Amikacin Therapy in Japanese Pediatric Patients: Narrative Review
Abstract
:1. Introduction
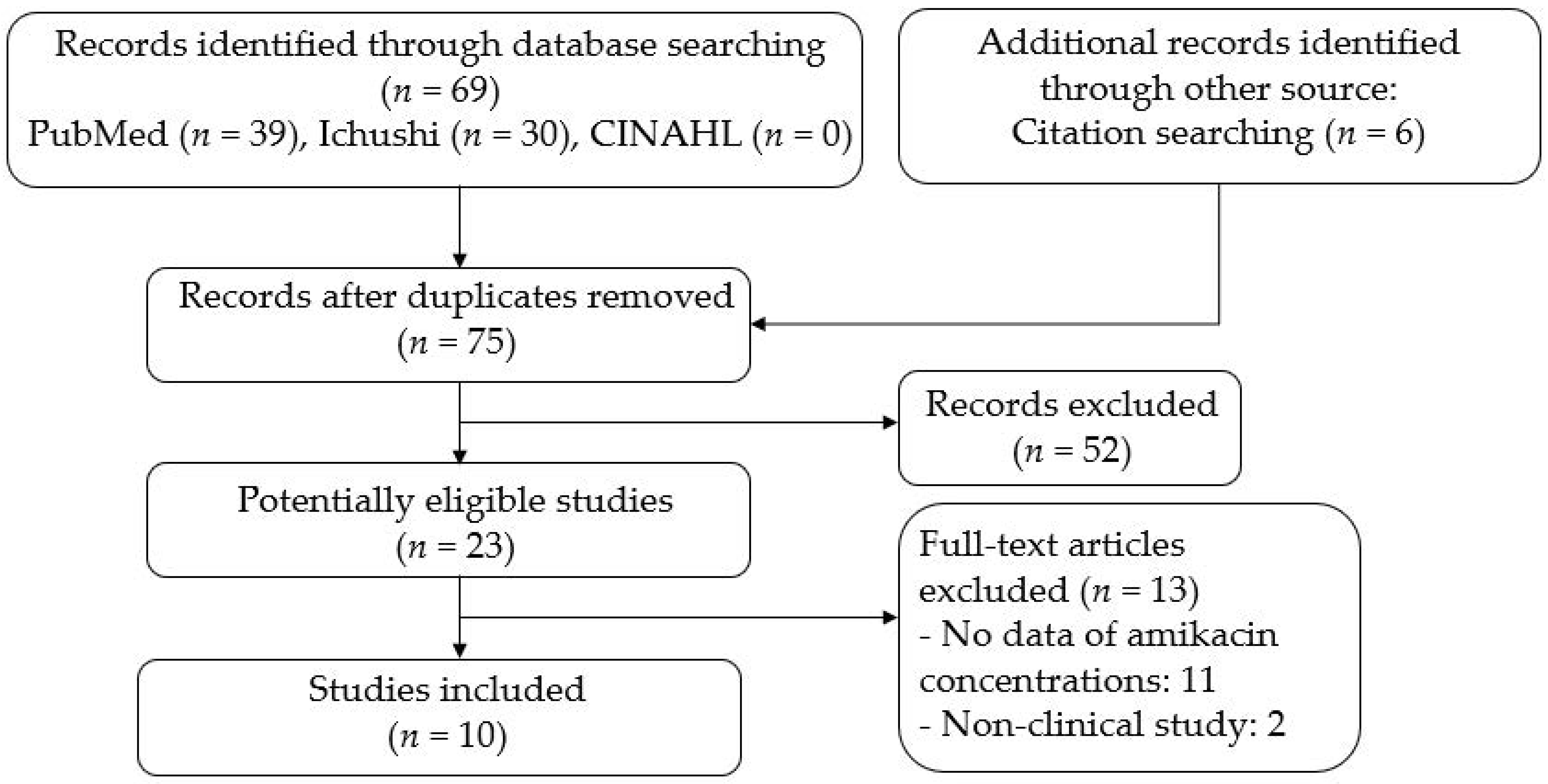
2. Materials and Methods
2.1. Literature Serch
2.2. Study Selection
2.3. Data Extraction
3. Results
3.1. Characteristics of Studies in Japanese Pediatric Patients Treated with Amikacin
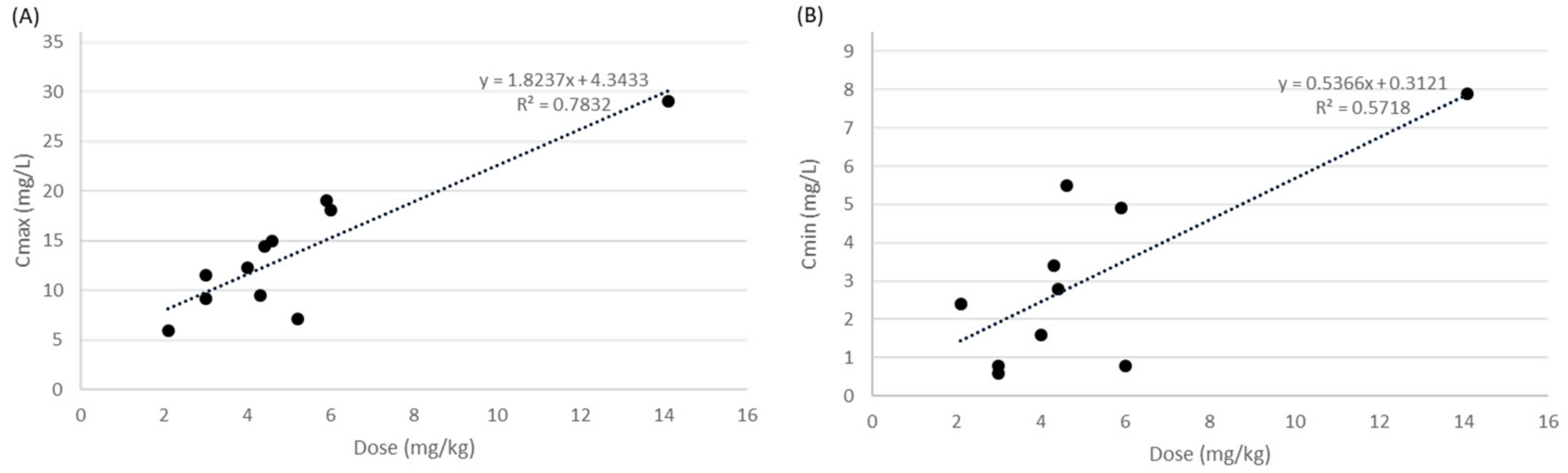
3.2. Overview of Amikacin Therapy and Clinical Outcomes in Japanese Pediatric Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention, Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 28 December 2021).
- King, A. Antibiotic Resistance will Kill 300 Million People by 2050. Available online: https://www.scientificamerican.com/article/antibiotic-resistance-will-kill-300-million-people-by-2050/ (accessed on 28 December 2021).
- Kumana, C.R.; Yuen, K.Y. Parenteral aminoglycoside therapy. Selection, administration and monitoring. Drugs 1994, 47, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Zelenitsky, S.A.; Harding, G.K.; Sun, S.; Ubhi, K.; Ariano, R.E. Treatment and outcome of Pseudomonas aeruginosa bacteremia: An antibiotic pharmacodynamic analysis. J. Antimicrob. Chemother. 2003, 52, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Engler, D.; Schellack, N.; Naude, A.; Gous, A.G. Use of amikacin in neonates and related ototoxicity. Neonatology 2013, 17, 24–27. [Google Scholar]
- Zaske, D.E.; Strate, R.G.; Kohls, P.R. Amikacin pharmacokinetics: Wide interpatient variation in 98 patients. J. Clin. Pharmacol. 1991, 31, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Boucher, B.A.; Coffey, B.C.; Kuhl, D.A.; Tolley, E.A.; Fabian, T.C. Algorithm for assessing renal dysfunction risk in critically ill trauma patients receiving aminoglycosides. Am. J. Surg. 1990, 160, 473–480. [Google Scholar] [CrossRef]
- Cella, M.; Knibbe, C.; Donhof, M.; Della, P. What is the right dose for children? Br. J. Clin. Pharmacol. 2010, 70, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.N. Modeling approaches to dose estimation in children. Br. J. Clin. Pharmacol. 2005, 59, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, I. Prediction of drug clearance in children: Impact of allometric exponents, body weight, and age. Ther. Drug. Monit. 2007, 29, 271–278. [Google Scholar] [CrossRef]
- Anderson, B.J.; Holford, N.H. Tips and traps analyzing pediatric PK data. Pediatr. Anaesth. 2011, 21, 222–237. [Google Scholar] [CrossRef] [PubMed]
- The guidelines of preferred reporting items for systematic review and meta-analysis (PRISMA) statement. Available online: http://prisma-statement.org (accessed on 28 December 2021).
- Nakamura, T.; Hashimoto, I.; Sawada, Y.; Mikami, J.; Saitoh, M.; Hottanda, K.; Bekki, E.; Nishidai, H.; Nakanishi, M.; Kasai, Y. Clinical studies on amikacin for infectious diseases following intravenous drip infusion. Jpn. J. Antibiot. 1982, 35, 897–908. [Google Scholar]
- Hashira, S.; Koike, Y.; Fujii, R. Fundamental study of amikacin in newborn. Jpn. J. Antibiot. 1987, 40, 1129–1134. [Google Scholar] [PubMed]
- Iwai, N.; Sasaki, A.; Taneda, Y.; Mizoguchi, F.; Nakamura, H.; Kawamura, M.; Tauchi, N.; Ozaki, T.; Ichikawa, T.; Matsui, S. Pharmacokinetics in neonates and infants following administration of amikacin. Jpn. J. Antibiot. 1987, 40, 1157–1175. [Google Scholar]
- Kuroki, S.; Okura, K.; Haruta, T.; Kobayashi, Y. Investigation of the use of amikacin in newborns. Jpn. J. Antibiot. 1987, 40, 1192–1199. [Google Scholar]
- Masumi, R.; Hirama, Y.; Narita, A.; Nakazawa, S.; Iwasaki, A.; Niino, K.; Sato, H.; Nakazawa, S.; Tazoe, K.; Chikaoka, H. Studies on intravenous administration of amikacin to neonates. Jpn. J. Antibiot. 1987, 40, 1146–1156. [Google Scholar] [PubMed]
- Motohiro, T.; Tanaka, K.; Kawakami, A.; Koga, T.; Shimada, Y.; Tomita, S.; Sakata, Y.; Fujimoto, T.; Nishiyama, T.; Kuda, N. Pharmacokinetics of amikacin in children and neonates. Jpn. J. Antibiot. 1987, 40, 1200–1214. [Google Scholar] [PubMed]
- Nanri, S.; Sunakawa, K.; Yamashita, N.; Akita, H.; Hotta, M.; Jozaki, K.; Iwata, S.; Iwasaki, Y.; Kanemitsu, T.; Tojo, M.; et al. A pharmacokinetic study in neonates (mature and premature) administered with amikacin through intravenous drip infusion. Jpn. J. Antibiot. 1987, 40, 1135–1145. [Google Scholar] [PubMed]
- Nishimura, T.; Tabuki, K.; Takashima, T. Pharmacokinetic and clinical studies on amikacin in neonates. Jpn. J. Antibiot. 1987, 40, 1183–1191. [Google Scholar]
- Yura, J.; Hayashi, S.; Tsuruga, N.; Hashimoto, T.; Murata, Y.; Kamiya, Y. Pharmacokinetics of amikacin in the pediatric surgical field. Jpn. J. Antibiot. 1987, 40, 1176–1182. [Google Scholar] [PubMed]
- Endo, A.; Nemoto, A.; Hanawa, K.; Maebayashi, Y.; Hasebe, Y.; Kobayashi, M.; Naito, A.; Kobayashi, Y.; Yamamoto, S.; Isobe, K. Relationship between amikacin blood concentration and ototoxicity in low birth weight infants. J. Infect. Chemother. 2019, 24, 17–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouton, J.W.; Dudley, M.N.; Derendorf, H.; Drusano, G.L. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: An update. J. Antimicrob. Chemother. 2005, 55, 601–607. [Google Scholar] [CrossRef] [Green Version]
- Hartman, S.J.F.; Bruggemann, R.J.; Orriens, L.; Dia, N.; Schreuder, M.; de Wildt, S.N. Pharmacokinetics and target attainment of antibiotics in critically ill children: A systematic review of current literature. Clin. Pharmacokinet. 2020, 59, 173–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gijsen, M.; Vlasselaers, D.; Spriet, I.; Allegaert, K. Pharmacokinetics of antibiotics in pediatric intensive care: Fostering variability to attain precision medicine. Antibiotics 2021, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Bressolle, F.; Gouby, A.; Martinez, J.M.; Joubert, P.; Saissi, G.; Guillaud, R.; Gomeni, R. Population pharmacokinetics of amikacin in critically ill patients. Antimicrob. Agents Chemother. 1996, 40, 1682–1689. [Google Scholar] [CrossRef] [Green Version]
- Sherwin, C.M.T.; Wead, S.; Stockmann, C.; Healy, D.; Spigarelli, M.G.; Neely, A.; Kagan, R. Amikacin population pharmacokinetics among paediatric burn patients. Burns 2014, 40, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Hagihara, M.; Hirai, J.; Sakanashi, D.; Suematsu, H.; Nishiyama, N.; Koizumi, Y.; Yamagishi, Y.; Matsuura, K.; Mikamo, H. Evaluation of amikacin pharmacokinetics and pharmacodynamics for optimal initial dosing regimen. Drugs R&D 2017, 17, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Pancoast, S.J. Aminoglycoside antibiotics in clinical use. Med. Clin. North Am. 1988, 72, 581–612. [Google Scholar] [CrossRef]
- Craig, W.A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998, 26, 1–10. [Google Scholar] [CrossRef]
- Lacy, M.K.; Nicolau, D.P.; Nightingale, C.H.; Quintiliani, R. The pharmacodynamics of aminoglycosides. Clin. Infect. Dis. 1998, 27, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.D.; Leitman, P.S.; Smith, C.R. Clinical response to aminoglycoside therapy: Importance of the ratio of peak concentration to minimal inhibitor concentration. J. Infect. Dis. 1987, 155, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Illamola, S. Development of a Population Pharmacokinetic Model to Determine the Optimal Doses of Amikacin in the Treatment of Neonatal Sepsis. Ph.D. Thesis; University of Barcelona: Barcelona, Spain, 2012. Available online: http://diposit.ub.edu/dspace/bitstream/2445/41598/5/01.SMI_PhD_THESIS.pdf. (accessed on 28 December 2021).
- Contopoulos-Ioannidis, D.G.; Giotis, N.D.; Baliatsa, D.V.; Ioannidis, J.P.A. Extended-interval aminoglycoside administration for children: A meta-analysis. Pediatrics 2004, 114, e111–e118. [Google Scholar] [CrossRef] [Green Version]
- Bailey, T.C.; Lieele, J.R.; Littenberg, B.; Reichley, R.M.; Dunagan, W.C. A meta-analysis of extended-interval dosing versus multiple daily dosing of aminoglycosides. Clin. Infect. Dis. 1997, 24, 786–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takesue, Y.; Ohmagari, N.; Okada, K.; Kasahara, K.; Seki, M.; Takakura, S. Practice guidelines for therapeutic drug monitoring of antimicrobial drugs revised edition. Jpn. J. Chemother. 2016, 64, 387–477. [Google Scholar]
- Alhadab, A.A.; Ahmed, M.A.; Brundage, R.C. Amikacin pharmacokinetic-pharmacodynamic analysis in pediatric cancer patients. Antimicrob. Agents Chemother. 2018, 62, e01781-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, P.A. Freedom from ototoxicity in aminoglycoside treated neonates: A mistake notion. Laryngoscope 1981, 91, 1985–1994. [Google Scholar] [CrossRef]
- Eviatar, L.; Eviatar, A. Aminoglycoside ototoxicity in the neonatal period: Possible etiologic factor in delayed postural control. Otolorngol. Head Neck Surg. 1984, 89, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Hagihara, M.; Okudaira, M.; Asai, N.; Koizumi, Y.; Yamagishi, Y.; Mikamo, H. Systematic review and meta-analysis to explore optimal therapeutic range of vancomycin trough level for infected paediatric patients with Gram-positive pathogens to reduce mortality and nephrotoxicity risk. Int. J. Antimicrob. Agents 2021, 58, 106393. [Google Scholar] [CrossRef] [PubMed]
- Hanai, Y.; Hamada, Y.; Kimura, T.; Matsumoto, K.; Takahashi, Y.; Fujii, S.; Nishizawa, K.; Miyazaki, Y.; Takesue, Y. Favorable effects of voriconazole trough concentrations exceeding 1 μg/mL on treatment success and all-cause mortality: A systematic review and meta-analysis. J. Fungi 2021, 7, 306. [Google Scholar] [CrossRef]
- Yamada, T.; Fujii, S.; Shigemi, A.; Takesue, Y. A meta-analysis of the target trough concentration of gentamicin and amikacin for reducing the risk of nephrotoxicity. J. Infect. Chemother. 2021, 27, 256–261. [Google Scholar] [CrossRef]
| Study | Study Design | Setting | No of Patients | Age | Body Weight (kg) | Renal Function | Type of Infection | Bacteria | MIC of AMK (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| Nakamura T, 1982 | Case report | Single- center | 3 | 9 years (8–15 years) | 38 (29–57) | NR | Peritonitis | E. coli | 1.56 |
| Hashira S, 1987 | PK study | Single- center | 21 | 5 days (0–25 days) | 2.8 (0.6–3.9) | NR | Pneumonia (n = 7); sepsis (n = 3); others (n = 11) | NR | NR |
| Iwai N, 1987 | PK study | Single- center | 24 | 25.5 days (8–365 days) | 3.4 (1.9–11.5) | NR | NR | NR | NR |
| Kuroki S, 1987 | Case report | Single- center | 2 | 45.0 days (26–64 days) | 4.6 (3.8–5.3) | Scr 0.45 (0.40–0.50) | UTI | E. coli | Sensitivity against AMK |
| Masumi R, 1987 | Case report | Single- center | 2 | 0.5 days (0–1 days) | 2.7 (2.4–2.9) | NR | Pneumonia | NR | NR |
| Motohiro T, 1987 | PK study | Single- center | 15: children, n = 6; neonate, n = 9 | Children, 9.3 years (7–11 years); neonate, 12 days (4–18 days) | Children, 25.0 (21.1–31.9); neonate, 2.1 (1.4–3.3) | NR | NR | NR | NR |
| Nanri S, 1987 | PK study | Single- center | 13 | 2.9 days (0–11 days) | 2.6 (1.9–4.1) | NR | NR | NR | NR |
| Nishimura T, 1987 | Case report | Single- center | 1 | 13 days | NR | NR | SSTI | S. aureus | 0.78 |
| Yura J, 1987 | Case report | Single- center | 1 | 18 days | 1.7 | NR | SSI | NR | NR |
| Endo A, 2019 | Retrospective study | Single- center | 20 | GA, 30 ± 5.1 weeks | 1.3 ± 0.8 | NR | Sepsis; pneumonia; intraperitoneal infection | NR | NR |
| Study | Regimen | Treatment Duration (day) | Sampling Time after AMK Administration | Cmax (mg/L) | Cmin (mg/L) | Cmax/MIC | Clinical Effect | Bacteriological Effect | Adverse Event |
|---|---|---|---|---|---|---|---|---|---|
| Nakamura T, 1982 | 5.3 mg/kg (3.5–6.9 mg/kg) every 12 h | 5 (4–9) | Cmax: 1.5 h Cmix: NR | 7.0 (4.6–9.8) | NR | 4.5 (2.9–6.3) | All patients cured. | Bacteriological cure rate was 100%. | None |
| Hashira S, 1987 | 5.0 mg/kg (2.0–7.5 mg/kg) every 12 h | NR | Cmax: 0.5–1 h Cmin: 12 h | 14.4 (4.5–37.7) | 1.9 (0.6–9.3) | NR | NR | NR | NR |
| Iwai N, 1987 | 3.0 mg/kg (1.4–6.0 mg/kg) every 24 h | NR | Cmax: 0.5–1 h Cmin: 8 h | 8.7 (2.6–28.5) | 1.3 (0.8–5.2) | NR | NR | NR | NR |
| Kuroki S, 1987 | 5.9 mg/kg (5.7–6.1 mg/kg) every 8 h | 7 (7–7) | Cmax: 0.5–1 h Cmin: 8 h | 19.0 (18.0–20.0) | 4.9 (3.8–6) | NR | All patients cured. | Bacteriological cure rate was 100%. | None |
| Masumi R, 1987 | 2.1 mg/kg (1.6–2.5 mg/kg) every 12 h | 3 (3–3) | Cmax: 0.5–1 h Cmin: 8–12 h | 5.9 (3.8–8.0) | 2.4 (2.0–2.7) | NR | All patients cured. | NR | None |
| Motohiro T, 1987 | Children, 3.0 mg/kg (2.0–4.0 mg/kg) every 24 h; neonate, 4.3 mg/kg (3.0–6.0 mg/kg) every 24 h | NR | Cmax: 0.5–1 h Cmin: 6 h | Children, 11.5 (8.2–13.9); neonate, 9.5 (6.1–16.2) | Children, 0.6 (0.3–1.1); neonate, 3.4 (1.7–6.6) | NR | NR | NR | NR |
| Nanri S, 1987 | 4.6 mg/kg (3.0–6.0 mg/kg) every 24 h | NR | Cmax: 0.5–1 h Cmin: 6 h | 15.0 (6.3–26.3) | 5.5 (2.1–10.4) | NR | NR | NR | NR |
| Nishimura T, 1987 | 3 mg/kg every 8 h | 7 | Cmax: 0.5 h Cmin: 8 h | 9.1 | 0.8 | 11.7 | Cured | The culture on day 5 showed negative. | None |
| Yura J, 1987 | 6.0 mg/kg every 24 h | NR | Cmax: 0.5 h Cmin: 8 h | 18.1 | 0.8 | NR | NR | NR | NR |
| Endo A, 2019 | 14.1 ± 2.6 mg/kg every 24 h | 10.1 ± 4.1 | Cmax: 1–1.5 h Cmin: 23–24 h | 29.1 (19.4–42.5) | 7.9 (1.8–28.4) | NR | All patients cured. | NR | 20% (4/20, 3 patients of them had trough concentrations ≥ 10 mg/L) |
| Study | Study Design | Setting | No of Patients | Age | Body Weight (kg) | Renal Function | Type of Infection | Bacteria | MIC of AMK (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| Bressolle F, 1996 | PK study | Single- center | 36 | 5.7 years (6 months–15 years) | 20.4 ± 13.6 | Scr 49.1 ± 17.5 μmol/L | Pneumonia (n = 4); UTI (n = 11); bone and joint (n = 5); sepsis (n = 2); SSTI (n = 5); gastrointestinal tract (n = 6); Others (n = 3) | E. coli (n = 10); P. aeruginosa (n = 2); M. catarrhalis (n = 1); Klebsiella sp (n = 2); S. aureus (n = 5); S. epidermidis (n = 1); Streptococcus sp (n = 3); Salmonella sp (n = 5); E. cloacae (n = 1) | NR |
| Sherwin CMT, 2014 | PK study | Single- center | 73 | Median 4.5 years (0.6–17 years) | Median 20 (8–90) | NR | Burn | NR | NR |
| Study | Regimen | Treatment Duration (day) | Cmax (mg/L) | Cmin (mg/L) | Cmax/MIC | Clinical Effect | Bacteriological Effect | Adverse Event |
|---|---|---|---|---|---|---|---|---|
| Bressolle F, 1996 | 70–1500 mg every 24 h | 8.8 ± 3.0 | 40.7 ± 15.8 | 0.97 ± 0.66 | NR | Two patients died. | NR | NR |
| Sherwin CMT, 2014 | 16.4 ± 3.9 mg/kg/day (4.9–22.3 mg/kg/day) | NR | 33.2 ± 9.4 | 3.8 ± 4.6 | NR | NR | NR | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, H.; Hamada, Y. Amikacin Therapy in Japanese Pediatric Patients: Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 1972. https://doi.org/10.3390/ijerph19041972
Kato H, Hamada Y. Amikacin Therapy in Japanese Pediatric Patients: Narrative Review. International Journal of Environmental Research and Public Health. 2022; 19(4):1972. https://doi.org/10.3390/ijerph19041972
Chicago/Turabian StyleKato, Hideo, and Yukihiro Hamada. 2022. "Amikacin Therapy in Japanese Pediatric Patients: Narrative Review" International Journal of Environmental Research and Public Health 19, no. 4: 1972. https://doi.org/10.3390/ijerph19041972
APA StyleKato, H., & Hamada, Y. (2022). Amikacin Therapy in Japanese Pediatric Patients: Narrative Review. International Journal of Environmental Research and Public Health, 19(4), 1972. https://doi.org/10.3390/ijerph19041972







