Oral Malignant Non-Hodgkin Lymphoma: A Retrospective Single-Center Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Data Collection Method and Study Variables
2.3. Statistical Analysis
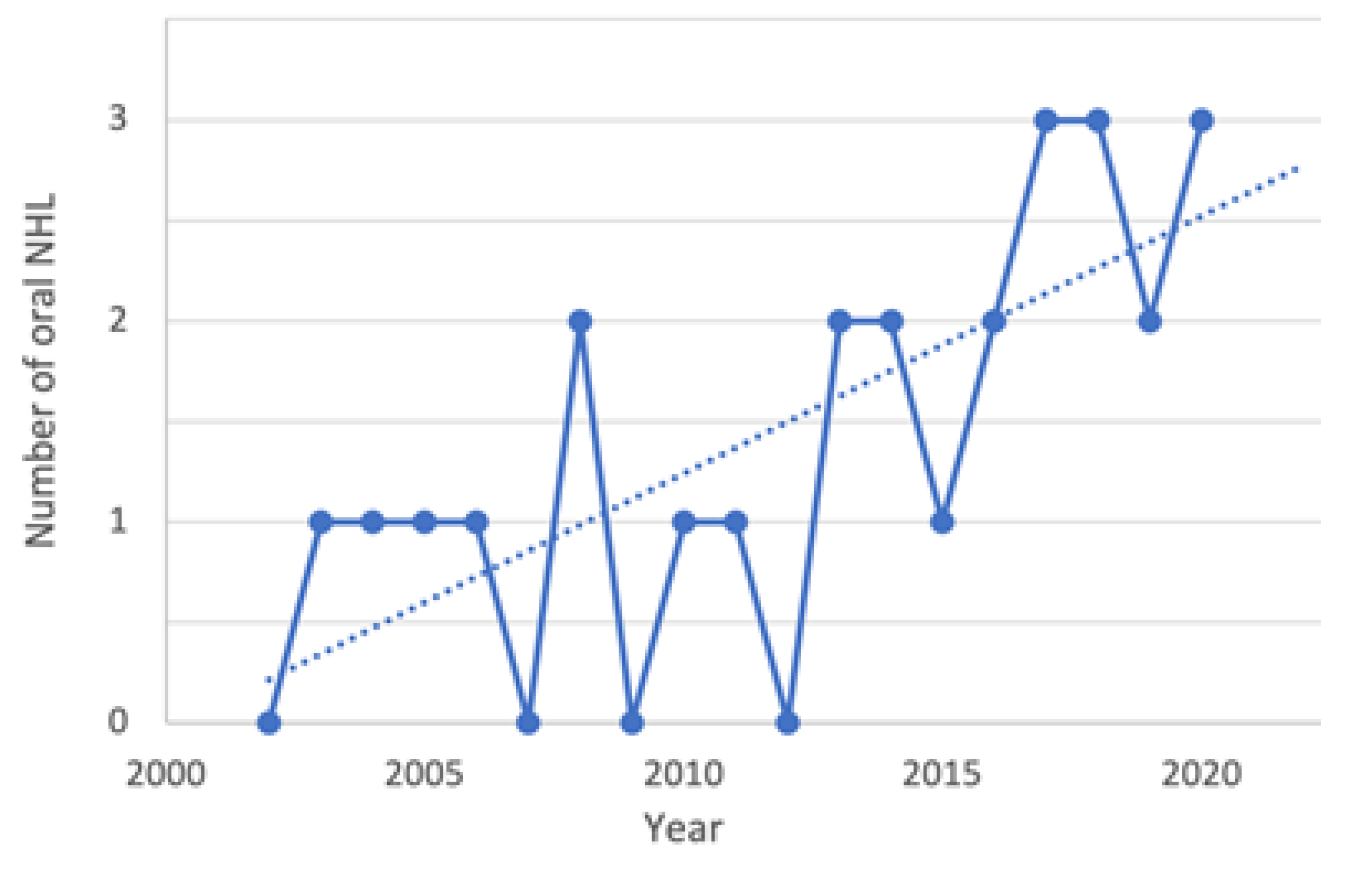
3. Results
3.1. Study Sample
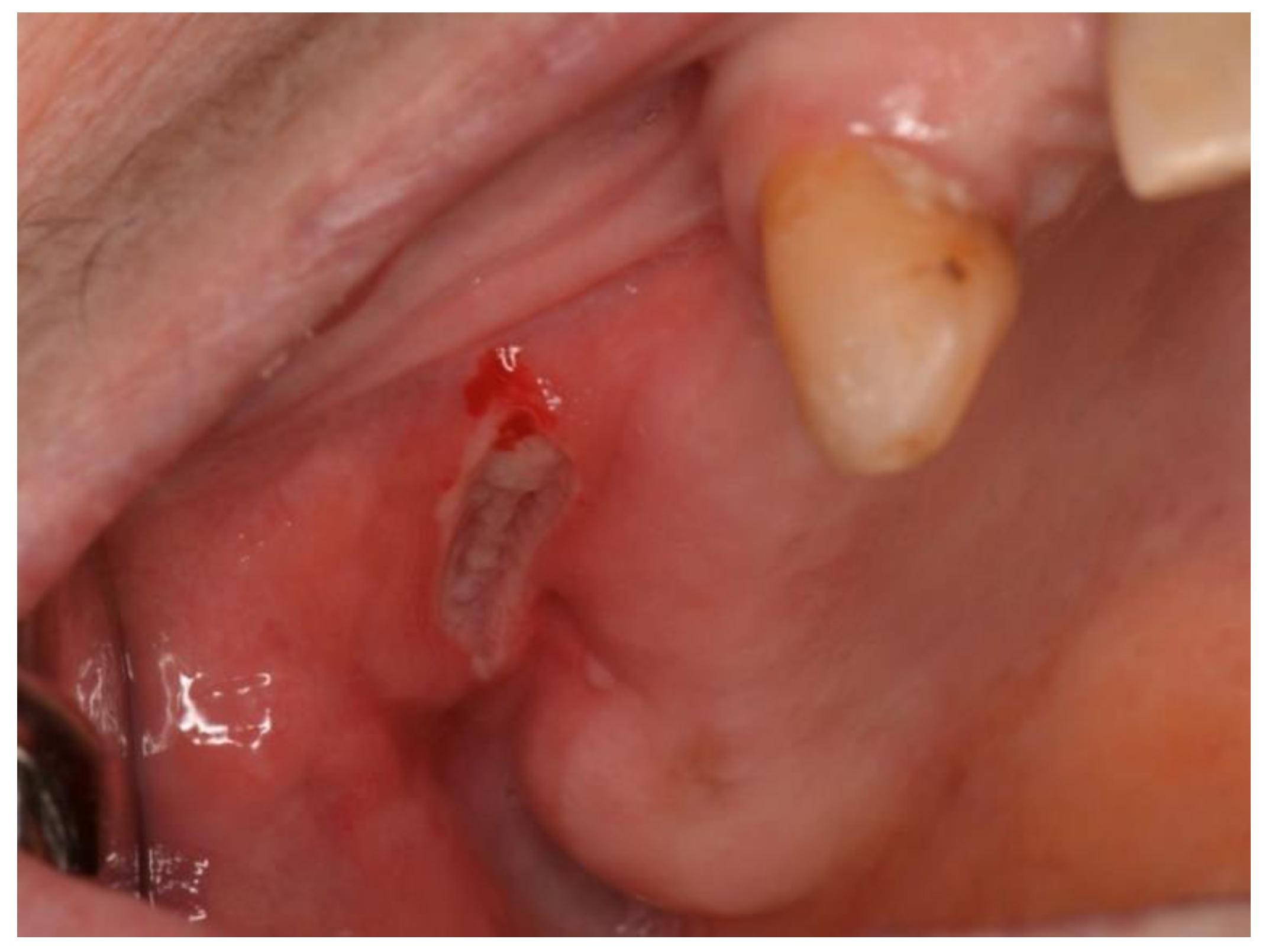
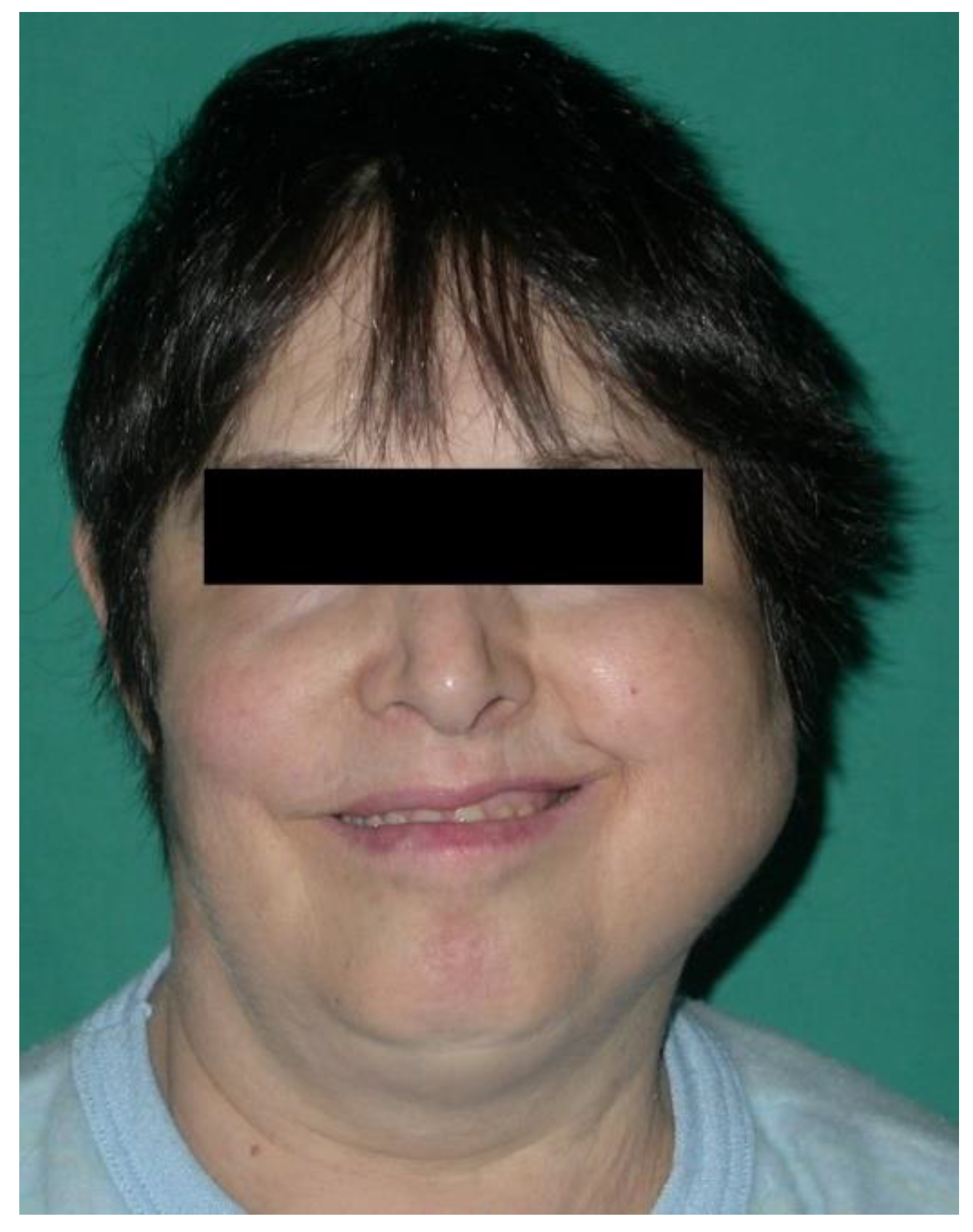
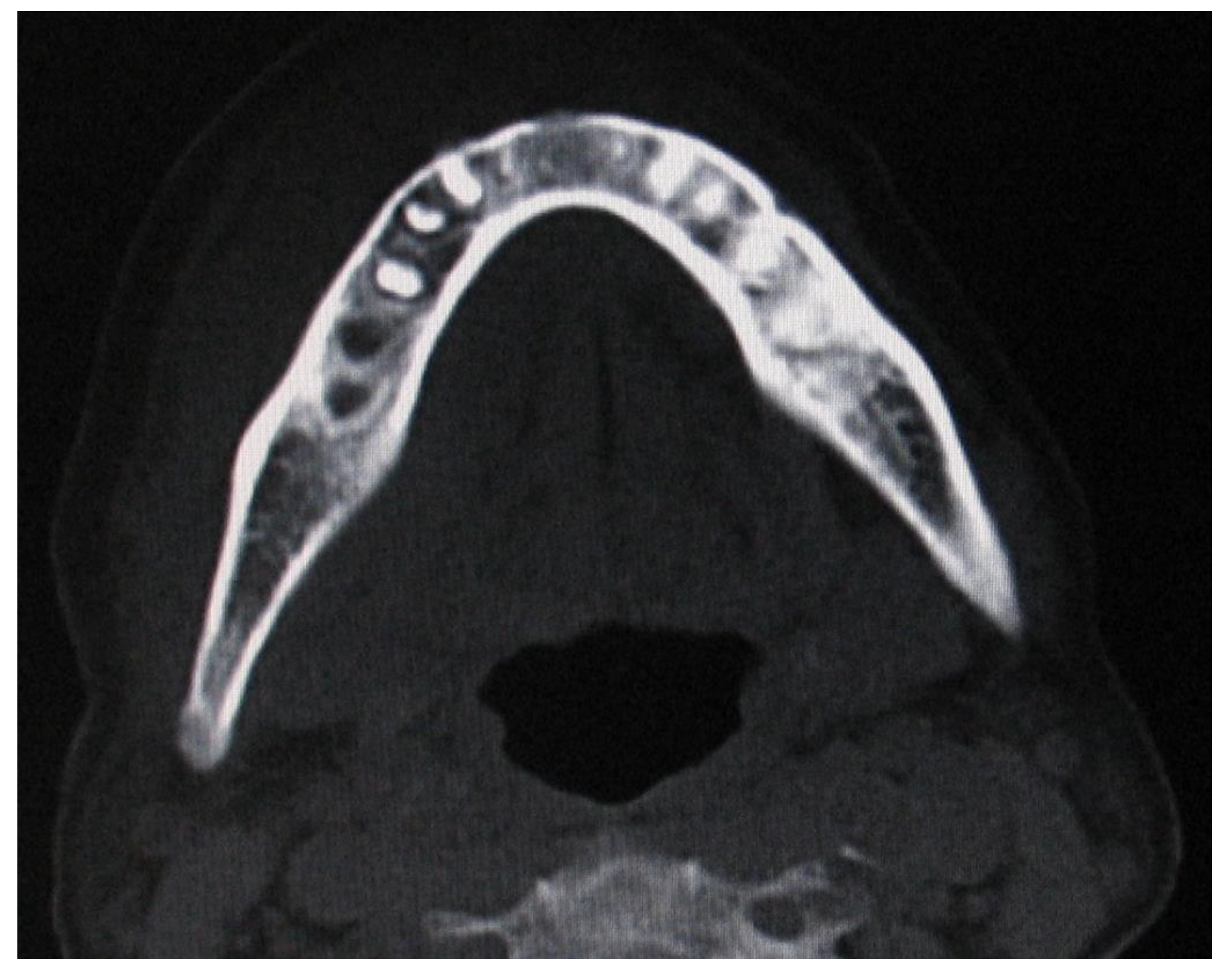
3.2. NHL Manifestations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mawardi, H.; Cutler, C.; Treister, N. Medical management update: Non-hodgkin lymphoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 107, e19–e33. [Google Scholar] [CrossRef]
- Iguchi, H.; Wada, T.; Matsushita, N.; Oishi, M.; Yamane, H. Anatomic distribution of hematolymphoid malignancies in the head and neck: 7 years of experience with 122 patients in a single institution. Acta Oto-Laryngol. 2012, 132, 1224–1231. [Google Scholar] [CrossRef]
- Budhy, T.I.; Soenarto, S.D.; Yaacob, H.B.; Ngeow, W.C. Changing incidence of oral and maxillofacial tumors in East Java, Indonesia, 1987–1992. Part 2: Malignant tumours. Br. J. Oral Maxillofac. Surg. 2001, 39, 460–464. [Google Scholar] [CrossRef][Green Version]
- Harris, N.L.; Jaffe, E.S.; Stein, H.; Banks, P.M.; Chan, J.K.; Cleary, M.L.; Gatter, K.C. A revised European-American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood 1994, 84, 1361. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Chi, H.-S.; Lee, K.-W.; Chiang, F.-Y.; Tai, C.-F.; Wang, L.-F.; Yang, S.-F.; Lin, S.-F.; Kuo, W.-R. Head and neck extranodal lymphoma in a single institute: A 17-year retrospective analysis. Kaohsiung J. Med Sci. 2012, 28, 435–441. [Google Scholar] [CrossRef]
- Hart, S.; Horsman, J.M.; Radstone, C.R.; Hancock, H.; Goepel, J.R.; Hancock, B.W. Localized extranodal lymphoma of the head and neck: The Sheffield Lymphoma Group experience (1971–2000). Clin. Oncol. 2004, 16, 186–192. [Google Scholar] [CrossRef]
- Bussu, F.; Hohaus, S.; Bastanza, G.; Bozzoli, V.; Tisi, M.; Martini, M.; Paludetti, G.; Almadori, G. Clinical and prognostic features of lymphomas arising in the head and neck region: Our experience of preferential association of different histotypes with various sites of origin in ninety patients. Clin. Otolaryngol. 2013, 38, 248–253. [Google Scholar] [CrossRef]
- Alli, N.; Meer, S. Head and neck lymphomas: A 20-year review in an oral pathology unit, Johannesburg, South Africa, a country with the highest global incidence of HIV/AIDS. Oral Oncol. 2017, 67, 17–23. [Google Scholar] [CrossRef]
- Guastafierro, S.; Falcone, U.; Celentano, M.; Cappabianca, S.; Giudice, A.; Colella, G. Primary mantle-cell non-Hodgkin’s lymphoma of the tongue. Int. J. Hematol. 2008, 88, 206–208. [Google Scholar] [CrossRef]
- Söderholm, A.L.; Lindqvist, C.; Heikinheimo, K.; Forssell, K.; Happonen, R.P. Non-Hodgkin’s lymphomas presenting through oral symptoms. Int. J. Oral Maxillofac. Surg. 1990, 19, 131–134. [Google Scholar] [CrossRef]
- Scherfler, S.; Freier, K.; Seeberger, R.; Bacon, C.; Hoffmann, J.; Thiele, O.C. Cranio-maxillofacial non-Hodgkin’s lymphoma: Clinical and histological presentation. J. Cranio-Maxillofac. Surg. 2011, 40, e211–e213. [Google Scholar] [CrossRef]
- van der Waal, R.; Huijgens, P.; van der Valk, P.; van der Waal, I. Characteristics of 40 primary extranodal non-Hodgkin lymphomas of the oral cavity in perspective of the new WHO classification and the International Prognostic Index. Int. J. Oral Maxillofac. Surg. 2005, 34, 391–395. [Google Scholar] [CrossRef]
- Kemp, S.; Gallagher, G.; Kabani, S.; Noonan, V.; O’Hara, C. Oral non-Hodgkin’s lymphoma: Review of the literature and World Health Organization classification with reference to 40 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 105, 194–201. [Google Scholar] [CrossRef]
- Das, U.; Chennagiri, S.P.; Sirsath, N.T.; Lakshmaiah, K.; Lokanatha, D.; Ramarao, C. Primary extranodal non-Hodgkin’s lymphoma of oral cavity—A single centre retrospective study. J. Cancer Res. Ther. 2014, 10, 945–950. [Google Scholar] [CrossRef]
- Giudice, A.; Liborio, F.; Averta, F.; Barone, S.; Fortunato, L. Oral lichenoid reaction: An uncommon side effect of rituximab. Case Rep. Dent. 2019, 2019, 3154856. [Google Scholar] [CrossRef]
- Giudice, A.; Bennardo, F.; Barone, S.; Antonelli, A.; Figliuzzi, M.M.; Fortunato, L. Can autofluorescence guide surgeons in the treatment of medication-related osteonecrosis of the jaw? A prospective feasibility study. J. Oral Maxillofac. Surg. 2018, 76, 982–995. [Google Scholar] [CrossRef]
- Fortunato, L.; Amato, M.; Simeone, M.; Bennardo, F.; Barone, S.; Giudice, A. Numb chin syndrome: A reflection of malignancy or a harbinger of MRONJ? A multicenter experience. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 389–394. [Google Scholar] [CrossRef]
- Lyons, S.F.; Liebowitz, D.N. The roles of human viruses in the pathogenesis of lymphoma. Semin. Oncol. 1998, 25, 461–475. [Google Scholar]
- Young, G.A.R.; Iland, H.J. Clinical perspectives in lymphoma. Intern. Med. J. 2007, 37, 478–484. [Google Scholar] [CrossRef]
- Epstein, J.B.; Epstein, J.D.; Le, N.D.; Gorsky, M. Characteristics of oral and paraoral malignant lymphoma: A population-based review of 361 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2001, 92, 519–525. [Google Scholar] [CrossRef]
- Jacobs, C.; Hoppe, R.T. Non-Hodgkin’s lymphomas of head and neck extranodal sites. Int. J. Radiat. Oncol. Biol. Phys. 1985, 11, 357–364. [Google Scholar] [CrossRef]
- Agrawal, M.G.; Agrawal, S.M.; Kambalimath, D.H. Non-Hodgkins lymphoma of maxilla: A rare entity. Natl. J. Maxillofac. Surg. 2011, 2, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.; Ziebart, T.; Sagheb, K.; Rahimi-Nedjat, R.K.; Manz, A.; Hess, G. Malignant lymphomas in the head and neck region—A retrospective, single-center study over 41 years. Int. J. Med. Sci. 2015, 12, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Giordano, M.; Russo, C.; Puzzo, L.; Trainiti, M.; Consoli, A.S.; Catania, V.E. Lymphoma of cheek: A case report. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 4–7. [Google Scholar] [PubMed]
- Silva, T.D.B.; Ferreira, C.B.T.; Leite, G.B.; Pontes, J.R.D.M.; Antunes, H.S. Oral manifestations of lymphoma: A systematic review. Ecancermedicalscience 2016, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Biamonte, F.; Buffone, C.; Santamaria, G.; Battaglia, A.M.; Mignogna, C.; Fortunato, L.; Costanzo, F.S.; Giudice, A. Gene expression analysis of autofluorescence margins in leukoplakia and oral carcinoma: A pilot study. Oral Dis. 2021, 27, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Kolokotronis, A.; Konstantinou, N.; Christakis, I.; Papadimitriou, P.; Matiakis, A.; Zaraboukas, T.; Antoniades, D. Localized B-cell non-Hodgkin’s lymphoma of oral cavity and maxillofacial region: A clinical study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 99, 303–310. [Google Scholar] [CrossRef]
- Ali, A.; Al-Belushi, B.S.; Waly, M.; Al-Moundhri, M.; Burney, I.A. Dietary and lifestyle factors and risk of non-hodgkin’s lymphoma in oman. Asian Pac. J. Cancer Prev. 2013, 14, 841–848. [Google Scholar] [CrossRef]
- Triantafillidou, K.; Dimitrakopoulos, J.; Iordanidis, F.; Gkagkalis, A. Extranodal non-hodgkin lymphomas of the oral cavity and maxillofacial region: A clinical study of 58 cases and review of the literature. J. Oral Maxillofac. Surg. 2012, 70, 2776–2785. [Google Scholar] [CrossRef]
- Basirat, M.; Rabiei, M.; Bashardoust, N. Incidence of head and neck lymphoma in Guilan province, Iran. Asian Pac. J. Cancer Prev. 2016, 17, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Nocini, P.; Muzio, L.L.; Fior, A.; Staibano, S.; Mignogna, M.D. Primary non-Hodgkin’s lymphoma of the jaws: Immunohistochemical and genetic review of 10 cases. J. Oral Maxillofac. Surg. 2000, 58, 636–644. [Google Scholar] [CrossRef]
- Tseng, C.H.; Wang, W.C.; Chen, C.Y.; Hsu, H.J.; Chen, Y.K. Clinical manifestations of oral lymphomas e retrospective study of 15 cases in a Taiwanese population and a review of 592 cases from the literature. J. Formos. Med. Assoc. 2020, 120 Pt 2, 361–370. [Google Scholar] [CrossRef]
- Deng, D.; Wang, Y.; Liu, W.; Qian, Y. Oral and maxillofacial non-Hodgkin lymphomas: Case report with review of literature. Medicine 2017, 96, e7890. [Google Scholar] [CrossRef] [PubMed]
- Storck, K.; Brandstetter, M.; Keller, U.; Knopf, A. Clinical presentation and characteristics of lymphoma in the head and neck region. Head Face Med. 2019, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Eisenbud, L.; Sciubba, J.; Mir, R.; Sachs, S.A. Oral presentations in non-Hodgkin’s lymphoma: A review of thirty-one cases. Part I. Data analysis. Oral Surg. Oral Med. Oral Pathol. 1983, 56, 15. [Google Scholar] [CrossRef]
- Picard, A.; Cardinne, C.; Denoux, Y.; Wagner, I.; Chabolle, F.; Bach, C. Extranodal lymphoma of the head and neck: A 67-case series. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2015, 132, 71–75. [Google Scholar] [CrossRef]
- Guevara-Canales, J.; Morales-Vadillo, R.; Sacsaquispe-Contreras, S.; Barrionuevo, C.; Montes, J.; Cava-Vergiú, C.E.; Soares, F.A.; Chaves-Netto, H.; Chaves, M. Malignant lymphoma of the oral cavity and the maxillofacial region: Overall survival prognostic factors. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e619–e626. [Google Scholar] [CrossRef]
- Richards, A.; Costelloe, M.A.; Eveson, J.W.; Scully, C.; Irvine, G.H.; Rooney, N. Oral mucosal non-Hodgkin’s lymphoma—A dangerous mimic. Oral Oncol. 2000, 36, 556–558. [Google Scholar] [CrossRef]
- Fortunato, L.; Barone, S.; Bennardo, F.; Giudice, A. Management of facial pyoderma gangrenosum using platelet-rich fibrin: A technical report. J. Oral Maxillofac. Surg. 2018, 76, 1460–1463. [Google Scholar] [CrossRef]
- Li, S.; Young, K.H.; Medeiros, L.J. Diffuse large B-cell lymphoma. Pathology 2018, 50, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Corti, M.; Villafañe, M.F.; Bistmans, A.; Campitelli, A.; Narbaitz, M.; Baré, P. Oral cavity and extraoral plasmablastic lymphomas in AIDS patients: Report of five cases and review of the literature. Int. J. STD AIDS 2011, 22, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.G.; Fisher, R.I. The epidemiology of non-Hodgkin’s lymphoma. Oncogene 2004, 23, 6524–6534. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Onishi, N.; Morito, T.; Takata, K.; Mizobuchi, K.; Nagatsuka, H.; Ichimura, K.; Tanaka, T.; Tamura, M.; Yoshino, T. Patients with localized primary non-tonsillar oral diffuse large B-cell lymphoma exhibit favorable prognosis despite a non-germinal center B-cell-like phenotype. Cancer Sci. 2009, 100, 42–46. [Google Scholar] [CrossRef]

| Case | Year of Diagnosis | Gender | Age (Years) | Occupational Exposure | Site | Signs and Symptoms | VAS | Diagnosis | Serological Test | Treatment/ |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2003 | M | 68 | N/A | Cheek | Swelling, pain | 5 | DLBCL | EBV+ | Outcome |
| 2 | 2004 | M | 46 | N/A | Palate | Swelling, ulceration | - | BL | - | CT + RT/died |
| 3 | 2005 | F | 52 | Yes | Cheek | Swelling, mucosal bleeding | - | DLBCL | - | N/A |
| 4 | 2006 | M | 56 | Yes | Gingiva | Swelling, ulceration | - | PBL | HIV+ | CT + RT/alive |
| 5 | 2008 | F | 66 | Yes | Tongue | Swelling, pain | 2 | DLBCL | - | N/A |
| 6 | 2008 | F | 82 | N/A | Palate | Swelling, ulceration | - | SLL | - | CT/alive |
| 7 | 2010 | M | 45 | Yes | Tongue | Swelling, ulceration | - | DLBCL | EBV+ | N/A |
| 8 | 2011 | M | 42 | Yes | Buccal mucosa | Mucosal bleeding, difficulties in swallowing | - | ENMZL | EBV+ | CT/alive |
| 9 | 2013 | M | 49 | N/A | Buccal mucosa | Swelling, ulceration | - | PBL | HIV+ | N/A |
| 10 | 2013 | F | 51 | Yes | Buccal mucosa | Mucosal bleeding, ulceration | - | PBL | HIV+ | CT/alive |
| 11 | 2014 | M | 55 | No | Tongue | Swelling, difficulties in swallowing | - | PBL | HIV+ | CT + RT/alive |
| 12 | 2014 | F | 73 | N/A | Buccal mucosa | Swelling | - | DLBCL | - | N/A |
| 13 | 2015 | M | 43 | Yes | Cheek | Swelling, ulceration | - | DLBCL | - | CT + RT/died |
| 14 | 2016 | F | 61 | Yes | Palate | Swelling, ulceration | - | PTCL | EBV+ | CT + RT/alive |
| 15 | 2016 | F | 76 | No | Buccal mucosa | Pain, ulceration | 5 | DLBCL | - | CT/died |
| 16 | 2017 | M | 45 | N/A | Gingiva | Bleeding | - | FL | - | CT + RT/alive |
| 17 | 2017 | M | 72 | Yes | Buccal mucosa | Paresthesia | - | ENMZL | EBV+ | N/A |
| 18 | 2017 | M | 54 | Yes | Tongue | Swelling | - | DLBCL | - | N/A |
| 19 | 2018 | F | 70 | No | Gingiva | Swelling, pain, ulceration | 3 | PBL | - | CT + RT/alive |
| 20 | 2018 | F | 60 | N/A | Mandible | Swelling, pain, tooth mobility, paresthesia | 5 | DLBCL | - | CT/died |
| 21 | 2018 | M | 58 | Yes | Buccal mucosa | Swelling, ulceration | - | MCL | - | CT + RT/alive |
| 22 | 2019 | M | 53 | Yes | Buccal mucosa | Swelling | - | PBL | HIV+ | N/A |
| 23 | 2019 | M | 64 | Yes | Tongue | Swelling, difficulties in swallowing | - | DLBCL | EBV+ | CT/died |
| 24 | 2020 | M | 44 | Yes | Buccal mucosa | Swelling | - | PBL | - | CT/alive |
| 25 | 2020 | F | 74 | No | Buccal mucosa | Swelling, ulceration | - | DLBCL | - | CT/died |
| 26 | 2020 | F | 51 | Yes | Palate | Swelling, pain, ulceration | 4 | PTCL | - | CT/alive |
| Patients | n | 26 | 100% |
| Gender | Male | 15 | 57.6% |
| Female | 11 | 42.3% | |
| Age (years) | Mean (range) | 58 (42–82) | |
| Occupational exposure | Yes | 15 | 57.7% |
| No | 4 | 15.4% | |
| Not availlable | 7 | 26.9% | |
| Site | Buccal mucosa | 10 | 38.4% |
| Tongue | 5 | 19.2% | |
| Gingiva | 3 | 11.5% | |
| Cheek | 3 | 11.5% | |
| Palate | 4 | 15.3% | |
| Mandible | 1 | 3.8% | |
| Signs and symptoms | Swelling | 21 | 80.7% |
| Ulceration | 13 | 50% | |
| Pain (VAS mode) | 6 (5) | 23% | |
| Paresthesia | 2 | 7.6% | |
| Difficulties in swallowing | 3 | 11.5% | |
| Tooth mobility | 1 | 3.8% | |
| Mucosal bleeding | 4 | 15.3% | |
| Diagnosis | DLBCL | 11 | 42.3% |
| PBL | 7 | 26.9% | |
| ENMZL | 2 | 7.6% | |
| PTCL | 2 | 7.6% | |
| BL | 1 | 3.8% | |
| FL | 1 | 3.8% | |
| SLL | 1 | 3.8% | |
| MCL | 1 | 3.8% | |
| Serological test | EBV+ | 6 | 23% |
| HIV+ | 5 | 19.2% | |
| Treatment | CT | 10 | 38.4% |
| CT + RT | 8 | 30.8% | |
| Not availlable | 8 | 30.8% | |
| Outcome | Alive | 11 | 42.3% |
| Died | 7 | 26.9% | |
| Not availlable | 8 | 30.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barone, S.; Buffone, C.; Ferrillo, M.; Pasqua, F.; Parrotta, S.; Salviati, M.; Bennardo, F.; Antonelli, A. Oral Malignant Non-Hodgkin Lymphoma: A Retrospective Single-Center Study. Int. J. Environ. Res. Public Health 2022, 19, 2605. https://doi.org/10.3390/ijerph19052605
Barone S, Buffone C, Ferrillo M, Pasqua F, Parrotta S, Salviati M, Bennardo F, Antonelli A. Oral Malignant Non-Hodgkin Lymphoma: A Retrospective Single-Center Study. International Journal of Environmental Research and Public Health. 2022; 19(5):2605. https://doi.org/10.3390/ijerph19052605
Chicago/Turabian StyleBarone, Selene, Caterina Buffone, Martina Ferrillo, Federica Pasqua, Stefano Parrotta, Marianna Salviati, Francesco Bennardo, and Alessandro Antonelli. 2022. "Oral Malignant Non-Hodgkin Lymphoma: A Retrospective Single-Center Study" International Journal of Environmental Research and Public Health 19, no. 5: 2605. https://doi.org/10.3390/ijerph19052605
APA StyleBarone, S., Buffone, C., Ferrillo, M., Pasqua, F., Parrotta, S., Salviati, M., Bennardo, F., & Antonelli, A. (2022). Oral Malignant Non-Hodgkin Lymphoma: A Retrospective Single-Center Study. International Journal of Environmental Research and Public Health, 19(5), 2605. https://doi.org/10.3390/ijerph19052605








