.04 | 0.02 | n.s. | n.s. | n.s. | n.s. |
| MEAN | 0.022 | 0.015 | 0.040 | 0.024 | 0.021 | 0.013 | 0.031 | 0.019 | 0.024 | 0.012 | 0.034 | 0.019 | 0.031 | 0.004 | 0.018 | 0.003 |
| CADMIUM mg/kg * | ||||||||||||||||
| Bream | 0.002 | 0.001 | 0.002 | 0.003 | 0.001 | 0.000 | 0.001 | 0.000 | 0.004 | 0.002 | 0.004 | 0.003 | n.s. | n.s. | n.s. | n.s. |
| Roach | 0.003 | 0.002 | 0.006 | 0.006 | 0.003 | 0.002 | 0.007 | 0.004 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Perch | 0.002 | 0.001 | 0.004 | 0.003 | 0.011 | 0.020 | 0.008 | 0.008 | 0.002 | 0.000 | n.s. | n.s. | 0.004 | 0.001 | 0.002 | 0.000 |
| Pike | 0.003 | 0.001 | 0.003 | 0.001 | 0.003 | 0.003 | n.s. | n.s. | 0.002 | 0.002 | 0.004 | 0.005 | n.s. | n.s. | n.s. | n.s. |
| MEAN | 0.002 | 0.001 | 0.004 | 0.004 | 0.005 | 0.012 | 0.006 | 0.006 | 0.003 | 0.002 | 0.004 | 0.004 | 0.004 | 0.001 | 0.002 | 0.000 |
| MERCURY mg/kg * | ||||||||||||||||
| Bream | 0.005 | 0.002 | 0.004 | 0.002 | 0.007 | 0.004 | 0.004 | 0.002 | 0.005 | 0.002 | 0.004 | 0.003 | n.s. | n.s. | n.s. | n.s. |
| Roach | 0.018 | 0.023 | 0.031 | 0.031 | 0.021 | 0.032 | 0.033 | 0.028 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Perch | 0.013 | 0.003 | 0.013 | 0.003 | 0.010 | 0.002 | 0.011 | 0.004 | 0.011 | 0.004 | n.s. | n.s. | 0.012 | 0.004 | 0.012 | 0.003 |
| Pike | 0.028 | 0.003 | 0.018 | 0.005 | 0.015 | 0.003 | n.s. | n.s. | 0.018 | 0.009 | 0.017 | 0.007 | n.s. | n.s. | n.s. | n.s. |
| MEAN | 0.014 | 0.014 | 0.015 | 0.016 | 0.015 | 0.020 | 0.017 | 0.020 | 0.012 | 0.009 | 0.012 | 0.009 | 0.012 | 0.004 | 0.012 | 0.003 |
| Species | SPRING | SUMMER | AUTUMN | WINTER | |||||
|---|---|---|---|---|---|---|---|---|---|
| F v Mf | T v Mt | F v Mf | T v Mt | F v Mf | T v Mt | F v Mf | T v Mt | ||
| Zn | Bream | 0.276 | −0.021 | −0.530 | −0.809 | −0.836 | −0.504 | n.s. | n.s. |
| Roach | 0.156 | 0.404 | −0.220 | −0.560 | 0.250 | n.s. | −0.387 | 0.489 | |
| Perch | −0.907 | −0.632 | 0.432 | −0.334 | n.s. | n.s. | n.s. | n.s. | |
| Pike | −0.343 | −0.436 | −0.952 | n.s. | 0.183 | 0.618 | n.s. | n.s. | |
| Al | Bream | 0.659 | −0.200 | 0.223 | −0.592 | −0.886 | −0.175 | n.s. | n.s. |
| Roach | 0.149 | −0.178 | 0.348 | 0.472 | −0.296 | n.s. | −0.821 | 0.625 | |
| Perch | −0.546 | −0.686 | 0.477 | −0.361 | n.s. | n.s. | n.s. | n.s. | |
| Pike | −0.916 | 0.007 | 0.970 | n.s. | 0.699 | −0.595 | n.s. | n.s. | |
| Fe | Bream | −0.554 | −0.525 | 0.139 | −0.518 | 0.385 | −0.745 | n.s. | n.s. |
| Roach | 0.485 | 0.662 | 0.445 | 0.254 | 0.992 | n.s. | −0.335 | 0.705 | |
| Perch | −0.368 | 0.911 | −0.477 | −0.052 | n.s. | n.s. | n.s. | n.s. | |
| Pike | 0.076 | 0.575 | 0.966 | n.s. | −0.110 | −0.516 | n.s. | n.s. | |
| Mn | Bream | −0.534 | −0.309 | −0.262 | −0.826 | −0.314 | −0.997 | n.s. | n.s. |
| Roach | 0.176 | 0.32 | 0.565 | −0.154 | −0.906 | n.s. | −0.204 | −0.227 | |
| Perch | 0.561 | 0.595 | 0.617 | 0.074 | n.s. | n.s. | n.s. | n.s. | |
| Pike | 0.422 | 0.043 | 0.114 | n.s. | 0.052 | 0.855 | n.s. | n.s. | |
| Cu | Bream | −0.719 | −0.190 | 0.757 | 0.127 | −0.116 | −0.895 | n.s. | n.s. |
| Roach | −0.010 | 0.390 | −0.777 | −0.515 | −0.925 | n.s. | −0.543 | 0.317 | |
| Perch | −0.963 | 0.376 | 0.948 | −0.448 | n.s. | n.s. | n.s. | n.s. | |
| Pike | 0.940 | 0.487 | 0.634 | n.s. | 0.633 | −0.419 | n.s. | n.s. | |
| Ni | Bream | −0.514 | −0.393 | 0.877 | 0.815 | −0.995 | 0.998 | n.s. | n.s. |
| Roach | 0.263 | 0.208 | −0.509 | −0.165 | −0.513 | n.s. | 0.813 | −0.328 | |
| Perch | 0.593 | −0.015 | −0.429 | −0.211 | n.s. | n.s. | n.s. | n.s. | |
| Pike | 0.217 | −0.313 | 0.779 | n.s. | 0.902 | 0.219 | n.s. | n.s. | |
| Li | Bream | 0.739 | −0.342 | 0.593 | −0.377 | −0.805 | −0.944 | n.s. | n.s. |
| Roach | 0.229 | 0.124 | −0.163 | 0.332 | 0.513 | n.s. | 0.769 | 0.965 | |
| Perch | −0.644 | 0.598 | −0.451 | 0.578 | n.s. | n.s. | n.s. | n.s. | |
| Pike | −0.615 | 0.169 | −0.627 | n.s. | −0.013 | −0.121 | n.s. | n.s. | |
| Cr | Bream | −0.870 | 0.299 | 0.314 | 0.683 | 0.494 | 0.734 | n.s. | n.s. |
| Roach | 0.891 | 0.152 | 0.983 | 0.229 | −0.324 | n.s. | −0.044 | −0.849 | |
| Perch | −0.679 | 0.647 | 0.634 | 0.618 | n.s. | n.s. | n.s. | n.s. | |
| Pike | 0.145 | 0.249 | −0.970 | n.s. | 0.277 | 0.418 | n.s. | n.s. | |
| Pb | Bream | −0.104 | −0.249 | −0.251 | 0.671 | 0.964 | 0.977 | n.s. | n.s. |
| Roach | 0.407 | 0.574 | −0.891 | −0.670 | 0.541 | n.s. | 0.418 | −0.502 | |
| Perch | 0.231 | −0.060 | 0.611 | −0.071 | n.s. | n.s. | n.s. | n.s. | |
| Pike | −0.097 | 0.121 | 0.731 | n.s. | 0.657 | −0.788 | n.s. | n.s. | |
| Cd | Bream | 0.462 | −0.055 | −0.452 | −0.198 | −0.851 | 0.991 | n.s. | n.s. |
| Roach | 0.050 | 0.247 | 0.517 | −0.176 | 0.733 | n.s. | −0.506 | −0.482 | |
| Perch | 0.573 | −0.061 | −0.118 | 0.486 | n.s. | n.s. | n.s. | n.s. | |
| Pike | 0.985 | −0.503 | −0.873 | n.s. | −0.205 | −0.319 | n.s. | n.s. | |
| Hg | Bream | −0.385 | −0.414 | 0.174 | -0.493 | −0.839 | −0.277 | n.s. | n.s. |
| Roach | 0.176 | −0.09 | −0.724 | −0.452 | −0.958 | n.s. | 0.469 | −0.603 | |
| Perch | 0.163 | 0.239 | 0.667 | −0.498 | n.s. | n.s. | n.s. | n.s. | |
| Pike | −0.549 | −0.805 | 0.484 | n.s. | −0.528 | 0.247 | n.s. | n.s. | |
| Recommended Dietary Allowance % | Adequate Intake% | Provisional Tolerable Weekly Intake % | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn | Fe | Cu | Li | Mn | Al | Pb | Cd | Hg | |||||||||||||||||
| Women | Men | Women | Men | Adult | Adult | Women | Men | ||||||||||||||||||
| x | SD | x | SD | x | SD | x | SD | x | SD | x | SD | x | SD | x | SD | x | SD | x | SD | x | SD | x | SD | ||
| Bream | F | 24 | 13 | 17 | 10 | 5.6 | 4.0 | 4.1 | 2.9 | 6.8 | 3.4 | 0.3 | 0.2 | 5.1 | 3.5 | 4.0 | 2.7 | 0.9 | 0.5 | 0.05 | 0.03 | 0.02 | 0.01 | 0.07 | 0.03 |
| T | 3 | 2 | 2 | 1 | 4.3 | 3.0 | 3.1 | 2.1 | 2.4 | 1.0 | 0.4 | 0.4 | 0.6 | 0.4 | 0.5 | 0.3 | 0.8 | 0.4 | 0.05 | 0.03 | 0.02 | 0.02 | 0.05 | 0.03 | |
| Perch | F | 26 | 14 | 19 | 10 | 4.2 | 2.2 | 3.0 | 1.6 | 1.4 | 0.3 | 0.5 | 0.4 | 1.0 | 0.2 | 0.8 | 0.1 | 0.8 | 0.2 | 0.03 | 0.01 | 0.02 | 0.01 | 0.25 | 0.33 |
| T | 12 | 8 | 9 | 6 | 4.5 | 2.9 | 3.3 | 2.1 | 2.1 | 1.7 | 0.6 | 0.6 | 1.9 | 2.5 | 1.5 | 2.0 | 1.4 | 0.5 | 0.10 | 0.06 | 0.04 | 0.04 | 0.05 | 0.02 | |
| Roach | F | 23 | 9 | 16 | 7 | 5.1 | 2.4 | 3.7 | 1.7 | 5.2 | 2.0 | 0.6 | 0.7 | 2.8 | 1.4 | 2.2 | 1.1 | 0.8 | 0.2 | 0.06 | 0.02 | 0.04 | 0.08 | 0.14 | 0.04 |
| T | 17 | 13 | 12 | 9 | 5.0 | 4.1 | 3.7 | 3.0 | 3.5 | 2.4 | 0.4 | 0.4 | 1.1 | 0.7 | 0.9 | 0.5 | 0.7 | 0.2 | 0.06 | 0.03 | 0.03 | 0.04 | 0.15 | 0.04 | |
| Pike | F | 39 | 37 | 29 | 27 | 8.7 | 2.6 | 6.3 | 1.9 | 3.0 | 1.1 | 0.3 | 0.1 | 4.3 | 2.6 | 3.4 | 2.1 | 1.6 | 0.8 | 0.04 | 0.02 | 0.02 | 0.01 | 0.24 | 0.11 |
| T | 11 | 6 | 8 | 5 | 4.8 | 1.1 | 3.5 | 0.8 | 2.1 | 0.9 | 0.4 | 0.2 | 2.0 | 0.3 | 1.5 | 0.3 | 2.8 | 0.5 | 0.08 | 0.03 | 0.02 | 0.03 | 0.22 | 0.07 | |
| Species | Zn | Ni | Fe | Mn | Cr | Cu | Al | Li | Pb | Cd | Hg | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimated Daily Intake (EDI) | |||||||||||||
| Bream | F | x | 26.89 | 0.038 | 6.404 | 1.304 | 0.039 | 0.875 | 17.93 | 0.045 | 0.012 | 0.001 | 0.003 |
| SD | 15.06 | 0.038 | 4.546 | 0.896 | 0.018 | 0.434 | 9.82 | 0.030 | 0.008 | 0.001 | 0.001 | ||
| T | x | 3.90 | 0.082 | 4.922 | 0.153 | 0.025 | 0.309 | 15.26 | 0.058 | 0.011 | 0.001 | 0.002 | |
| SD | 2.34 | 0.127 | 3.374 | 0.107 | 0.019 | 0.130 | 8.05 | 0.054 | 0.009 | 0.001 | 0.001 | ||
| M | x | 1.55 | 0.015 | 0.837 | 0.125 | 0.032 | 0.082 | 9.03 | 0.026 | 0.009 | 0.001 | 0.001 | |
| SD | 0.59 | 0.013 | 0.341 | 0.095 | 0.018 | 0.032 | 3.95 | 0.011 | 0.008 | 0.001 | 0.001 | ||
| Roach | F | x | 25.90 | 0.099 | 5.820 | 0.711 | 0.075 | 0.675 | 15.88 | 0.080 | 0.016 | 0.003 | 0.006 |
| SD | 10.64 | 0.100 | 2.696 | 0.348 | 0.057 | 0.259 | 4.70 | 0.096 | 0.005 | 0.005 | 0.002 | ||
| T | x | 19.58 | 0.083 | 5.757 | 0.295 | 0.073 | 0.453 | 13.95 | 0.060 | 0.016 | 0.002 | 0.006 | |
| SD | 14.40 | 0.093 | 4.695 | 0.170 | 0.078 | 0.303 | 3.10 | 0.058 | 0.008 | 0.003 | 0.002 | ||
| M | x | 3.61 | 0.027 | 1.012 | 0.090 | 0.038 | 0.138 | 7.43 | 0.020 | 0.008 | 0.003 | 0.001 | |
| SD | 2.29 | 0.026 | 0.557 | 0.040 | 0.027 | 0.043 | 2.29 | 0.019 | 0.004 | 0.005 | 0.001 | ||
| Perch | F | x | 29.71 | 0.059 | 4.779 | 0.261 | 0.053 | 0.179 | 16.72 | 0.076 | 0.007 | 0.001 | 0.010 |
| SD | 16.02 | 0.028 | 2.516 | 0.040 | 0.039 | 0.035 | 4.90 | 0.050 | 0.004 | 0.001 | 0.013 | ||
| T | x | 19.58 | 0.083 | 5.757 | 0.295 | 0.073 | 0.453 | 13.95 | 0.060 | 0.016 | 0.002 | 0.006 | |
| SD | 14.40 | 0.093 | 4.695 | 0.170 | 0.078 | 0.303 | 3.10 | 0.058 | 0.008 | 0.003 | 0.002 | ||
| M | x | 2.42 | 0.067 | 0.719 | 0.114 | 0.029 | 0.082 | 12.54 | 0.037 | 0.008 | 0.002 | 0.006 | |
| SD | 1.52 | 0.113 | 0.503 | 0.040 | 0.019 | 0.043 | 7.10 | 0.032 | 0.007 | 0.002 | 0.003 | ||
| Pike | F | x | 45.07 | 0.027 | 9.962 | 1.108 | 0.059 | 0.390 | 32.25 | 0.040 | 0.010 | 0.001 | 0.009 |
| SD | 42.23 | 0.022 | 2.966 | 0.676 | 0.016 | 0.135 | 16.13 | 0.014 | 0.006 | 0.001 | 0.004 | ||
| T | x | 12.83 | 0.020 | 5.443 | 0.508 | 0.027 | 0.273 | 56.66 | 0.051 | 0.020 | 0.002 | 0.009 | |
| SD | 7.15 | 0.024 | 1.204 | 0.085 | 0.007 | 0.110 | 10.86 | 0.028 | 0.008 | 0.002 | 0.003 | ||
| M | x | 3.48 | 0.051 | 0.875 | 0.123 | 0.036 | 0.086 | 10.18 | 0.026 | 0.007 | 0.001 | 0.003 | |
| SD | 1.53 | 0.076 | 0.968 | 0.101 | 0.018 | 0.043 | 4.34 | 0.012 | 0.004 | 0.001 | 0.001 | ||
| Target Hazard Quotient (THQ) | |||||||||||||
| Bream | F | x | 0.090 | 0.002 | 0.009 | 0.009 | 0.013 | 0.022 | 0.045 | 0.002 | 0.003 | 0.001 | 0.027 |
| SD | 0.050 | 0.002 | 0.006 | 0.006 | 0.006 | 0.011 | 0.025 | 0.002 | 0.002 | 0.001 | 0.012 | ||
| T | x | 0.013 | 0.004 | 0.007 | 0.001 | 0.008 | 0.008 | 0.038 | 0.003 | 0.003 | 0.001 | 0.019 | |
| SD | 0.008 | 0.006 | 0.005 | 0.001 | 0.006 | 0.003 | 0.020 | 0.003 | 0.002 | 0.001 | 0.011 | ||
| M | x | 0.005 | 0.001 | 0.001 | 0.001 | 0.011 | 0.002 | 0.023 | 0.001 | 0.003 | 0.001 | 0.012 | |
| SD | 0.002 | 0.001 | 0.000 | 0.001 | 0.006 | 0.001 | 0.010 | 0.001 | 0.002 | 0.001 | 0.007 | ||
| Roach | F | x | 0.086 | 0.005 | 0.008 | 0.005 | 0.025 | 0.017 | 0.040 | 0.004 | 0.004 | 0.003 | 0.058 |
| SD | 0.035 | 0.005 | 0.004 | 0.002 | 0.019 | 0.006 | 0.012 | 0.005 | 0.001 | 0.005 | 0.016 | ||
| T | x | 0.065 | 0.004 | 0.008 | 0.002 | 0.024 | 0.011 | 0.035 | 0.003 | 0.004 | 0.002 | 0.062 | |
| SD | 0.048 | 0.005 | 0.007 | 0.001 | 0.026 | 0.008 | 0.008 | 0.003 | 0.002 | 0.003 | 0.018 | ||
| M | x | 0.012 | 0.001 | 0.001 | 0.001 | 0.013 | 0.003 | 0.019 | 0.001 | 0.002 | 0.003 | 0.013 | |
| SD | 0.008 | 0.001 | 0.001 | 0.000 | 0.009 | 0.001 | 0.006 | 0.001 | 0.001 | 0.005 | 0.014 | ||
| Perch | F | x | 0.099 | 0.003 | 0.007 | 0.002 | 0.018 | 0.004 | 0.042 | 0.004 | 0.002 | 0.001 | 0.099 |
| SD | 0.053 | 0.001 | 0.004 | 0.000 | 0.013 | 0.001 | 0.012 | 0.003 | 0.001 | 0.001 | 0.133 | ||
| T | x | 0.065 | 0.004 | 0.008 | 0.002 | 0.024 | 0.011 | 0.035 | 0.003 | 0.004 | 0.002 | 0.062 | |
| SD | 0.048 | 0.005 | 0.007 | 0.001 | 0.026 | 0.008 | 0.008 | 0.003 | 0.002 | 0.003 | 0.018 | ||
| M | x | 0.008 | 0.003 | 0.001 | 0.001 | 0.010 | 0.002 | 0.031 | 0.002 | 0.002 | 0.002 | 0.056 | |
| SD | 0.005 | 0.006 | 0.001 | 0.000 | 0.006 | 0.001 | 0.018 | 0.002 | 0.002 | 0.002 | 0.032 | ||
| Pike | F | x | 0.150 | 0.001 | 0.014 | 0.008 | 0.020 | 0.010 | 0.081 | 0.002 | 0.003 | 0.001 | 0.094 |
| SD | 0.141 | 0.001 | 0.004 | 0.005 | 0.005 | 0.003 | 0.040 | 0.001 | 0.002 | 0.001 | 0.043 | ||
| T | x | 0.043 | 0.001 | 0.008 | 0.004 | 0.009 | 0.007 | 0.142 | 0.003 | 0.006 | 0.002 | 0.086 | |
| SD | 0.024 | 0.001 | 0.002 | 0.001 | 0.002 | 0.003 | 0.027 | 0.001 | 0.002 | 0.002 | 0.029 | ||
| M | x | 0.012 | 0.003 | 0.001 | 0.001 | 0.012 | 0.002 | 0.025 | 0.001 | 0.002 | 0.001 | 0.027 | |
| SD | 0.005 | 0.004 | 0.001 | 0.001 | 0.006 | 0.001 | 0.011 | 0.001 | 0.001 | 0.001 | 0.011 | ||
| Total Target Hazard Quotient (TTHQ) | |||||||||||||
| Bream | F | 0.223 | |||||||||||
| T | 0.106 | ||||||||||||
| M | 0.060 | ||||||||||||
| Roach | F | 0.255 | |||||||||||
| T | 0.222 | ||||||||||||
| M | 0.069 | ||||||||||||
| Perch | F | 0.280 | |||||||||||
| T | 0.222 | ||||||||||||
| M | 0.119 | ||||||||||||
| Pike | F | 0.384 | |||||||||||
| T | 0.309 | ||||||||||||
| M | 0.087 | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokorska-Niewiada, K.; Witczak, A.; Protasowicki, M.; Cybulski, J. Estimation of Target Hazard Quotients and Potential Health Risks for Toxic Metals and Other Trace Elements by Consumption of Female Fish Gonads and Testicles. Int. J. Environ. Res. Public Health 2022, 19, 2762. https://doi.org/10.3390/ijerph19052762
Pokorska-Niewiada K, Witczak A, Protasowicki M, Cybulski J. Estimation of Target Hazard Quotients and Potential Health Risks for Toxic Metals and Other Trace Elements by Consumption of Female Fish Gonads and Testicles. International Journal of Environmental Research and Public Health. 2022; 19(5):2762. https://doi.org/10.3390/ijerph19052762
Chicago/Turabian StylePokorska-Niewiada, Kamila, Agata Witczak, Mikołaj Protasowicki, and Jacek Cybulski. 2022. "Estimation of Target Hazard Quotients and Potential Health Risks for Toxic Metals and Other Trace Elements by Consumption of Female Fish Gonads and Testicles" International Journal of Environmental Research and Public Health 19, no. 5: 2762. https://doi.org/10.3390/ijerph19052762
APA StylePokorska-Niewiada, K., Witczak, A., Protasowicki, M., & Cybulski, J. (2022). Estimation of Target Hazard Quotients and Potential Health Risks for Toxic Metals and Other Trace Elements by Consumption of Female Fish Gonads and Testicles. International Journal of Environmental Research and Public Health, 19(5), 2762. https://doi.org/10.3390/ijerph19052762






