Sarcopenic Obesity Tendency and Nutritional Status Is Related to the Risk of Sarcopenia, Frailty, Depression and Quality of Life in Patients with Dementia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Demographic Data and Anthropometric Assessments
2.3. Blood Sample Collection and Hematological Measurements
2.4. Nutritional Status, Frailty, Depression and Quality of Life Measurements
2.5. Muscle Strength and Endurance Assessments
2.6. Statistical Analyses
3. Results
3.1. Characteristics of Patients
3.2. Nutritional Status, Frailty, Depression, Quality of Life, Muscle Mass, Strength and Endurance
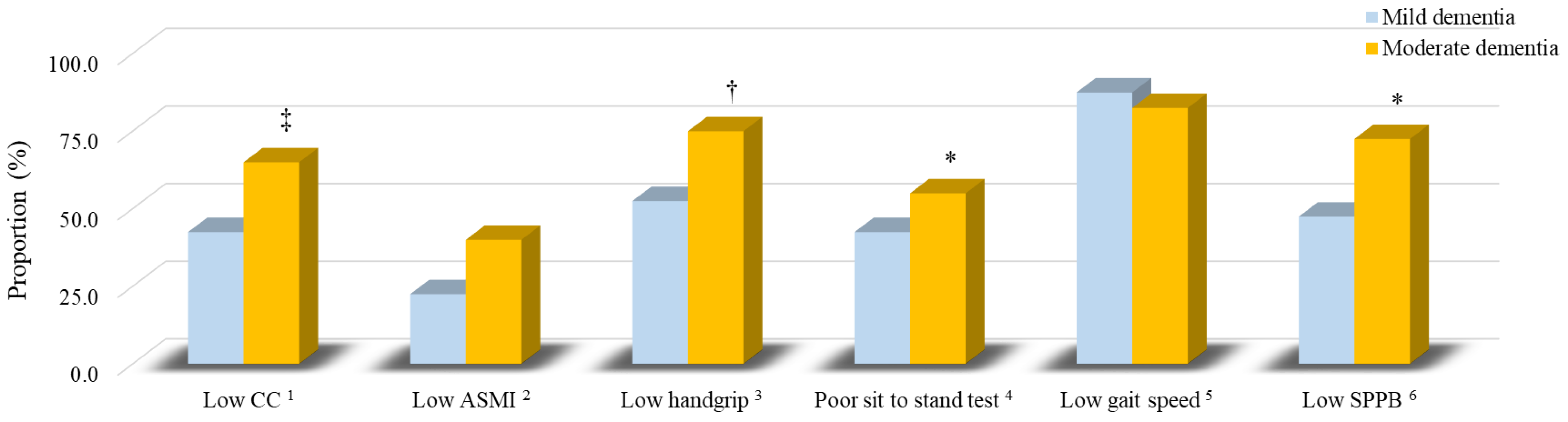
3.3. The Sarcopenia Components of Patients
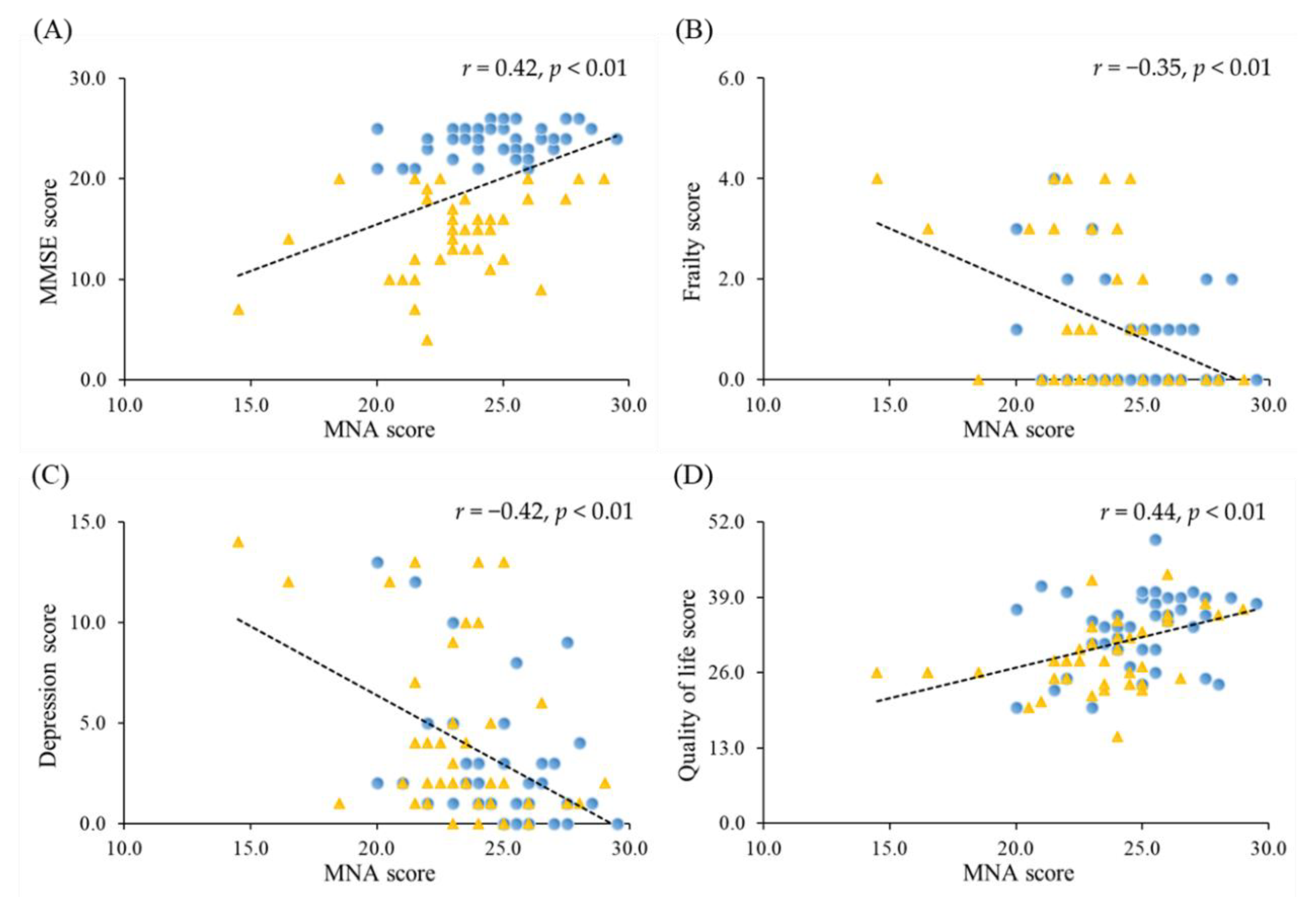
3.4. Correlations between Nutrition Status and the Severity of Dementia, Frailty, Depression and Quality of Life
3.5. Correlations between Nutrition Status, Frailty, Depression, Quality of Life and Muscle Function
3.6. Associations between Nutritional Status and Frailty, Depression, Quality of Life and Sarcopenia
3.7. The Proportion of Sarcopenic Obesity in Patients with Dementia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 12 August 2021).
- Baumgart, M.; Snyder, H.M.; Carrillo, M.C.; Fazio, S.; Kim, H.; Johns, H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015, 11, 718–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.Y.; Huang, C.S. Aging in Taiwan: Building a Society for Active Aging and Aging in Place. Gerontologist 2016, 56, 176–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taiwan Alzheimer Disease Association. People with Dementia in Taiwan. (April 2021). Available online: http://www.tada2002.org.tw/About/IsntDementia#bn1 (accessed on 7 February 2022).
- Scott, K.R.; Barrett, A.M. Dementia syndromes: Evaluation and treatment. Expert Rev. Neurother. 2007, 7, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Cortes, F.; Nourhashémi, F.; Guérin, O.; Cantet, C.; Gillette-Guyonnet, S.; Andrieu, S.; Ousset, P.J.; Vellas, B.; REAL-FR Group. Prognosis of Alzheimer’s disease today: A two-year prospective study in 686 patients from the REAL-FR Study. Alzheimers Dement. 2008, 4, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Volkert, D.; Chourdakis, M.; Faxen-Irving, G.; Frühwald, T.; Landi, F.; Suominen, M.H.; Vandewoude, M.; Wirth, R.; Schneider, S.M. ESPEN guidelines on nutrition in dementia. Clin. Nutr. 2015, 34, 1052–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Arai, Y. Aging-related frailty and sarcopenia. Frailty and sarcopenia in the centenarians. Clin. Calcium 2018, 28, 1235–1240. Available online: https://pubmed.ncbi.nlm.nih.gov/30146510/ (accessed on 12 August 2021).
- Matsumoto, H.; Tanimura, C.; Tanishima, S.; Osaki, M.; Noma, H.; Hagino, H. Sarcopenia is a risk factor for falling in independently living Japanese older adults: A 2-year prospective cohort study of the GAINA study. Geriatr. Gerontol. Int. 2017, 17, 2124–2130. [Google Scholar] [CrossRef]
- Yeung, S.S.Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.M.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, A.; Seino, S.; Abe, T.; Nofuji, Y.; Yokoyama, Y.; Amano, H.; Nishi, M.; Taniguchi, Y.; Narita, M.; Fujiwara, Y.; et al. Sarcopenia: Prevalence; associated factors; and the risk of mortality and disability in Japanese older adults. J. Cachexia Sarcopenia Muscle 2021, 12, 30–38. [Google Scholar] [CrossRef]
- Crocker, T.F.; Brown, L.; Clegg, A.; Farley, K.; Franklin, M.; Simpkins, S.; Young, J. Quality of life is substantially worse for community-dwelling older people living with frailty: Systematic review and meta-analysis. Qual. Life Res. 2019, 28, 2041–2056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobbens, R.J.J.; van Assen, M.A.L.M. Associations between multidimensional frailty and quality of life among Dutch older people. Arch. Gerontol. Geriatr. 2017, 73, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Cabett Cipolli, G.; Sanches Yassuda, M.; Aprahamian, I. Sarcopenia Is Associated with Cognitive Impairment in Older Adults: A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2019, 23, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; Hsu, T.H.; Wu, W.T.; Huang, K.C.; Han, D.S. Association Between Sarcopenia and Cognitive Impairment: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1164.e7–1164.e15. [Google Scholar] [CrossRef]
- Peng, T.C.; Chen, W.L.; Wu, L.W.; Chang, Y.W.; Kao, T.W. Sarcopenia and cognitive impairment: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 2695–2701. [Google Scholar] [CrossRef]
- Choi, K.M. Sarcopenia and sarcopenic obesity. Korean J. Intern. Med. 2016, 31, 1054–1060. [Google Scholar] [CrossRef] [Green Version]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare. Dementia Diagnostic Manual. (February 2017). Available online: https://www.mohw.gov.tw/dl-27189-8993c3ad-0f47-45e0-a602-6a4362faae9a.html (accessed on 12 December 2018).
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Nolan, J.M.; Mulcahy, R.; Power, R.; Moran, R.; Howard, A.N. Nutritional Intervention to Prevent Alzheimer’s Disease: Potential Benefits of Xanthophyll Carotenoids and Omega-3 Fatty Acids Combined. J. Alzheimers Dis. 2018, 64, 367–378. [Google Scholar] [CrossRef] [Green Version]
- Okorodudu, D.O.; Jumean, M.F.; Montori, V.M.; Romero-Corral, A.; Somers, V.K.; Erwin, P.J.; Lopez-Jimenez, F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: A systematic review and meta-analysis. Int. J. Obes. 2010, 34, 791–799. [Google Scholar] [CrossRef] [Green Version]
- Vellas, B.; Villars, H.; Abellan, G.; Soto, M.E.; Rolland, Y.; Guigoz, Y.; Morley, J.E.; Chumlea, W.; Salva, A.; Rubenstein, L.Z.; et al. Overview of the MNA—Its history and challenges. J. Nutr. Health Aging 2006, 10, 456–465. [Google Scholar] [PubMed]
- Ensrud, K.E.; Ewing, S.K.; Taylor, B.C.; Fink, H.A.; Cawthon, P.M.; Stone, K.L.; Hillier, T.A.; Cauley, J.A.; Hochberg, M.C.; Rodondi, N.; et al. Comparison of 2 frailty indexes for prediction of falls; disability; fractures; and death in older women. Arch. Intern. Med. 2008, 168, 382–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yesavage, J.A.; Sheikh, J.I. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin. Gerontol. J. Aging Ment. Health 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Logsdon, R.G.; Gibbons, L.E.; McCurry, S.M.; Teri, L. Quality of life in Alzheimer’s disease: Patient and caregiver reports. J. Ment. Health Aging 1999, 5, 21–32. [Google Scholar]
- Rikli, R.E.; Jones, C.J. Senior Fitness Test Manual. Champaign IL Hum. Kinet. 2013. Available online: https://www.google.com/books?hl=zh-TW&lr=&id=NXfXxOFFOVwC&oi=fnd&pg=PR1&dq=Senior+Fitness+Test+Manual&ots=cU2-B9UphO&sig=74fpWG49JiOfIzxUWgVomiZsGrg (accessed on 12 December 2018).
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Roqué, M.; Salvà, A.; Vellas, B. Malnutrition in community-dwelling adults with dementia (NutriAlz Trial). J. Nutr. Health Aging 2013, 17, 295–299. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare. 2013–2016 National Nutrition Survey in Taiwan. (12 July 2019). Available online: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=3999&pid=11145 (accessed on 26 August 2021).
- Sanders, C.; Behrens, S.; Schwartz, S.; Wengreen, H.; Corcoran, C.D.; Lyketsos, C.G.; Tschanz, J.T. Nutritional Status is Associated with Faster Cognitive Decline and Worse Functional Impairment in the Progression of Dementia: The Cache County Dementia Progression Study1. J. Alzheimers Dis. 2016, 52, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Tagawa, R.; Watanabe, D.; Ito, K.; Ueda, K.; Nakayama, K.; Sanbongi, C.; Miyachi, M. Dose-response relationship between protein intake and muscle mass increase: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2020, 79, 66–75. [Google Scholar] [CrossRef]
- Shimokata, H.; Kuzuya, F. Aging; basal metabolic rate; and nutrition. Nihon Ronen Igakkai Zasshi 1993, 30, 572–576. [Google Scholar] [CrossRef]
- Bennett, S.; Thomas, A.J. Depression and dementia: Cause; consequence or coincidence? Maturitas 2014, 79, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.; Steffens, D.C. What are the causes of late-life depression? Psychiatr. Clin. N. Am. 2013, 36, 497–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soysal, P.; Veronese, N.; Thompson, T.; Kahl, K.G.; Fernandes, B.S.; Prina, A.M.; Solmi, M.; Schofield, P.; Koyanagi, A.; Tseng, P.T.; et al. Relationship between depression and frailty in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2017, 36, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailly, N.; Maître, I.; Van Wymelbeke, V. Relationships between nutritional status; depression and pleasure of eating in aging men and women. Arch. Gerontol. Geriatr. 2015, 61, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Engelheart, S.; Andrén, D.; Repsilber, D.; Bertéus, F.H.; Brummer, R.J. Nutritional status in older people—An explorative analysis. Clin. Nutr. ESPEN 2021, 46, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Tay, L.B.; Chua, M.P.; Tay, E.L.; Chan, H.N.; Mah, S.M.; Latib, A.; Wong, C.Q.; Ng, Y.S. Multidomain Geriatric Screen and Physical Fitness Assessment Identify Prefrailty/Frailty and Potentially Modifiable Risk Factors in Community-Dwelling Older Adults. Ann. Acad. Med. Singap. 2019, 48, 171–180. [Google Scholar] [PubMed]
- Li, P.S.; Hsieh, C.J.; Miao, N.F. A Study of Physical Activity; Frailty; and Health-Related Quality of Life Among Community-Dwelling Older Adults in Taiwan. J. Nurs. Res. 2020, 28, e124. [Google Scholar] [CrossRef]
- Groessl, E.J.; Kaplan, R.M.; Rejeski, W.J.; Katula, J.A.; Glynn, N.W.; King, A.C.; Anton, S.D.; Walkup, M.; Lu, C.J.; Reid, K.; et al. Physical Activity and Performance Impact Long-term Quality of Life in Older Adults at Risk for Major Mobility Disability. Am. J. Prev. Med. 2019, 56, 141–146. [Google Scholar] [CrossRef]
- Sjöberg, L.; Karlsson, B.; Atti, A.R.; Skoog, I.; Fratiglioni, L.; Wang, H.X. Prevalence of depression: Comparisons of different depression definitions in population-based samples of older adults. J. Affect. Disord. 2017, 221, 123–131. [Google Scholar] [CrossRef]

| Mild Dementia (N = 40) | Moderate Dementia (N = 40) | p Value | |
|---|---|---|---|
| MMSE score | 23.9 ± 1.6 (24.0) | 14.6 ± 4.1 (15.0) | <0.01 |
| Age (years) | 76.0 ± 6.4 (76.0) | 76.9 ± 6.2 (78.0) | 0.54 |
| Female (n, %) | 26 (65.0%) | 32 (80.0%) | 0.21 |
| BMI (kg/m2) | 24.6 ± 3.8 (24.3) | 23.8 ± 3.9 (23.5) | 0.32 |
| Waist (cm) | 91.9 ± 9.5 (92.3) | 89.5 ± 10.4 (89.0) | 0.29 |
| Male | 91.9 ± 8.3 (92.8) | 90.4 ± 4.9 (90.0) | 0.66 |
| Female | 91.9 ± 10.3 (92.0) | 89.3 ± 11.4 (86.8) | 0.37 |
| Body fat (%) | 28.4 ± 7.5 (29.1) | 30.5 ± 5.4 (30.9) | 0.17 |
| Male | 22.3 ± 6.5 (23.0) | 24.0 ± 3.2 (24.4) | 0.48 |
| Female | 31.8 ± 5.7 (31.8) | 32.1 ± 4.5 (32.2) | 0.80 |
| Obesity (n, %) 1 | 18 (45.0%) | 26 (65.0%) | 0.09 |
| Male | 3 (21.4%) | 4 (50.0%) | 0.34 |
| Female | 15 (57.7%) | 22 (68.8%) | 0.44 |
| SBP (mmHg) | 130.8 ± 17.2 (131.0) | 128.4 ± 17.9 (129.5) | 0.53 |
| DBP (mmHg) | 74.1 ± 10.0 (72.5) | 71.9 ± 10.7 (72.0) | 0.34 |
| Albumin (g/L) | 45.0 ± 2.0 (45.0) | 44.0 ± 3.0 (44.0) | 0.16 |
| Fasting glucose (mmol/L) | 6.4 ± 1.4 (6.1) | 6.7 ± 1.8 (6.1) | 0.84 |
| Total cholesterol (mmol/L) | 4.9 ± 0.9 (4.7) | 4.8 ± 1.0 (4.5) | 0.45 |
| GPT (U/L) | 20.3 ± 13.1 (16.5) | 19.1 ± 7.9 (18.5) | 0.71 |
| Creatinine (µmol/L) | 75.1 ± 15.9 (73.4) | 79.6 ± 23.9 (71.6) | 0.64 |
| Education | 0.16 | ||
| None | 4 (10.0%) | 11 (27.5%) | |
| Elementary school | 18 (45.0%) | 15 (37.5%) | |
| Junior high school | 8 (20.0%) | 7 (17.5%) | |
| Senior high school | 7 (17.5%) | 7 (17.5%) | |
| University or above | 3 (7.5%) | 0 (0.0%) | |
| Tobacco use | 4 (10.0%) | 6 (15.0%) | 0.74 |
| Alcohol use | 3 (7.5%) | 5 (12.5%) | 0.71 |
| Exercise habit 2 | 26 (65.0%) | 21 (52.5%) | 0.36 |
| Family history of dementia (n, %) | 9 (22.5%) | 5 (12.5%) | 0.57 |
| Mild Dementia (N = 40) | Moderate Dementia (N = 40) | p Value | |
|---|---|---|---|
| MNA score (points) | 24.9 ± 2.2 (25.0) | 23.3 ± 2.8 (23.5) | <0.01 |
| MNA < 24 (n, %) | 11 (27.5%) | 22 (55.0%) | 0.02 |
| Frailty score (points) | 0.75 ± 1.08 (0.00) | 1.30 ± 1.52 (1.00) | 0.15 |
| Frailty ≥ 2 (n, %) | 8 (20.0%) | 14 (35.0%) | 0.21 |
| Depression score (points) | 2.8 ± 3.3 (2.0) | 4.3 ± 4.5 (2.0) | 0.11 |
| Depression ≥ 5 (n, %) | 8 (20.0%) | 13 (32.5%) | 0.31 |
| Quality of life score (points) | 33.6 ± 6.5 (35.0) | 28.8 ± 5.9 (28.0) | <0.01 |
| Quality of life ≤ 26 (n, %) | 8 (20.0%) | 16 (40.0%) | 0.09 |
| Muscle mass | |||
| CC (cm) | |||
| Overall | 33.9 ± 2.8 (33.8) | 32.0 ± 2.7 (32.0) | <0.01 |
| Male | 34.4 ± 2.5 (34.3) | 32.5 ± 2.5 (32.3) | 0.10 |
| Female | 33.6 ± 3.0 (33.6) | 31.9 ± 2.8 (31.8) | 0.03 |
| Whole body muscle mass (kg) | |||
| Overall | 40.1 ± 6.9 (39.9) | 36.3 ± 6.4 (34.7) | 0.01 |
| Male | 46.4 ± 4.3 (47.0) | 45.0 ± 3.5 (45.1) | 0.42 |
| Female | 36.7 ± 5.4 (36.9) | 34.1 ± 4.9 (33.9) | 0.06 |
| ASMI (kg/m2) | |||
| Overall | 6.7 ± 0.9 (6.8) | 6.1 ± 1.1 (6.1) | 0.01 |
| Male | 7.2 ± 0.6 (7.3) | 6.8 ± 0.6 (6.8) | 0.16 |
| Female | 6.5 ± 0.9 (6.5) | 6.0 ± 1.1 (5.8) | 0.08 |
| Muscle strength | |||
| Handgrip strength (kg) | |||
| Overall | 20.6 ± 7.6 (20.5) | 16.1 ± 6.9 (16.8) | <0.01 |
| Male | 28.6 ± 4.1 (27.8) | 22.0 ± 9.2 (25.3) | 0.03 |
| Female | 16.2 ± 5.0 (17.3) | 14.6 ± 5.4 (15.6) | 0.24 |
| Leg-back strength (kg) | |||
| Overall | 45.3 ± 22.8 (43.0) | 34.9 ± 18.6 (28.5) | 0.02 |
| Male | 67.8 ± 20.4 (65.3) | 53.3 ± 28.9 (51.0) | 0.18 |
| Female | 33.2 ± 12.5 (27.5) | 30.1 ± 11.1 (26.0) | 0.29 |
| Muscle endurance | |||
| Dumbbells curls (reps) | |||
| Overall | 13.8 ± 6.1 (14.0) | 11.0 ± 7.7 (10.0) | 0.04 |
| Male | 16.4 ± 7.2 (18.0) | 13.8 ± 12.7 (15.0) | 0.53 |
| Female | 12.4 ± 5.0 (12.0) | 10.3 ± 5.9 (10.0) | 0.15 |
| Sit to stand test (s) | |||
| Overall | 13.6 ± 5.6 (11.0) | 18.2 ± 10.8 (16.0) | 0.09 |
| Male | 12.7 ± 5.1 (10.5) | 12.7 ± 8.9 (10.5) | 0.59 |
| Female | 14.1 ± 6.0 (11.5) | 19.7 ± 11.0 (17.0) | 0.06 |
| Gait speed (m/s) | |||
| Overall | 0.72 ± 0.24 (0.71) | 0.66 ± 0.22 (0.67) | 0.26 |
| Male | 0.77 ± 0.28 (0.72) | 0.83 ± 0.24 (0.80) | 0.65 |
| Female | 0.69 ± 0.22 (0.71) | 0.61 ± 0.19 (0.67) | 0.19 |
| SPPB (scores) | |||
| Overall | 9.3 ± 2.7 (10.0) | 7.2 ± 3.9 (8.5) | 0.02 |
| Male | 10.1 ± 2.1 (10.5) | 8.4 ± 4.5 (10.0) | 0.55 |
| Female | 8.8 ± 2.9 (9.0) | 6.9 ± 3.7 (8.0) | 0.08 |
| MNA Score | Frailty Score | Depression Score | Quality of Life Score | |
|---|---|---|---|---|
| R1 (p Value) | ||||
| Muscle mass | ||||
| CC (cm) | 0.45 (<0.01) | −0.07 (0.56) | −0.21 (0.06) | 0.17 (0.14) |
| Whole body muscle mass (kg) | 0.35 (<0.01) | 0.10 (0.36) | −0.14 (0.23) | −0.10 (0.40) |
| ASMI (kg/m2) | 0.36 (<0.01) | 0.06 (0.60) | −0.11 (0.32) | 0.00 (1.00) |
| Muscle strength | ||||
| Handgrip strength (kg) | 0.26 (0.02) | −0.27 (0.02) | −0.19 (0.10) | 0.04 (0.75) |
| Leg-back strength (kg) | 0.10 (0.40) | −0.35 (<0.01) | −0.18 (0.11) | 0.06 (0.61) |
| Muscle endurance | ||||
| Dumbbells curls (reps) | 0.25 (0.03) | −0.62 (<0.01) | −0.44 (<0.01) | 0.35 (<0.01) |
| Sit to stand test (s) | −0.17 (0.17) | 0.48 (<0.01) | 0.43 (<0.01) | −0.31 (0.01) |
| Gait speed (m/s) | 0.24 (0.04) | −0.43 (<0.01) | −0.28 (0.01) | 0.17 (0.15) |
| SPPB (scores) | 0.16 (0.15) | −0.62 (<0.01) | −0.34 (<0.01) | 0.32 (<0.01) |
| Poor MNA 1 | |||||
|---|---|---|---|---|---|
| Unadjusted | Adjusted 12 | ||||
| Odds Ratios (95% CI) | p Value | Odds Ratios (95% CI) | p Value | Hosmer-Lemeshow Test Pr > Chi-Square | |
| Normal MNA 1 | 1.00 | - | 1.00 | - | |
| Frailty 2 | 4.76 (1.66–13.69) | <0.01 | 4.35 (1.41–13.39) | 0.01 | 0.75 |
| Depression 3 | 3.17 (1.13–8.90) | 0.03 | 2.62 (0.86–8.00) | 0.09 | 0.75 |
| Poor quality of life 4 | 2.73 (1.02–7.28) | <0.05 | 3.27 (1.04–10.33) | 0.04 | 0.37 |
| Sarcopenia components | |||||
| Low CC 5 | 3.11 (1.21–7.96) | 0.02 | 4.00 (1.39–11.45) | 0.01 | 0.92 |
| Low ASMI 6 | 2.00 (0.76–5.23) | 0.16 | 3.54 (1.09–11.53) | 0.04 | 0.18 |
| Low handgrip 7 | 2.52 (0.95–6.74) | 0.07 | 2.42 (0.82–7.18) | 0.11 | 0.79 |
| Poor sit to stand test 8 | 1.74 (0.61–4.95) | 0.30 | 1.49 (0.50–4.43) | 0.47 | 0.97 |
| Low gait speed 9 | 1.32 (0.23–7.70) | 0.76 | 0.30 (0.02–3.88) | 0.36 | 0.96 |
| Low SPPB 10 | 2.02 (0.79–5.17) | 0.14 | 1.61 (0.57–4.58) | 0.37 | 0.56 |
| Sarcopenia 11 | 2.24 (0.84–5.95) | 0.11 | 3.97 (1.19–13.27) | 0.03 | 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, H.-H.; Lai, T.-J.; Yen, C.-H.; Chang, P.-S.; Pan, J.-C.; Lin, P.-T. Sarcopenic Obesity Tendency and Nutritional Status Is Related to the Risk of Sarcopenia, Frailty, Depression and Quality of Life in Patients with Dementia. Int. J. Environ. Res. Public Health 2022, 19, 2492. https://doi.org/10.3390/ijerph19052492
Chou H-H, Lai T-J, Yen C-H, Chang P-S, Pan J-C, Lin P-T. Sarcopenic Obesity Tendency and Nutritional Status Is Related to the Risk of Sarcopenia, Frailty, Depression and Quality of Life in Patients with Dementia. International Journal of Environmental Research and Public Health. 2022; 19(5):2492. https://doi.org/10.3390/ijerph19052492
Chicago/Turabian StyleChou, Hsi-Hsien, Te-Jen Lai, Chi-Hua Yen, Po-Sheng Chang, Ji-Cyun Pan, and Ping-Ting Lin. 2022. "Sarcopenic Obesity Tendency and Nutritional Status Is Related to the Risk of Sarcopenia, Frailty, Depression and Quality of Life in Patients with Dementia" International Journal of Environmental Research and Public Health 19, no. 5: 2492. https://doi.org/10.3390/ijerph19052492
APA StyleChou, H.-H., Lai, T.-J., Yen, C.-H., Chang, P.-S., Pan, J.-C., & Lin, P.-T. (2022). Sarcopenic Obesity Tendency and Nutritional Status Is Related to the Risk of Sarcopenia, Frailty, Depression and Quality of Life in Patients with Dementia. International Journal of Environmental Research and Public Health, 19(5), 2492. https://doi.org/10.3390/ijerph19052492






