Neurophysiologic Reactions during Heart Rate Variability Biofeedback Session in Adolescents with Different Risk of Internet Addiction
Abstract
:1. Introduction
2. Materials and Methods
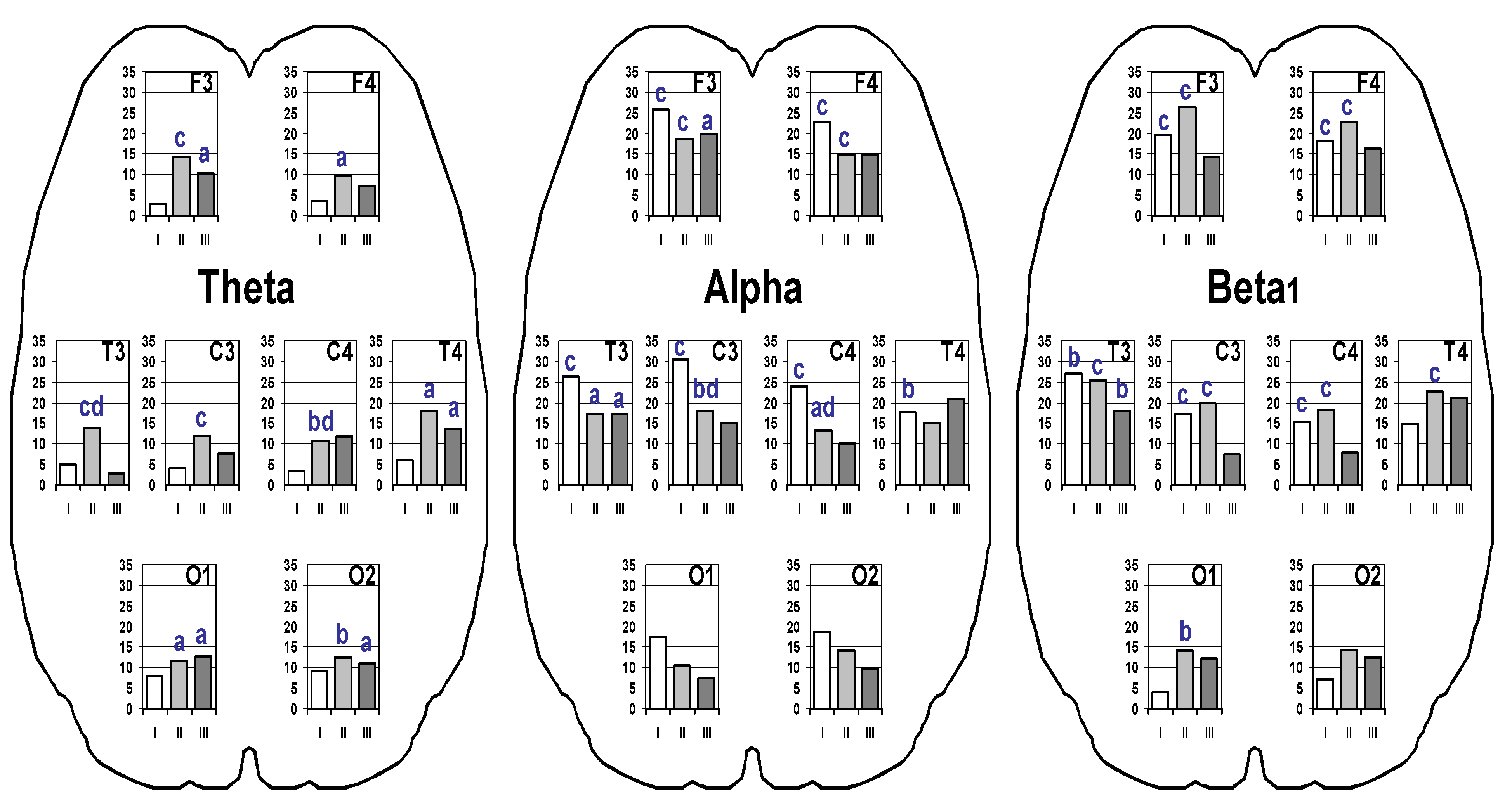
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuss, D.J.; Griffiths, M.D.; Binder, J.F. Internet addiction in students: Prevalence and risk factors. Comput. Hum. Behav. 2013, 29, 959–966. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Li, A.Y.-L. Internet Addiction Prevalence and Quality of (Real) Life: A Meta-Analysis of 31 Nations Across Seven World Regions. Cyberpsychol. Behav. Soc. Netw. 2014, 17, 755–760. [Google Scholar] [CrossRef] [Green Version]
- Young, K.S. Assessment issues with internet-addicted children and adolescents. In Internet Addiction in Children and Adolescents: Risk Factors, Assessment, and Treatment; Young, K.S., de Abreu, C.N., Eds.; Springer Publishing Company: New York, NY, USA, 2017; pp. 143–160. [Google Scholar] [CrossRef]
- Zhang, M.W.B.; Lim, R.B.C.; Lee, C.; Ho, R.C.M. Prevalence of Internet Addiction in Medical Students: A Meta-analysis. Acad. Psychiatry 2018, 42, 88–93. [Google Scholar] [CrossRef]
- Malygin, V.; Merkurieva, Y. Differentiated Intervention Model for Internet Addiction in Adolescents. Couns. Psychol. Psychother. 2020, 28, 142–163. [Google Scholar] [CrossRef]
- Cerniglia, L.; Zoratto, F.; Cimino, S.; Laviola, G.; Ammanti, M.; Adriani, W. Internet Addiction in adolescence: Neurobiological, psychosocial and clinical issues. Neurosci.Biobehav. Rev. 2016, 76, 174–184. [Google Scholar] [CrossRef]
- Kim, N.; Hughes, T.; Park, C.G.; Quinn, L.; Kong, I.D. Altered Autonomic Functions and Distressed Personality Traits in Male Adolescents with Internet Gaming Addiction. Cyberpsychol. Behav. Soc. Netw. 2016, 19, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Sepede, G.; Tavino, M.; Santacroce, R.; Fiori, F.; Salerno, R.M.; Di Giannantonio, M. Functional magnetic resonance imaging of internet addiction in young adults. World J. Radiol. 2016, 8, 210–225. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.C.W. Autonomic Nervous System and Brain Circuitry for Internet Addiction. In Internet Addiction. Neuroscientific Approaches and Therapeutical Implications Including Smartphone Addiction; Montag, C., Reuter, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 161–180. [Google Scholar]
- Lin, P.-C.; Kuo, S.-Y.; Lee, P.-H.; Sheen, T.-C.; Chen, S.-R. Effects of Internet Addiction on Heart Rate Variability in School-Aged Children. J. Cardiovasc. Nurs. 2014, 29, 493–498. [Google Scholar] [CrossRef]
- Lehrer, P.; Kaur, K.; Sharma, A.; Shah, K.; Huseby, R.; Bhavsar, J.; Sgobba, P.; Zhang, Y. Heart Rate Variability Biofeedback Improves Emotional and Physical Health and Performance: A Systematic Review and Meta Analysis. Appl. Psychophysiol. Biofeedback 2020, 45, 109–129. [Google Scholar] [CrossRef]
- Blumenstein, B.; Breslav, I.; Bar-Eli, M.; Tenenbaum, G.; Weinstein, Y. Regulation of mental states and biofeedback techniques: Effects on breathing pattern. Appl. Psychophysiol. Biofeedback 1995, 20, 169–183. [Google Scholar] [CrossRef]
- Poskotinova, L.; Demin, D.; Krivonogova, E. Short-term HRV Biofeedback: Perspectives in Environmental Physiology and Medicine. Int. J. Biomed. 2017, 7, 24–27. [Google Scholar] [CrossRef]
- Baevsky, R.; Ivanov, G.; Chireykin, L.; Gavrilushkin, A.; Dovgalevsky, P.; Kukushkin, Y.; Mironova, T.; Prilutsky, D.; Semenov, A.; Fedorov, V.; et al. Analysis of heart rate variability when using different electrocar-diographic systems (part 1). J. Arrhythmology 2002, 24, 65–86. [Google Scholar]
- Demin, D.; Poskotinova, L. Changes in the Spectral Characteristics of the Electroencephalogram during Biocontrol of Heart Rate Variability Parameters in Healthy Subjects. Neurosci. Behav. Physiol. 2018, 48, 913–916. [Google Scholar] [CrossRef]
- Demin, D.; Poskotinova, L.; Grjibovski, A.M.; Varakina, Z. Neurophysiological Effects of Heart Rate Variability Biofeedback Training in Adolescents: A Russian Study. Int. J. Epidemiol. 2015, 44, 216. [Google Scholar] [CrossRef]
- Skok, A.B.; Shubina, O.S.; Shtark, M.B. Neurobiofeedback in the treatment of addictive disorders and attention deficite syndrome: Basis and approaches. In Biofeedback-4: Theory and Practice; CERIS: Novosibirsk, Russia, 2002; pp. 142–151. [Google Scholar]
- Poskotinova, L.; Krivonogova, O.; Zaborsky, O. Cardiovascular response to physical exercise and risk of Internet-addiction in 15-16 year-old adolescents. J. Behav. Addict 2021, 10, 347–351. [Google Scholar] [CrossRef]
- Krivonogova, O.; Krivonogova, E.; Poskotinova, L. Heart rate variability, time estimation and Internet-dependent behavior in 16-17-year-old adolescents: A Study in Russian Arctic. Life 2021, 11, 497. [Google Scholar] [CrossRef]
- The WHO Child Growth Standards. Available online: https://www.who.int/childgrowth/standards/en (accessed on 15 January 2022).
- Chen, S.; Weng, L.; Su, Y.; Wu, H.; Yang, P. Development of a Chinese Internet addiction scale and its psychometric study. Chin. J. Psychol. 2003, 45, 279–294. [Google Scholar]
- Malygin, V.L.; Feklysov, K.A.; Iskandirova, A.B.; Antonenko, A.A. Methodological approaches to the early detection of Internet-dependent behavior. Med. Psihol. Ross. 2011, 6, 32–33. [Google Scholar]
- Method for Correcting Autonomic Nervous Misbalance States with Varicard Complex for Processing Cardiointervalograms and Analyzing Cardiac Rhythm Variability, Operating under Computer Software Program with Biofeedback. Available online: https://patentimages.storage.googleapis.com/2a/b4/c7/68fcfa35cce6e1/RU2317771C2.pdf (accessed on 15 January 2022).
- Travis, F.; Haaga, D.A.; Hagelin, J. A self-referential default brain state: Patterns of coherence, power and eLORETA sources during eyes-closed rest and transcendental meditation practice. Cogn. Process. 2010, 11, 21–30. [Google Scholar] [CrossRef]
- Kim, D.-K.; Lee, K.-M.; Kim, J.; Whang, M.-C.; Kang, S.W. Dynamic correlations between heart and brain rhythm during Autogenic meditation. Front. Hum. Neurosci. 2013, 7, 414. [Google Scholar] [CrossRef] [Green Version]
- Maier, S.F.; Watkins, L.R. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010, 1355, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palma, J.-A.; Benarroch, E.E. Neural control of the heart: Recent concepts and clinical correlations. Neurology 2014, 83, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D.; Mathias, C.J.; Josephs, O.; O’Doherty, J.; Zanini, S.; Dewar, B.; Cipolotti, L.; Shallice, T.; Dolan, R. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain 2003, 126, 2139–2152. [Google Scholar] [CrossRef] [Green Version]
- Shoemaker, J.K.; Goswami, R. Forebrain neurocircuitry associated with human reflex cardiovascular control. Front. Physiol. 2015, 6, 240. [Google Scholar] [CrossRef] [Green Version]
- Ikemoto, S.; Yang, C.; Tan, A. Basal ganglia circuit loops, dopamine and motivation: A review and enquiry. Behav. Brain Res. 2015, 290, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Ezzyat, Y.; Wanda, P.A.; Levy, D.F.; Kadel, A.; Aka, A.; Pedisich, I.; Sperling, M.R.; Sharan, A.D.; Lega, B.C.; Burks, A.; et al. Closed-loop stimulation of temporal cortex rescues functional networks and improves memory. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Engel, A.K.; Fries, P. Beta-band oscillations—signalling the status quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef]
- Demin, D.B.; Poskotinova, L.V.; Krivonogova, E.V. Eeg Reactions during Heart Rate Variability Biofeedback Procedure in Adolescents with Different Autonomic Tone Living in Northern Areas. Hum. Ecol. 2016, 23, 23–30. [Google Scholar] [CrossRef] [Green Version]
| Variables | Group | Baseline | HRV BF |
|---|---|---|---|
| SI, units | I | 119.0 (71.9; 208.4) | 78.1 (47.0,129.4) b |
| II | 115.2 (78.7; 193.6) | 60.2 (41.3; 101.4) b | |
| III | 183.3 (106.8; 329.1) | 83.6 (52.1; 114.0) b | |
| TP, ms2 | I | 1899 (1468; 2923) | 83.6 (52.1; 114.0) b |
| II | 1895 (1350; 2961) | 6135 (3865; 8793) b | |
| III | 1565 (1170; 2277) | 5078 (3445; 6743) b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demin, D.; Poskotinova, L. Neurophysiologic Reactions during Heart Rate Variability Biofeedback Session in Adolescents with Different Risk of Internet Addiction. Int. J. Environ. Res. Public Health 2022, 19, 2759. https://doi.org/10.3390/ijerph19052759
Demin D, Poskotinova L. Neurophysiologic Reactions during Heart Rate Variability Biofeedback Session in Adolescents with Different Risk of Internet Addiction. International Journal of Environmental Research and Public Health. 2022; 19(5):2759. https://doi.org/10.3390/ijerph19052759
Chicago/Turabian StyleDemin, Denis, and Liliya Poskotinova. 2022. "Neurophysiologic Reactions during Heart Rate Variability Biofeedback Session in Adolescents with Different Risk of Internet Addiction" International Journal of Environmental Research and Public Health 19, no. 5: 2759. https://doi.org/10.3390/ijerph19052759
APA StyleDemin, D., & Poskotinova, L. (2022). Neurophysiologic Reactions during Heart Rate Variability Biofeedback Session in Adolescents with Different Risk of Internet Addiction. International Journal of Environmental Research and Public Health, 19(5), 2759. https://doi.org/10.3390/ijerph19052759






