Effect of Humin and Chemical Factors on CO2-Fixing Acetogenesis and Methanogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Humin Extraction and Preparation of Chemically Reduced Humin
2.2. Microbial Consortia
2.3. Effect of Various Factors on Acetogenesis and Methanogenesis
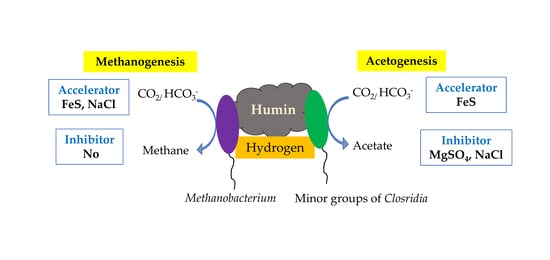
2.3.1. Experiment 1: Effect of Humin on Acetogenesis and Methanogenesis
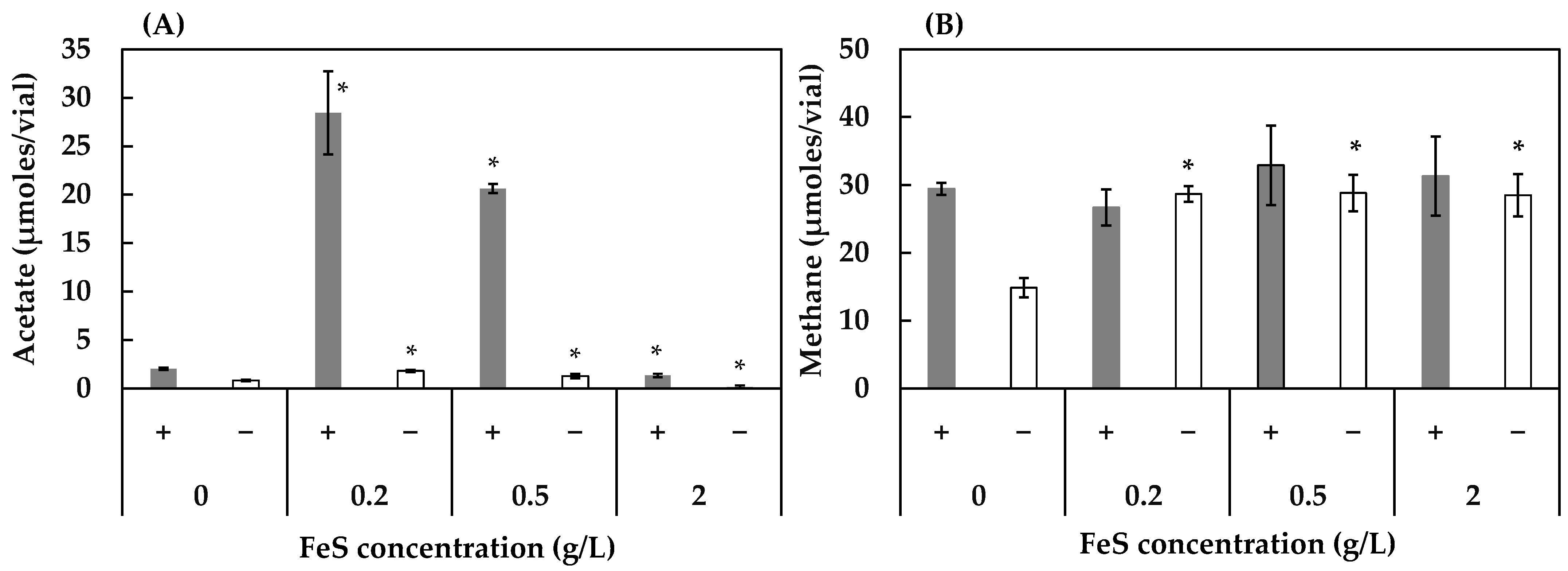
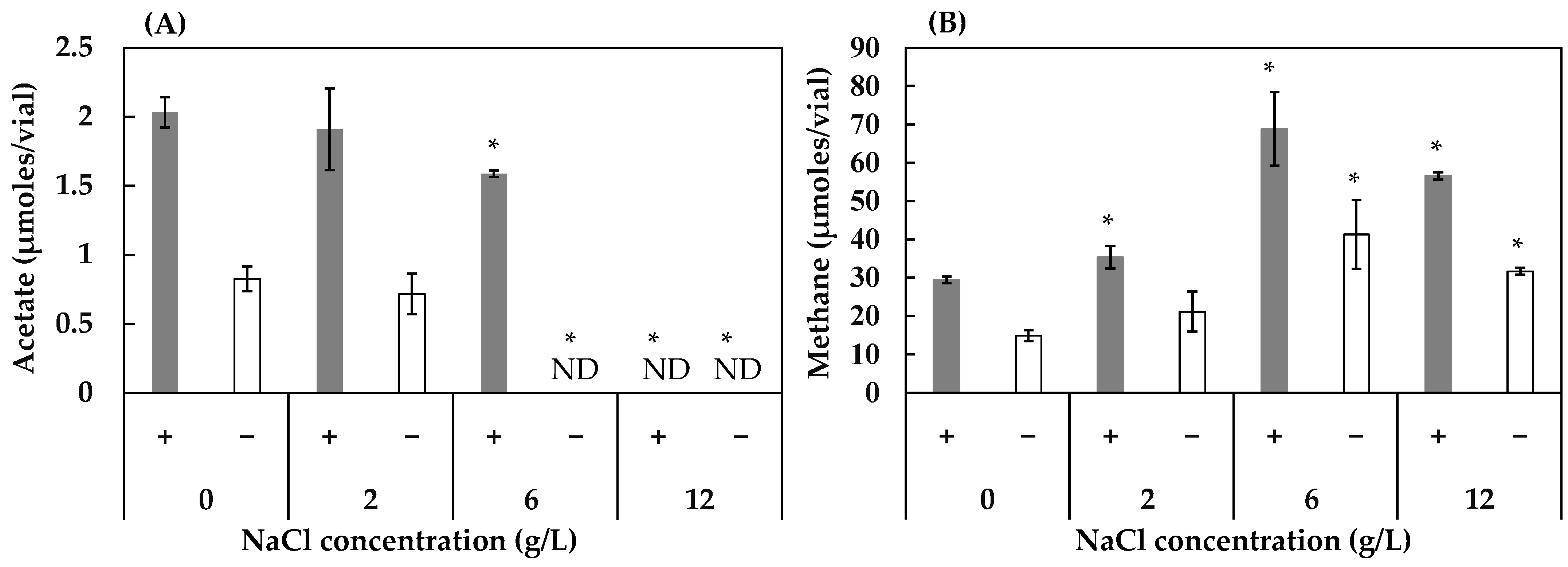
2.3.2. Experiment 2: Effect of Different Concentrations of FeS, NaCl, and MgSO4 on Acetogenesis and Methanogenesis
2.4. Chemical Analysis
2.5. Microbial Community Analysis
2.6. Estimation of Electron Equivalents
2.7. Statistical Analysis
3. Results
3.1. Effect of Humin on CO2-Fixing Acetogenesis and Methanogenesis
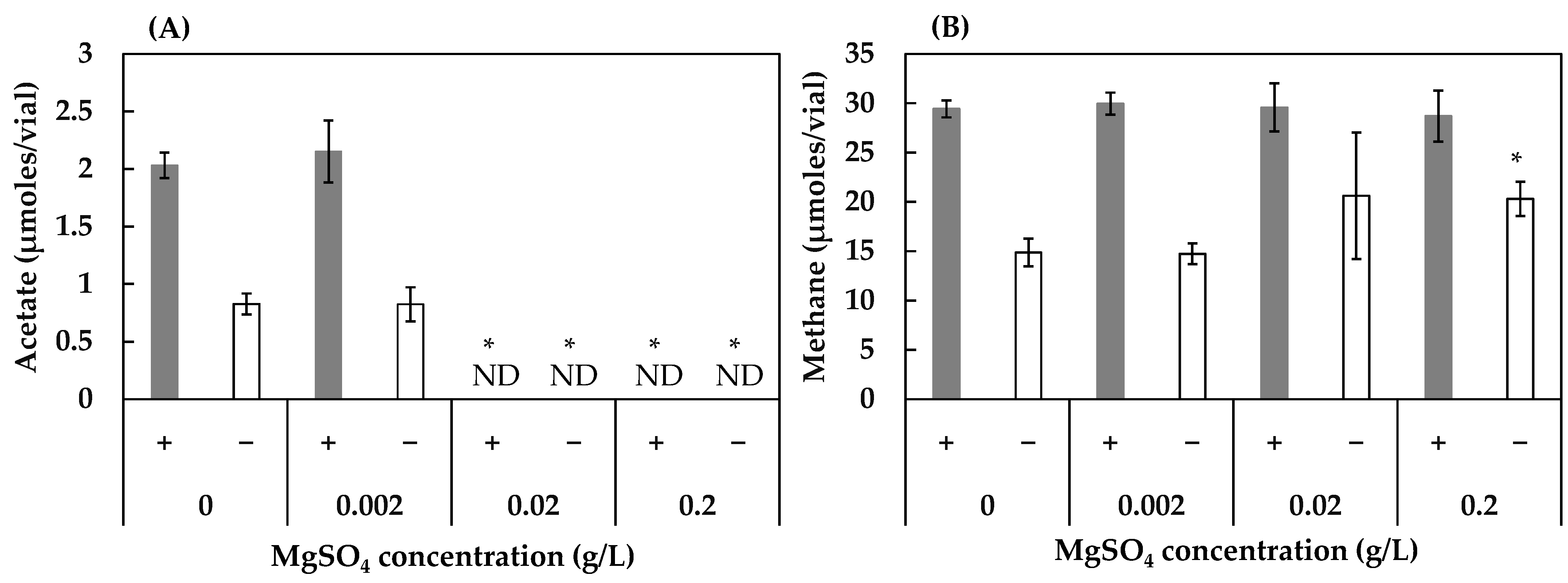
3.2. Effect of Concentrations of FeS, NaCl, and MgSO4 on Acetogenesis and Methanogenesis
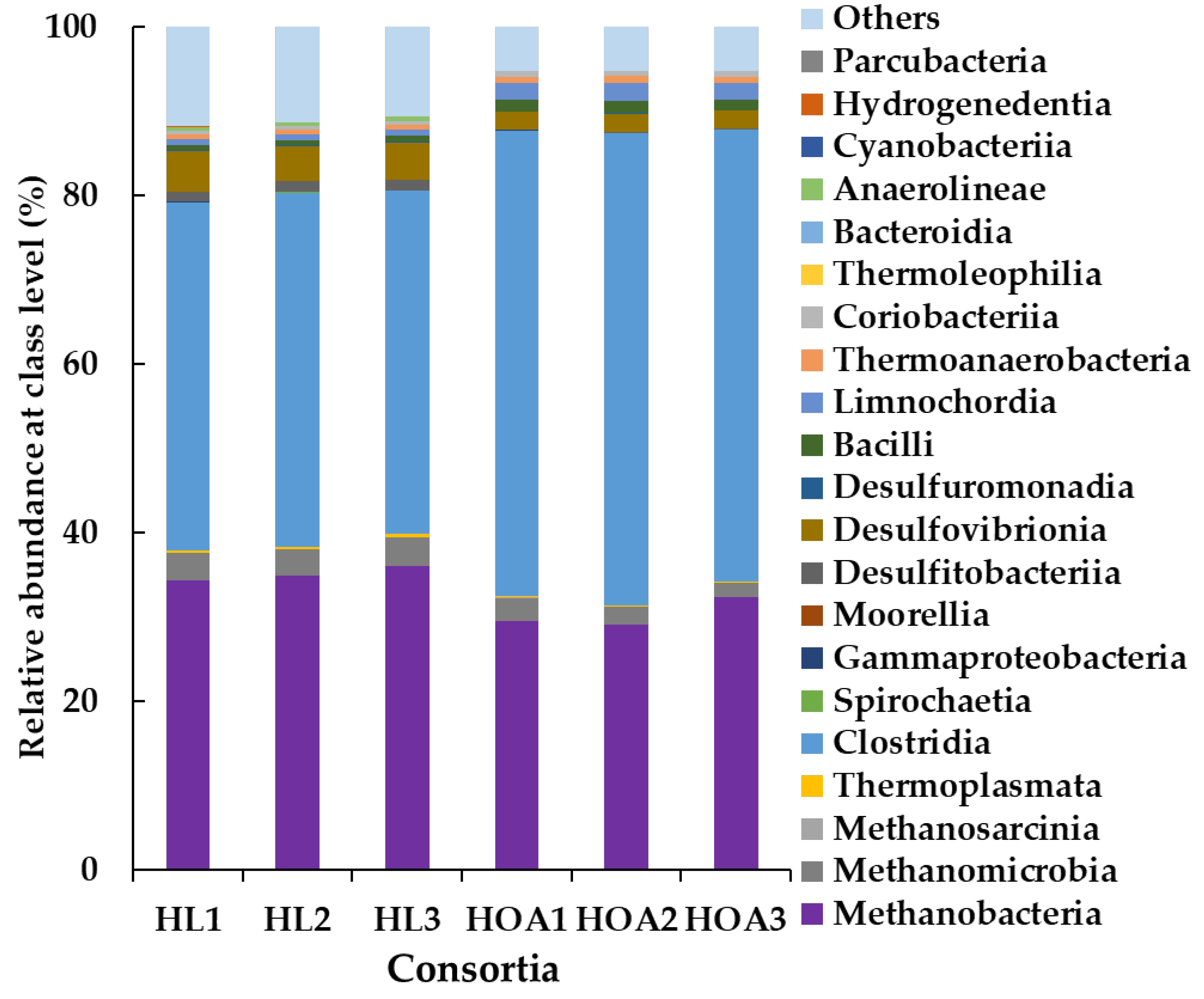
3.3. Microbial Community Structure of HL and HOA Consortium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Research and Innovation 2011: Carbon Dioxide Utilization: Electrochemical Conversion of CO2—Opportunities and Challenges; Det Norske Veritas. Available online: https://www.yumpu.com/en/document/read/51370595/carbon-dioxide-utilization-dnv (accessed on 25 December 2021).
- Intergovernmental Panel on Climate Change 2014: The Physical Science Basis. Working Group III Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; Available online: https://www.ipcc.ch/report/ar5/wg3/ (accessed on 25 December 2021).
- ElMekawy, A.; Hegab, H.M.; Mohanakrishna, G.; Elbaz, A.F.; Bulut, M.; Pant, D. Technological advances in CO2 conversion electro-biorefinery: A step towards commercialization. Bioresour. Technol. 2016, 215, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, P.; Gazzani, M.; Mazzotti, M. The role of carbon capture and utilization, carbon capture and storage, and biomass to enable a net-zero-CO2 emissions chemical industry. Ind. Eng. Chem. Res. 2020, 59, 7033–7045. [Google Scholar] [CrossRef] [Green Version]
- Šoštarič, M.; Klinar, M.; Bricelj, M.; Golob, J.; Berovič, M.; Likozar, B. Growth, lipid extraction and thermal degradation of the microalga Chlorella vulgaris. New Biotechnol. 2012, 29, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Eleršek, T.; Flisar, K.; Likozar, B.; Klemenčič, M.; Golob, J.; Kotnik, T.; Miklavčič, D. Electroporation as a solvent-free green technique for non-destructive extraction of proteins and lipids from Chlorella vulgaris. Front. Bioeng. Biotechnol. 2020, 8, 443. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhao, Y.; Yang, J. Recent advances in developing artificial autotrophic microorganism for reinforcing CO2 fixation. Front. Microbiol. 2020, 11, 592631. [Google Scholar] [CrossRef]
- Drake, H.L.; Gößner, A.S.; Daniel, S.L. Old Acetogens, New Light. Ann. N. Y. Acad. Sci. 2008, 1125, 100–128. [Google Scholar] [CrossRef]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood–Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta 2008, 1784, 1873–1898. [Google Scholar] [CrossRef] [Green Version]
- Borrel, G.; Adam, P.S.; Gribaldo, S. Methanogenesis and the Wood–Ljungdahl Pathway: An ancient, versatile, and fragile association. Genome Biol. Evol. 2016, 8, 1706–1711. [Google Scholar] [CrossRef] [Green Version]
- Philips, J.; Verbeeck, K.; Rabaey, K.; Arends, J.B.A. Electron transfer mechanisms in biofilms. In Microbial Electrochemical and Fuel Cells; Scott, K., Yu, E.H., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 67–113. [Google Scholar]
- Lovley, D.R.; Coates, J.D.; Blunt-Harris, E.L.; Phillips, E.J.P.; Woodward, J.C. Humic substances as electron acceptors for microbial respiration. Nature 1996, 382, 445–448. [Google Scholar] [CrossRef]
- Lovely, D.R.; Fraga, J.L.; Coates, J.D.; Blunt-Harris, E.L. Humics as an electron donor for anaerobic respiration. Environ. Microbiol. 1999, 1, 89–98. [Google Scholar] [CrossRef]
- Laskar, M.; Kasai, T.; Awata, T.; Katayama, A. Humin assists reductive acetogenesis in absence of other external electron donor. Int. J. Environ. Res. Public Health 2020, 17, 4211. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.M.; Kasai, T.; Yamaura, M.; Katayama, A. Humin: No longer inactive natural organic matter. Chemosphere 2021, 269, 128697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, D.; Xiao, Z.; Li, Z.; Suzuki, D.; Katayama, A. Characterization of humins from different natural sources and the effect on microbial reductive dechlorination of pentachlorophenol. Chemosphere 2015, 131, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Zhang, N.; Yu, X.; Zhang, Z.; Yang, S.; Zhang, C. Effect of humins from different sediments on microbial degradation of 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB153), and their polyphasic characterization. RSC Adv. 2017, 7, 6849. [Google Scholar] [CrossRef] [Green Version]
- Rickard, D. Kinetics of FeS precipitation: Part 1. Competing reaction mechanisms. Geochim. Cosmochim. Acta 1995, 59, 4367–4379. [Google Scholar] [CrossRef]
- Menon, S.; Ragsdale, W.S. Role of the [4Fe-4S] cluster in reductive activation of the cobalt center of the corrinoid iron-sulfur protein from Clostridium thermoaceticum during acetate biosynthesis. Biochemistry 1998, 37, 5689–5698. [Google Scholar] [CrossRef]
- Dar, S.A.; Kleerebezem, R.; Stams, A.J.M.; Kuenen, J.G.; Muyzer, G. Competition and coexistence of sulfate-reducing bacteria, acetogens and methanogens in a lab-scale anaerobic bioreactor as affected by changing substrate to sulfate ratio. Appl. Microbiol. Biotechnol. 2008, 78, 1045–1055. [Google Scholar] [CrossRef] [Green Version]
- Schink, B. Microbially driven redox reactions in anoxic environments: Pathways, energetics, and biochemical consequences. Eng. Life Sci. 2006, 6, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Schuchmann, K.; Müller, V. Energetics and application of heterotrophy in acetogenic bacteria. Appl. Environ. Microbiol. 2016, 82, 4056–4069. [Google Scholar] [CrossRef] [Green Version]
- Paulo, L.M.; Stams, A.J.M.; Sousa, D.Z. Methanogens, sulphate, and heavy metals: A complex system. Rev. Environ. Sci. Biotechnol. 2015, 14, 537–553. [Google Scholar] [CrossRef] [Green Version]
- Ntagia, E.; Chatzigiannidou, I.; Williamson, A.J.; Arends, J.B.A.; Rabaey, K. Homoacetogenesis and microbial community composition are shaped by pH and total sulfide concentration. Microb. Biotechnol. 2020, 13, 1026–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šmigán, P.; Majerník, A.; Greksák, M. Na+-driven ATP synthesis in Methanobacterium thermoautotrophicum and its differentiation from H+-driven ATP synthesis by rhodamine 6G. FEBS Lett. 1994, 349, 424–428. [Google Scholar] [CrossRef] [Green Version]
- Thauer, R.K.; Kaster, A.K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 2008, 6, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Ratnawati, E.; Undu, C.M. Characteristics and management of brackishwater pond soil in South Sulawesi Province, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2020, 564, 012021. [Google Scholar] [CrossRef]
- Pham, D.M.; Miyata, Y.; Awata, T.; Nakatake, M.; Zhang, C.F.; Kanda, K.; Ogawa, S.; Ohta, S.; Yagi, S.; Katayama, A. Development of sample preparation technique to characterize chemical structure of humin by synchrotron-radiation based X-ray photoelectron spectroscopy. Surf. Interface Anal. 2018, 51, 226–233. [Google Scholar] [CrossRef]
- Yoshida, N.; Yoshida, Y.; Handa, Y.; Kim, H.K.; Ichihara, S.; Katayama, A. Polyphasic characterization of a PCP-to-phenol dechlorinating microbial community enriched from paddy soil. Sci. Total Environ. 2007, 381, 233–242. [Google Scholar] [CrossRef]
- Li, Z.; Inoue, Y.; Suzuki, D.; Ye, L.; Katayama, A. Long-term anaerobic mineralization of pentachlorophenol in a continuous-flow system using only lactate as an external nutrient. Environ. Sci. Technol. 2013, 47, 1534–1541. [Google Scholar] [CrossRef]
- Widdel, F.; Kohring, G.W.; Mayer, F. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids: III. Characterization of the filamentous gliding Desulfonema limicola gen. nov., sp. nov. and Desulfonema magnum sp. nov. Arch. Microbiol. 1983, 134, 286–294. [Google Scholar] [CrossRef]
- Lo¨ Ffler, F.E.; Sanford, R.A.; Tiedje, J.M. Initial Characterization of a Reductive Dehalogenase from Desulfitobacterium chlororespirans Co23†. Appl. Environ. Microbiol. 1996, 62, 3809–3813. [Google Scholar] [CrossRef] [Green Version]
- Holliger, C.; Hahn, D.; Harmsen, H.; Ludwig, W.; Schumacher, W.; Tindall, B.; Vazque, F.; Weiss, N.; Zehnder, J.A. Dehalobacter restrictus gen. nov. and sp. nov., a strictly anaerobic bacterium that reductively dechlorinates tetra and trichloroethene in an anaerobic respiration. Arch. Microbiol. 1998, 169, 313–321. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glo¨ckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Bengelsdorf, F.R.; Beck, M.H.; Erz, C.; Hoffmeister, S.; Karl, M.M.; Riegler, P.; Wirth, S.; Poehlein, A.; Weuster-Botz, D.; Durre, P. Bacterial anaerobic synthesis gas (syngas) and CO2 + H2 fermentation. Adv. Appl. Microbiol. 2018, 103, 143–221. [Google Scholar] [PubMed]
- Garrity, G.M.; Bell, J.A.; Lilburn, T.G. Taxonomic Outline of the Prokaryotes Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 2004; pp. 13–14. [Google Scholar]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Biotechnol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Postgate, J.R.; Campbell, L.L. Classification of Desulfovibrio species, the nonsporulating sulfate-reducing bacteria. Bacteriol. Rev. 1966, 30, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Zavarzina, S.D.G.; Gavrilov, S.N.; Chistyakova, N.I.; Antonova, A.V.; Gracheva, M.A.; Merkel, A.Y.; Perevalova, A.A.; Chernov, M.S.; Zhilina, T.N.; Bychkov, A.Y.; et al. Syntrophic growth of alkaliphilic anaerobes controlled by ferric and ferrous minerals transformation coupled to acetogenesis. ISME J. 2020, 14, 425–436. [Google Scholar] [CrossRef]
- Zhang, C.; Katayama, A. Humin as an electron mediator for microbial reductive dehalogenation. Environ. Sci. Technol. 2012, 46, 6575–6583. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.; Xiao, Z.; Suzuki, D.; Katayama, A. Humin as an electron donor for enhancement of multiple microbial reduction reactions with different redox potentials in a consortium. J. Biosci. Bioeng. 2015, 119, 188–194. [Google Scholar] [CrossRef]
- Igarashi, K.; Kato, S. Extracellular electron transfer in acetogenic bacteria and its application for conversion of carbon dioxide into organic compounds. Appl. Microbiol. Biotechnol. 2017, 101, 6301–6307. [Google Scholar] [CrossRef] [PubMed]
- Ney, B.; Ahmed, F.H.; Carere, C.R.; Biswas, A.; Warden, A.C.; Morales, S.E.; Pandey, G.; Watt, S.J.; Oakeshott, J.G.; Taylor, M.C.; et al. The methanogenic redox cofactor F420 is widely synthesized by aerobic soil bacteria. ISME J. 2017, 11, 125–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.; Ferry, J.G. Electron bifurcation and confurcation in methanogenesis and reverse methanogenesis. Front. Microbiol. 2018, 9, 1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, M.; Jiang, Y.; Li, D. Production of acetate from carbon dioxide in bioelectrochemical systems based on autotrophic mixed culture. J. Microbiol. Biotechnol. 2013, 23, 1140–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanakrishna, G.; Seelam, J.S.; Vanbroekhoven, K.; Pant, D. An enriched electroactive homoacetogenic biocathode for the microbial electrosynthesis of acetate through carbon dioxide reduction. Faraday Discuss. 2015, 183, 445–462. [Google Scholar] [CrossRef]
- Bajrachary, S.; Vanbroekhoven, K.; Buisman, C.J.N.; Strik, D.P.B.T.B.; Pant, D. Bioelectrochemical conversion of CO2 to chemicals: CO2 as a next generation feedstock for electricity-driven bioproduction in batch and continuous modes. Faraday Discuss. 2017, 202, 433–449. [Google Scholar] [CrossRef]
- Bajrachary, S.; Yuliasni, R.; Vanbroekhoven, K.; Buisman, C.J.N.; Strik, D.P.B.T.B.; Pant, D. Long-term operation of microbial electrosynthesis cell reducing CO2 to multi-carbon chemicals with a mixed culture avoiding methanogenesis. Bioelectrochemistry 2017, 113, 26–34. [Google Scholar] [CrossRef]
- Gong, Y.; Tang, J.; Zhao, D. Application of iron sulfide particles for groundwater and soil remediation: A review. Water Res. 2016, 89, 309–320. [Google Scholar] [CrossRef]
- Bryce, C.; Blackwell, N.; Schmidt, C.; Otte, J.; Huang, Y.M.; Kleindienst, S.; Tomaszewski, E.; Schad, M.; Warter, V.; Peng, C.; et al. Microbial anaerobic Fe(II) oxidation—Ecology, mechanisms and environmental implications. Environ. Microbiol. 2018, 10, 3462–3483. [Google Scholar] [CrossRef] [Green Version]
- Gander, J.W.; Parkin, G.F.; Scherer, M.M. Kinetics of 1,1,1-Trichloroethane transformation by iron sulfide and a methanogenic consortium. Environ. Sci. Technol. 2002, 36, 4540–4546. [Google Scholar] [CrossRef]
- Fernández-Naveira, A.; Veiga, M.C.; Kennes, C. Effect of salinity on C1-gas fermentation by Clostridium carboxidivorans producing acids and alcohols. AMB Express 2019, 9, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jousset, A.; Bienhold, C.; Chatzinotas, A.; Gallien, L.; Gobet, A.; Kurm, V.; Küsel, K.; Rillig, M.C.; Rivett, D.W.; Salles, J.F.; et al. Where less may be more: How the rare biosphere pulls ecosystems strings. ISME J. 2017, 11, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.J.; Yu, L.; Heidelberg, J.F.; Kirchman, D.L. Activity of abundant and rare bacteria in a coastal ocean. Proc. Natl. Acad. Sci. USA 2011, 108, 12776–12781. [Google Scholar] [CrossRef] [Green Version]
- Musat, N.; Halm, H.; Winterboller, B.; Hoppe, P.; Peduzzi, S.; Hillion, F.; Horreard, F.; Amann, R.; Jorgensen, B.B.; Kuypers, M.M.M. A single-cell view on the ecophysiology of anaerobic phototrophic bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 17861–17866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
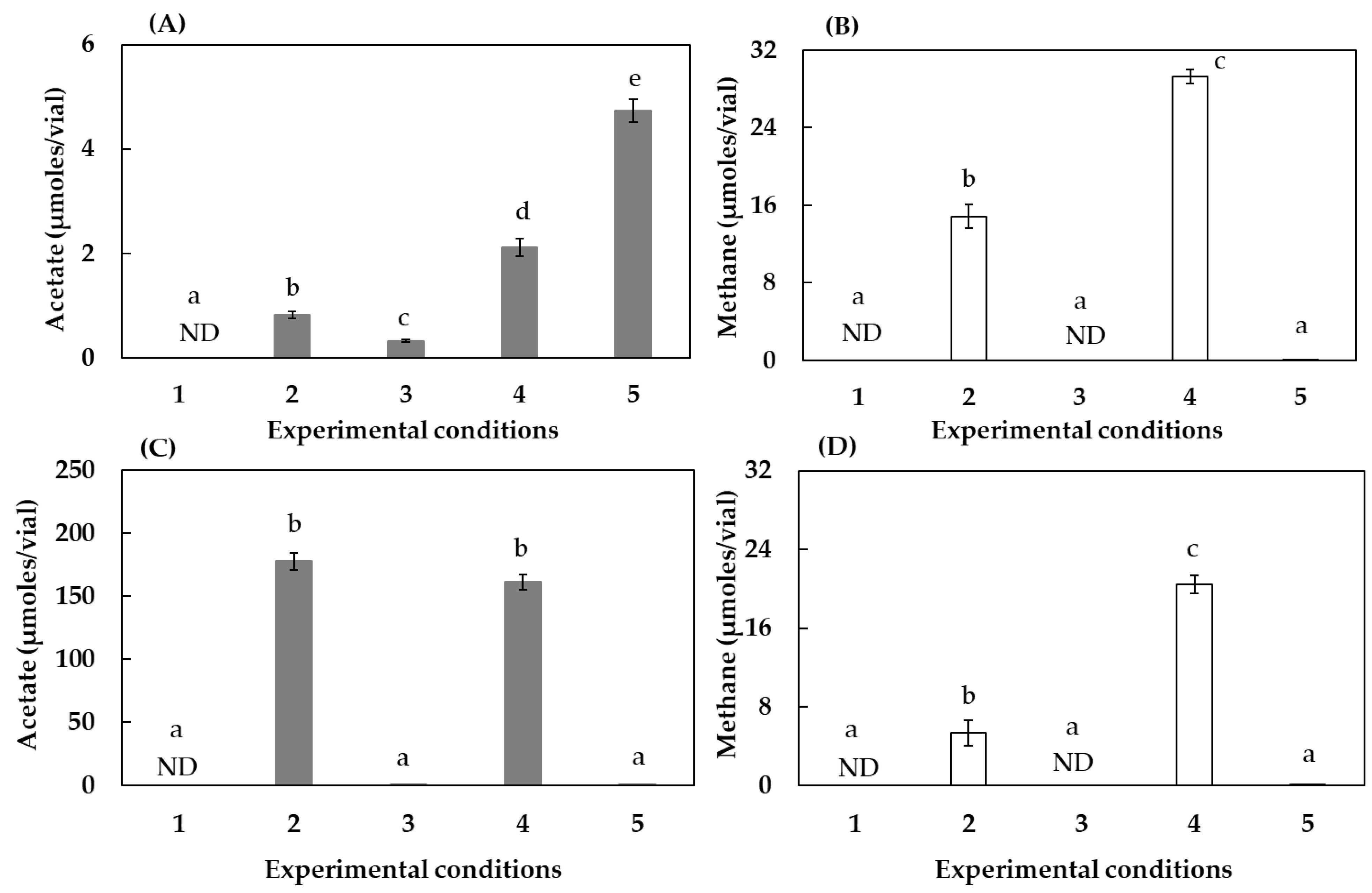

| Condition | Intact Humin | Reduced Humin | Buffer | The Composition of Gas in the Headspace |
|---|---|---|---|---|
| 1 | + | − | MOPS | N2 |
| 2 | − | − | HCO3− | H2/CO2 |
| 3 | + | − | HCO3− | N2/CO2 |
| 4 | + | − | HCO3− | H2/CO2 |
| 5 | − | + | HCO3− | N2/CO2 |
| Condition | Electron Equivalents from H2 Consumed (µeeq/vial) | Electron Equivalents from H2 Used for Reducing Humin (µeeq/vial) | Electron Equivalents Required for Acetate Production (µeeq/vial) | Electron Equivalents Required for Methane Production (µeeq/vial) | ||
|---|---|---|---|---|---|---|
| From H2 | From Humin | From H2 | From Humin | |||
| 2 | 176.8 ± 15.4 | n/a | 6.6 ± 0.5 | n/a | 118.9 ± 11.2 | n/a |
| 3 | n/a | n/a | n/a | 2.6 ± 0.2 | n/a | 0 |
| 4 | 241.2 ± 4.0 | 64.4 ± 18.2 | 6.6 ± 0.5 | 10.3 ± 0.9 | 118.9 ± 11.2 | 116.5 ± 5.9 |
| 5 | n/a | n/a | n/a | 37.9 ± 1.8 | n/a | 0 |
| Genus a | Relative Abundance (%) | |||||
|---|---|---|---|---|---|---|
| HL1 | HL2 | HL3 | HOA1 | HOA2 | HOA3 | |
| Hungateiclostridiaceae (uncultured bacterium) 1 | 0.01 | 0.01 | 0.01 | 0 | 0 | 0 |
| Hungateiclostridiaceae (uncultured bacterium) 2 | 0.07 | 0.07 | 0.07 | 0 | 0 | 0 |
| Caloramator | 2.09 | 1.94 | 1.56 | 0 | 0 | 0 |
| Oxobacter | 0.01 | 0.01 | 0.01 | 0 | 0 | 0 |
| Oscillospiraceae (no matching in genus level) 1 | 0.02 | 0.01 | 0.02 | 0 | 0 | 0 |
| Papillibacter | 0.01 | 0.01 | 0.01 | 0 | 0 | 0 |
| Hydrogenoanaerobacterium | 0.23 | 0.17 | 0.22 | 0 | 0 | 0 |
| Oscillospirales (uncultured Clostridium) 2 | 0.05 | 0.05 | 0.04 | 0 | 0 | 0 |
| Anaerovorax | 0.03 | 0.01 | 0.02 | 0 | 0 | 0 |
| Sedimentibacter 1 | 0.04 | 0.03 | 0.03 | 0 | 0 | 0 |
| Caldicoprobacter | 0 | 0 | 0 | 0.09 | 0.08 | 0.07 |
| Oscillospirales (UCG-010) 3 | 0 | 0 | 0 | 0.01 | 0.01 | 0.01 |
| Sedimentibacter 2 | 0 | 0 | 0 | 0.05 | 0.07 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, B.N.; Pham, D.M.; Kasai, T.; Awata, T.; Katayama, A. Effect of Humin and Chemical Factors on CO2-Fixing Acetogenesis and Methanogenesis. Int. J. Environ. Res. Public Health 2022, 19, 2546. https://doi.org/10.3390/ijerph19052546
Ha BN, Pham DM, Kasai T, Awata T, Katayama A. Effect of Humin and Chemical Factors on CO2-Fixing Acetogenesis and Methanogenesis. International Journal of Environmental Research and Public Health. 2022; 19(5):2546. https://doi.org/10.3390/ijerph19052546
Chicago/Turabian StyleHa, Biec Nhu, Duyen Minh Pham, Takuya Kasai, Takanori Awata, and Arata Katayama. 2022. "Effect of Humin and Chemical Factors on CO2-Fixing Acetogenesis and Methanogenesis" International Journal of Environmental Research and Public Health 19, no. 5: 2546. https://doi.org/10.3390/ijerph19052546
APA StyleHa, B. N., Pham, D. M., Kasai, T., Awata, T., & Katayama, A. (2022). Effect of Humin and Chemical Factors on CO2-Fixing Acetogenesis and Methanogenesis. International Journal of Environmental Research and Public Health, 19(5), 2546. https://doi.org/10.3390/ijerph19052546