Changes in Biomarkers of Tobacco Exposure among Cigarette Smokers Transitioning to ENDS Use: The Population Assessment of Tobacco and Health Study, 2013–2015
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Analytic Sample and User Characteristics
2.3. Quantification of Biomarkers of Tobacco Exposure (BOE)
2.4. Statistical Analysis
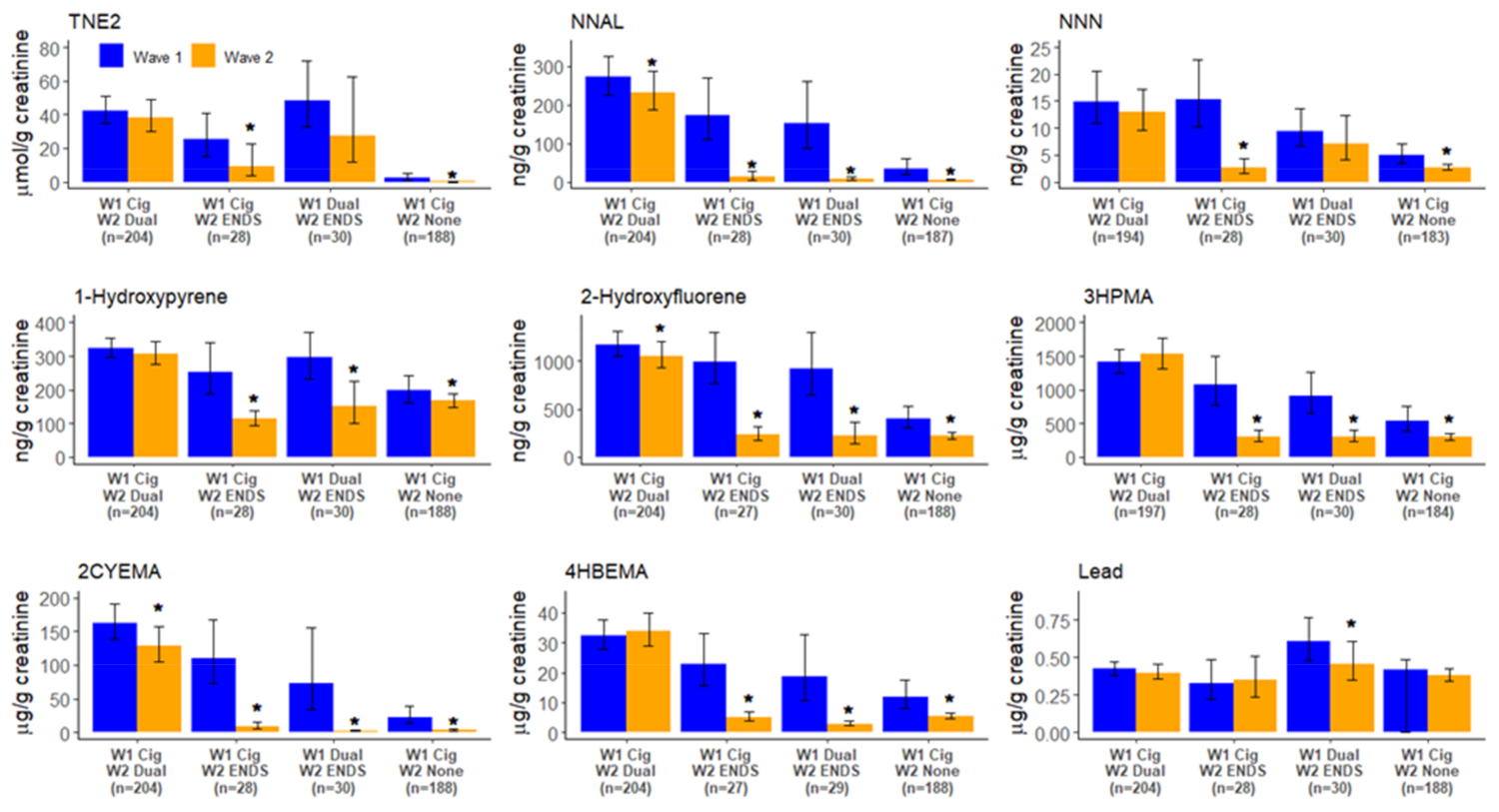
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Chang, C.M.; Edwards, S.H.; Arab, A.; Del Valle-Pinero, A.Y.; Yang, L.; Hatsukami, D.K. Biomarkers of tobacco exposure: Summary of an FDA-sponsored public workshop. Cancer Epidemiol. Biomark. Prev. 2017, 26, 291–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goniewicz, M.L.; Knysak, J.; Gawron, M.; Kosmider, L.; Sobczak, A.; Kurek, J.; Prokopowicz, A.; Jablonska-Czapla, M.; Rosik-Dulewska, C.; Havel, C.; et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 2014, 23, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jesús, V.R.; Bhandari, D.; Zhang, L.; Reese, C.; Capella, K.; Tevis, D.; Zhu, W.; Del Valle-Pinero, A.Y.; Lagaud, G.; Chang, J.T.; et al. Urinary biomarkers of exposure to volatile organic compounds from the Population Assessment of Tobacco and Health Study wave 1 (2013-2014). Int. J. Environ. Res. Public Health 2020, 17, 5408. [Google Scholar] [CrossRef] [PubMed]
- Keith, R.J.; Fetterman, J.L.; Orimoloye, O.A.; Dardari, Z.; Lorkiewicz, P.K.; Hamburg, N.M.; DeFilippis, A.P.; Blaha, M.J.; Bhatnagar, A. Characterization of volatile organic compound metabolites in cigarette smokers, electronic nicotine device users, dual users, and nonusers of tobacco. Nicotine Tob. Res. 2020, 22, 264–272. [Google Scholar] [CrossRef]
- Smith, D.M.; Shahab, L.; Blount, B.C.; Gawron, M.; Kosminder, L.; Sobczak, A.; Xia, B.; Sosnoff, C.S.; Goniewicz, M.L. Differences in exposure to nicotine, tobacco-specific nitrosamines, and volatile organic compounds among electronic cigarette users, tobacco smokers, and dual users from three countries. Toxics 2020, 8, 88. [Google Scholar] [CrossRef]
- Goniewicz, M.L.; Smith, D.M.; Edwards, K.C.; Blount, B.C.; Caldwell, K.L.; Feng, J.; Wang, L.; Christensen, C.; Ambrose, B.; Borek, N.; et al. Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA Netw. Open 2018, 1, e185937. [Google Scholar] [CrossRef] [Green Version]
- Lorkiewicz, P.; Riggs, D.W.; Keith, R.J.; Conklin, D.J.; Xie, Z.; Sutaria, S.; Lynch, B.; Srivastava, S.; Bhatnagar, A. Comparison of urinary biomarkers of exposure in humans using electronic cigarettes, combustible cigarettes, and smokeless tobacco. Nicotine Tob. Res. 2019, 21, 1228–1238. [Google Scholar] [CrossRef]
- Piper, M.E.; Baker, T.B.; Benowitz, N.L.; Kobinsky, K.H.; Jorenby, D.E. Dual users compared to smokers: Demographics, dependence, and biomarkers. Nicotine Tob. Res. 2019, 21, 1279–1284. [Google Scholar] [CrossRef]
- Rubinstein, M.L.; Delucchi, K.; Benowitz, N.L.; Ramo, D.E. Adolescent exposure to toxic volatile organic chemicals from e-cigarettes. Pediatrics 2018, 141, e20173557. [Google Scholar] [CrossRef] [Green Version]
- Hecht, S.S.; Carmella, S.G.; Kotandeniya, D.; Pillsbury, M.E.; Chen, M.; Ransom, B.W.; Vogel, R.I.; Thompson, E.; Murphy, S.E.; Hatsukami, D.K. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob. Res. 2015, 17, 704–709. [Google Scholar] [CrossRef]
- Shahab, L.; Goniewicz, M.L.; Blount, B.C.; Brown, J.; McNeill, A.; Alwis, K.U.; Feng, J.; Wang, L.; West, R. Nicotine, carcinogen, and toxin exposure in long-term e-cigarette and nicotine replacement therapy users: A cross-sectional study. Ann. Intern. Med. 2017, 166, 390–400. [Google Scholar] [CrossRef] [Green Version]
- Czoli, C.D.; Fong, G.T.; Goniewicz, M.L.; Hammond, D. Biomarkers of exposure among “dual users” of tobacco cigarettes and electronic cigarettes in Canada. Nicotine Tob. Res. 2019, 21, 1259–1266. [Google Scholar] [CrossRef]
- Goniewicz, M.L.; Gawron, M.; Smith, D.M.; Peng, M.; Jacob, P., 3rd; Benowitz, N.L. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: A longitudinal within-subjects observational study. Nicotine Tob. Res. 2017, 19, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Pulvers, K.; Emami, A.S.; Nollen, N.L.; Romero, D.R.; Strong, D.R.; Benowitz, N.L.; Ahluwalia, J.S. Tobacco consumption and toxicant exposure of cigarette smokers using electronic cigarettes. Nicotine Tob. Res. 2018, 20, 206–214. [Google Scholar] [CrossRef] [Green Version]
- D’Ruiz, C.D.; Graff, D.W.; Robinson, E. Reductions in biomarkers of exposure, impacts on smoking urge and assessment of product use and tolerability in adult smokers following partial or complete substitution of cigarettes with electronic cigarettes. BMC Public Health 2016, 16, 543. [Google Scholar] [CrossRef] [Green Version]
- McRobbie, H.; Phillips, A.; Goniewicz, M.L.; Smith, K.M.; Knight-West, O.; Przulj, D.; Hajek, P. Effects of switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein. Cancer Prev. Res. 2015, 8, 873–878. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, G.; Graff, D.W.; D’Ruiz, C.D. Reductions in biomarkers of exposure (BoE) to harmful or potentially harmful constituents (HPHCs) following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Toxicol. Mech. Methods 2016, 26, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Hyland, A.; Ambrose, B.K.; Conway, K.P.; Borek, N.; Lambert, E.; Carusi, C.; Taylor, K.; Crosse, S.; Fong, G.T.; Cummings, K.M.; et al. Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tob. Control 2017, 26, 371–378. [Google Scholar] [CrossRef]
- Caudill, S.P.; Schleicher, R.L.; Pirkle, J.L. Multi-rule quality control for the age-related eye disease study. Stat. Med. 2008, 27, 4094–4106. [Google Scholar] [CrossRef]
- Chang, J.T.; Anic, G.M.; Rostron, B.L.; Tanwar, M.; Chang, C.M. Cigarette Smoking Reduction and Health Risks: A Systematic Review and Meta-analysis. Nicotine Tob. Res. 2021, 23, 635–642. [Google Scholar] [CrossRef]
- Rostron, B.L.; Corey, C.G.; Chang, J.T.; van Bemmel, D.M.; Miller, M.E.; Chang, C.M. Changes in cigarettes per day and biomarkers of exposure among US adult smokers in the Population Assessment of Tobacco and Health Study waves 1 and 2 (2013-2015). Nicotine Tob. Res. 2020, 22, 1780–1787. [Google Scholar] [CrossRef] [Green Version]
- International Agency for Research on Cancer. Tobacco Smoke and Involuntary Smoking; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2004; Volume 83. [Google Scholar]
- Hornung, R.W.; Reed, L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- Harvanko, A.M.; St Helen, G.; Nardone, N.; Addo, N.; Benowitz, N.L. Twenty-four-hour subjective and pharmacological effects of ad-libitum electronic and combustible cigarette use among dual users. Addiction 2020, 115, 1149–1159. [Google Scholar] [CrossRef]
- St Helen, G.; Nardone, N.; Addo, N.; Dempsey, D.; Havel, C.; Jacob, P., 3rd; Benowitz, N.L. Differences in nicotine intake and effects from electronic and combustible cigarettes among dual users. Addiction 2020, 115, 757–767. [Google Scholar] [CrossRef]
- Huang, J.; Duan, Z.; Kwok, J.; Binns, S.; Vera, L.E.; Kim, Y.; Szczypka, G.; Emery, S.L. Vaping versus JUULing: How the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob. Control 2019, 28, 146–151. [Google Scholar] [CrossRef] [Green Version]
- King, B.A.; Gammon, D.G.; Marynak, K.L.; Rogers, T. Electronic cigarette sales in the United States, 2013–2017. JAMA 2018, 320, 1379–1380. [Google Scholar] [CrossRef]
- Boykan, R.; Messina, C.R.; Chateau, G.; Eliscu, A.; Tolentino, J.; Goniewicz, M.L. Self-reported use of tobacco, e-cigarettes, and marijuana versus urinary biomarkers. Pediatrics 2019, 143, e20183531. [Google Scholar] [CrossRef]

| W1 Exclusive Cigarette Smokers (n = 1899) | W1 Dual Cigarette/ENDS Users (n = 576) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | W2 Exclusive Cigarette Use (n = 1479) | W2 Dual Use (n = 204) | W2 Exclusive ENDS Use (n = 28) | W2 No Tobacco Use (n = 188) | W2 Exclusive Cigarette Use (n = 273) | W2 Dual Use (n = 242) | W2 Exclusive ENDS Use (n = 30) | W2 No Tobacco Use (n = 31) |
| Age, mean (SE) | 45.5 (0.6) | 41.6 (1.4) | 39.1 (3.6) a | 43.4 (2.1) | 42.7 (0.9) | 43.0 (0.9) | 44.8 (3.0) a | 41.0 (3.4) a |
| Sex | ||||||||
| Male | 46.6 (42.5, 50.8) | 34.5 (26.5, 43.5) | 71.8 (53.3, 85.0) a | 40.6 (31.0, 51.0) | 37.5 (30.6, 44.9) | 37.9 (30.7, 45.6) | 29.6 (13.8, 52.4) a | 27.9 (14.4, 47.1) a |
| Female | 53.4 (49.2, 57.5) | 65.5 (56.5, 73.5) | 28.2 (15.0, 46.7) a | 59.4 (49.0, 69.0) | 62.5 (55.1, 69.4) | 62.1 (54.4, 69.3) | 70.4 (47.6, 86.2) a | 72.1 (52.9, 85.6) a |
| Race/ethnicity | ||||||||
| Non-Hispanic White | 66.0 (61.4, 70.3) | 78.1 (69.5, 84.8) | 71.1 (41.6, 89.4) a | 59.4 (47.8, 70.0) | 76.7 (70.9, 81.5) | 81.7 (76.3, 86.1) | 86.3 (67.1, 95.1) a | 68.9 (48.3, 84.0) a |
| Other b | 34.0 (29.7, 38.6) | 21.9 (15.2, 30.5) | 28.9 (10.6, 58.4) a | 40.6 (30.0, 52.2) | 23.3 (18.5, 29.1) | 18.3 (13.9, 23.7) | 13.7 (4.9, 32.9) a | 31.1 (16.0, 51.7) a |
| Educational level | ||||||||
| Less than HS/GED | 27.3 (24.4, 30.4) | 25.6 (17.8, 35.3) | 18.0 (6.9, 39.3) a | 20.4 (14.4, 28.0) | 22.5 (17.9, 27.8) | 20.8 (15.8, 26.9) | 23.8 (11.2, 43.5) a | 17.2 (6.5, 38.3) a |
| HS graduate | 31.2 (27.3, 35.4) | 32.1 (22.5, 43.4) | 28.3 (11.0, 55.7) a | 32.1 (21.1, 45.5) | 26.6 (20.4, 33.9) | 22.0 (17.3, 27.7) | 12.0 (4.5, 28.1) a | 24.5 (12.2, 43.1) a |
| Some college or higher | 41.5 (37.5, 45.6) | 42.3 (32.5, 52.9) | 53.8 (31.5, 74.7) a | 47.6 (37.6, 57.7) | 50.9 (44, 57.7) | 57.1 (50.5, 63.5) | 64.3 (42.7, 81.2) a | 58.3 (38.2, 76.0) a |
| CPD, mean (SE) | ||||||||
| Wave 1 | 16.6 (1.1) | 17.0 (2.2) | 11.0 (1.8) a | 5.5 (0.8) | 14.3 (0.7) | 13.7 (0.6) | 8.8 (1.8) a | 8.6 (2.2) a |
| Wave 2 | 14.0 (0.7) | 14.1 (1.0) | NA | NA | 17.9 (3.0) | 13.6 (0.9) | NA | NA |
| W1 daily cigarette smoking | 83.7 (81.1, 86.0) | 91.9 (87.3, 94.9) | 86.5 (69.4, 94.7) a | 35.5 (24.2, 48.7) | 85.8 (80.8, 89.6) | 83.1 (77.8, 87.3) | 57.7 (34.9, 77.7) a | 44.0 (26.6, 63.0) a |
| W2 daily cigarette smoking | 82.6 (79.0, 85.6) | 82.5 (73.1, 89.1) | NA | NA | 88.1 (83.3, 91.7) | 76.6 (69.8, 82.2) | NA | NA |
| W1-W2 change in CPD | ||||||||
| Reduced CPD by ≥50% | 15.0 (12.1–18.5) | 18.8 (12.5–27.3) | NA | NA | 8.5 (5.4–13.3) | 18.7 (14.0–24.6) | NA | NA |
| Increased CPD by ≥50% | 18.4 (15.6–21.6) | 10.0 (6.6–14.9) | NA | NA | 19.8 (15.4–25.1) | 16.4 (12.0–21.9) | NA | NA |
| Change in CPD <50% | 66.6 (62.5–70.4) | 71.2 (62.5–78.6) | NA | NA | 71.7 (65.5–77.1) | 64.9 (57.9–71.3) | NA | NA |
| W2 daily ENDS use | NA | 13.3 (8.2, 20.9) | 80.3 (62.4, 91.0) a | NA | NA | 21.0 (15.6, 27.7) | 85.1 (66.2, 94.4) a | NA |
| W2 flavored ENDS use | NA | 58.2 (48.6, 67.2) | 80.5 (60.1, 91.9) a | NA | NA | 58.0 (49.5, 66.0) | 72.8 (51.9, 86.9) a | NA |
| W2 ENDS device type c | ||||||||
| Customizable | NA | 59.0 (50.1, 67.4) | 70.3 (40.6, 89.1) a | NA | NA | 61.1 (52.1, 69.4) | 74.6 (50.6, 89.4) a | NA |
| Non-customizable | NA | 41.0 (32.6, 49.9) | 29.7 (10.9, 59.4) a | NA | NA | 38.9 (30.6, 47.9) | 25.4 (10.6, 49.4) a | NA |
| W2 cigarette/ENDS frequency of use | ||||||||
| Daily cigarette, daily ENDS | NA | 7.0 (4.3, 11.4) | NA | NA | NA | 10.2 (6.6, 15.4) | NA | NA |
| Daily cigarettes, non-daily ENDS | NA | 75.4 (66.8, 82.4) | NA | NA | NA | 66.4 (58.6, 73.3) | NA | NA |
| Non-daily cigarette, daily ENDS | NA | 6.3 (2.5, 14.8) | NA | NA | NA | 10.8 (7.0, 16.4) | NA | NA |
| Non-daily cigarette, non-daily ENDS | NA | 11.2 (6.2, 19.4) | NA | NA | NA | 12.6 (8.5, 18.3) | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anic, G.M.; Rostron, B.L.; Hammad, H.T.; van Bemmel, D.M.; Del Valle-Pinero, A.Y.; Christensen, C.H.; Erives, G.; Faulcon, L.M.; Blount, B.C.; Wang, Y.; et al. Changes in Biomarkers of Tobacco Exposure among Cigarette Smokers Transitioning to ENDS Use: The Population Assessment of Tobacco and Health Study, 2013–2015. Int. J. Environ. Res. Public Health 2022, 19, 1462. https://doi.org/10.3390/ijerph19031462
Anic GM, Rostron BL, Hammad HT, van Bemmel DM, Del Valle-Pinero AY, Christensen CH, Erives G, Faulcon LM, Blount BC, Wang Y, et al. Changes in Biomarkers of Tobacco Exposure among Cigarette Smokers Transitioning to ENDS Use: The Population Assessment of Tobacco and Health Study, 2013–2015. International Journal of Environmental Research and Public Health. 2022; 19(3):1462. https://doi.org/10.3390/ijerph19031462
Chicago/Turabian StyleAnic, Gabriella M., Brian L. Rostron, Hoda T. Hammad, Dana M. van Bemmel, Arseima Y. Del Valle-Pinero, Carol H. Christensen, Gladys Erives, Lisa M. Faulcon, Benjamin C. Blount, Yuesong Wang, and et al. 2022. "Changes in Biomarkers of Tobacco Exposure among Cigarette Smokers Transitioning to ENDS Use: The Population Assessment of Tobacco and Health Study, 2013–2015" International Journal of Environmental Research and Public Health 19, no. 3: 1462. https://doi.org/10.3390/ijerph19031462






