The Effect of Exercise on Cardiometabolic Risk Factors in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.2.1. Participants
2.2.2. Intervention
2.2.3. Comparator
2.2.4. Outcome
2.3. Data Synthesis
2.4. Data Analysis
2.5. Methodological Quality
2.6. Risk of Bias Assessment and Certainty of Evidence
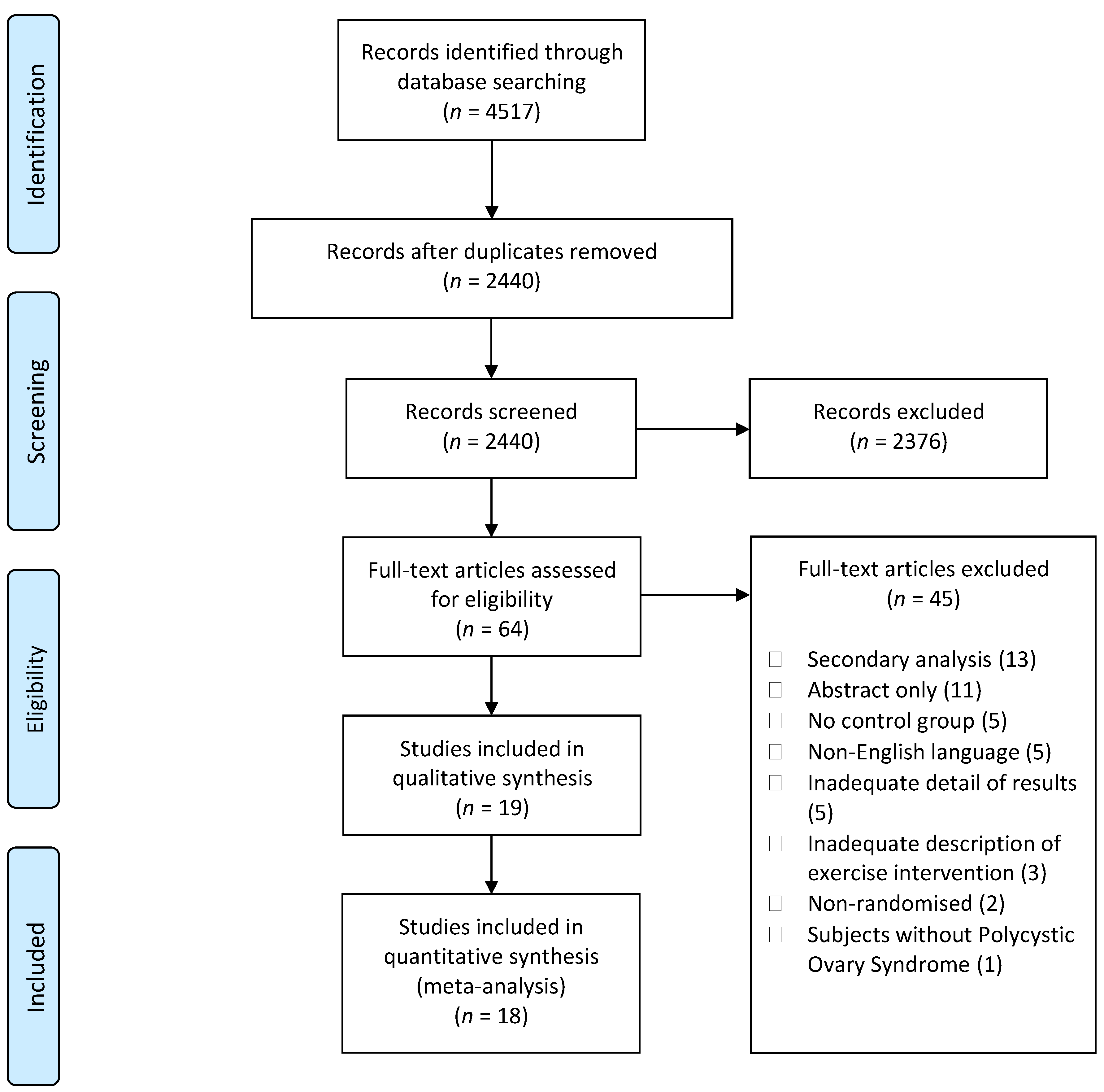
3. Results
3.1. Participant Characteristics
3.2. Intervention Characteristics
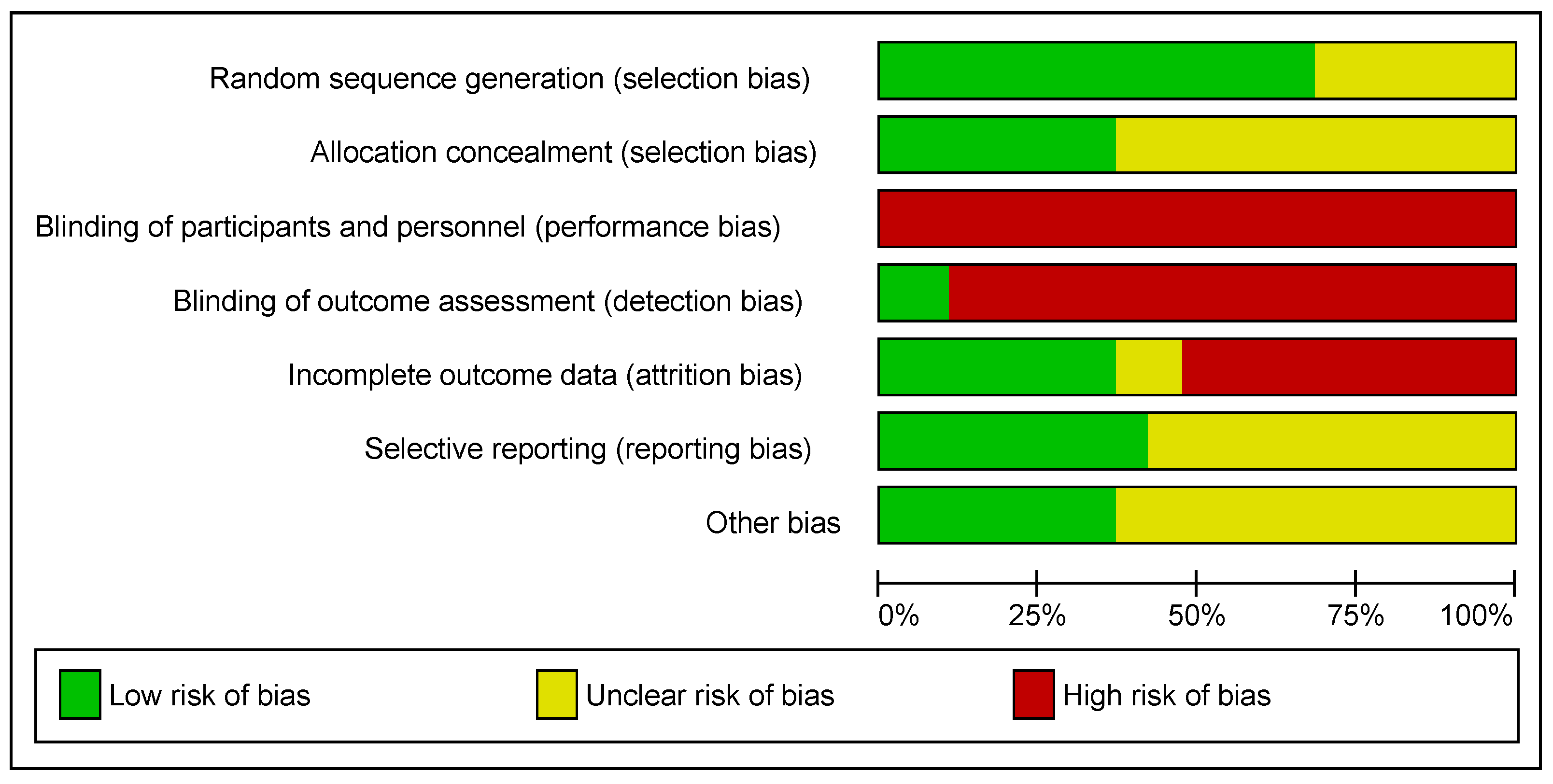
3.3. Methodological Quality, Risk of Bias, and Certainty of Evidence
3.4. Meta-Analysis
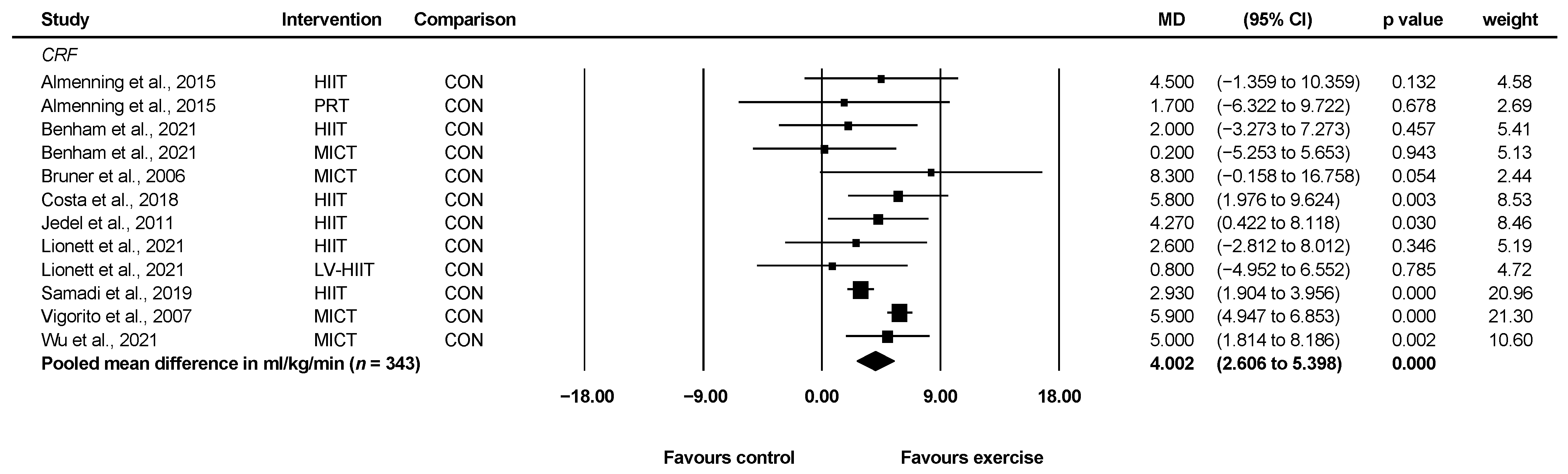
3.4.1. Cardiorespiratory Fitness
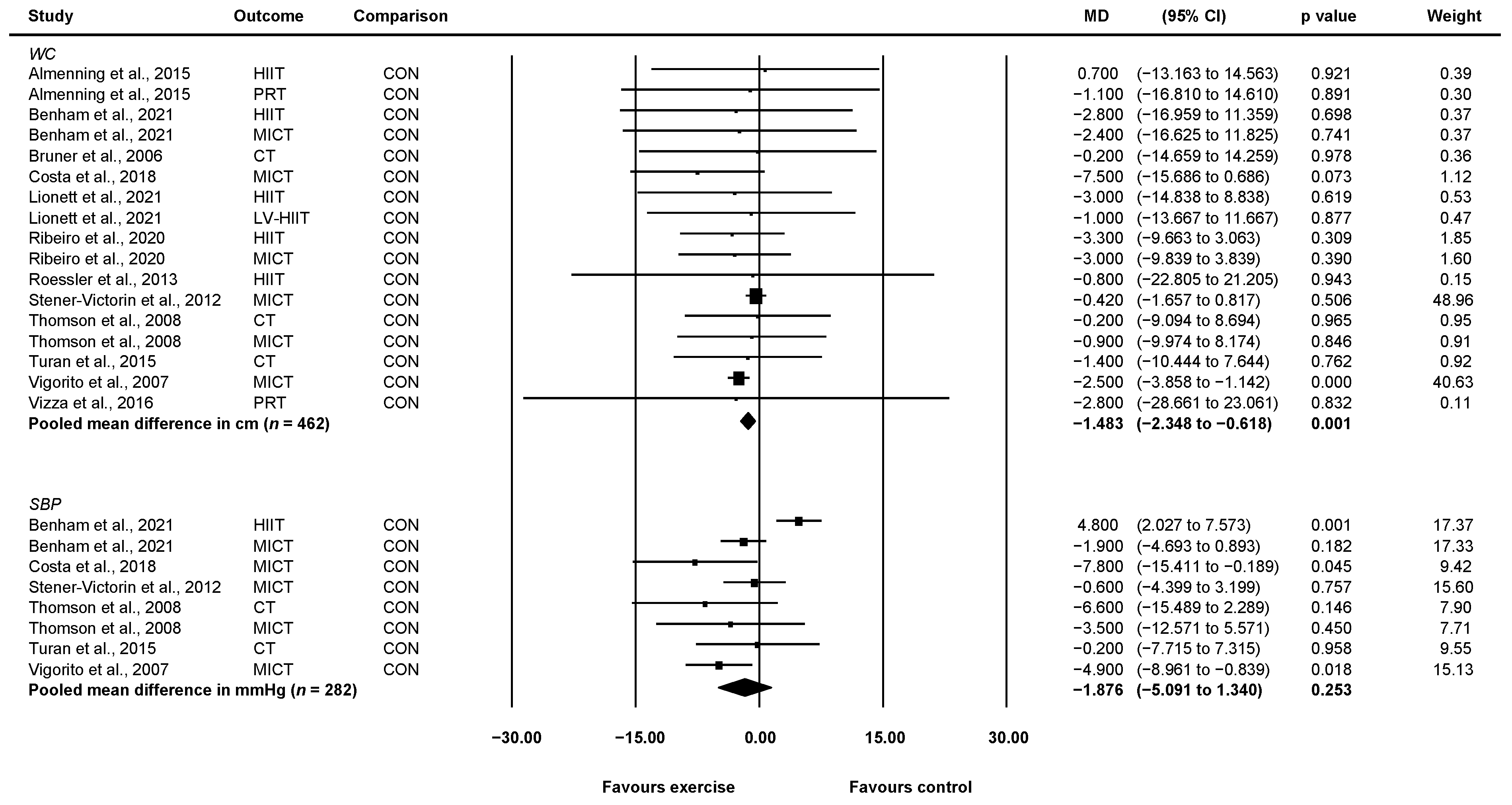
3.4.2. Waist Circumference
3.4.3. Systolic Blood Pressure
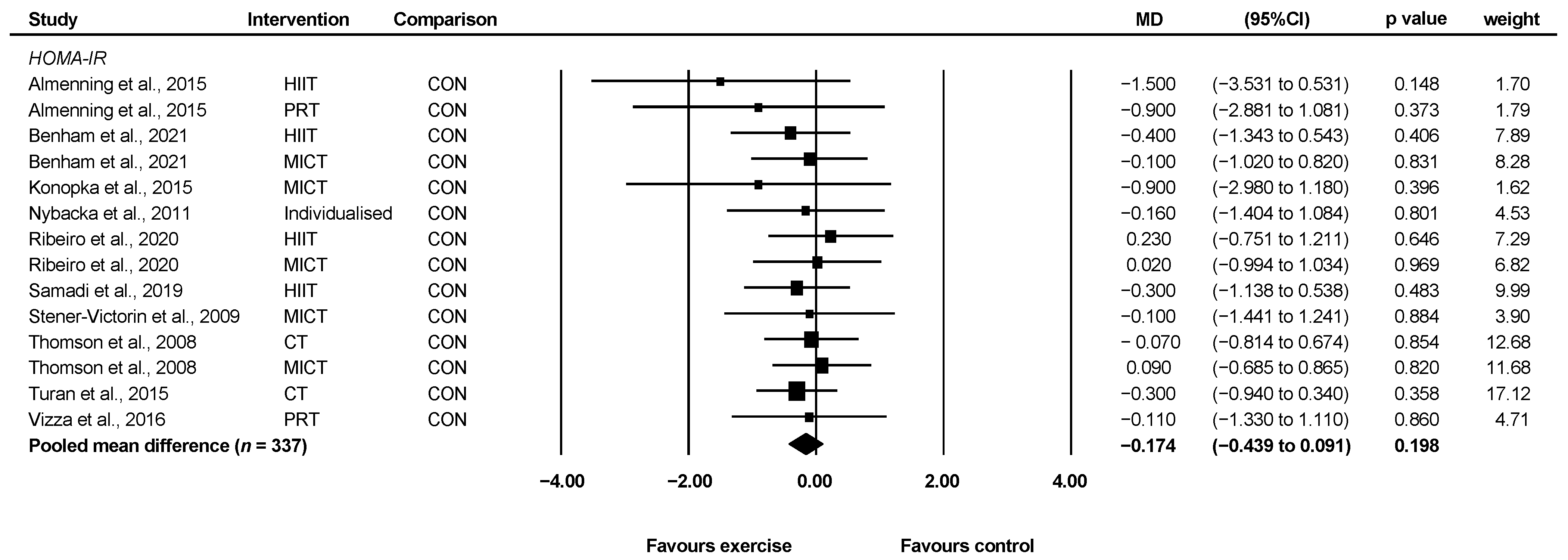
3.4.4. HOMA-IR
3.4.5. Fasting Blood Glucose
3.4.6. Triglycerides
3.4.7. HDL-C
3.5. Sub-Analyses
4. Discussion
4.1. Implications of the Research
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Reporting | Score |
| 0–1 |
| 0–1 |
| 0–1 |
| 0–1 |
| 0–1 |
| 0–1 |
| 0–1 |
| 0–1 |
| 0–1 |
| 0–1 |
| |
| 0–1 |
| 0–1 |
| 0–1 |
| |
| 0–1 |
| 0–1 |
| 0–1 |
| 0–1 |
| 0–1 |
| 0–1 |
| 0–1 |
| |
| 0–1 |
| 0–1 |
| 0–1 |
| 0–1 |
| 0–1 |
| 0–1 |
| |
| 0–1 |
| |
| 0–1 |
| 0–1 |
References
- Deswal, R.; Narwal, V.; Dang, A.; Pundir, C.S. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J. Hum. Reprod. Sci. 2020, 13, 261–271. [Google Scholar] [PubMed]
- Teede, H.J.; Hutchison, S.; Zoungas, S.; Meyer, C. Insulin resistance, the metabolic syndrome, diabetes, and cardiovascular disease risk in women with PCOS. Endocrine 2006, 30, 45–53. [Google Scholar] [CrossRef]
- Lim, S.S.; Davies, M.J.; Norman, R.J.; Moran, L.J. Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Updat. 2012, 18, 618–637. [Google Scholar] [CrossRef] [PubMed]
- DeUgarte, C.M.; Bartolucci, A.A.; Azziz, R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil. Steril. 2005, 83, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Misso, M.L.; Hopkins, W.G.; Shaw, C.S.; Teede, H.J.; Stepto, N.K. Insulin resistance in polycystic ovary syndrome: A systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum. Reprod. 2016, 31, 2619–2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambineri, A.; Patton, L.; Altieri, P.; Pagotto, U.; Pizzi, C.; Manzoli, L.; Pasquali, R. Polycystic ovary syndrome is a risk factor for type 2 diabetes: Results from a long-term prospective study. Diabetes 2012, 61, 2369–2374. [Google Scholar] [CrossRef] [Green Version]
- Osibogun, O.; Ogunmoroti, O.; Michos, E.D. Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends Cardiovasc. Med. 2020, 30, 399–404. [Google Scholar] [CrossRef]
- Donà, S.; Bacchi, E.; Moghetti, P. Is cardiorespiratory fitness impaired in PCOS women? A review of the literature. J. Endocrinol. Investig. 2017, 40, 463–469. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; on behalf of theInternational PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef] [Green Version]
- Sabag, A.; Little, J.P.; Johnson, N.A. Low-volume high-intensity interval training for cardiometabolic health. J. Physiol. 2021. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, 2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshre, T.R.; Group A-SPCW. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar]
- Zawadzski, J. Diagnostic criteria for polycystic ovary syndrome: Towards a rational approach. Polycystic Ovary Syndr. 1992, 39, 32–37. [Google Scholar]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.; Futterweit, W.; Janssen, O.E.; Legro, R.; Norman, R.; Taylor, A.E.; et al. Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An androgen excess society guideline. J. Clin. Endocrinol. Metab. 2006, 91, 4237–4245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norton, K.; Norton, L.; Sadgrove, D. Position statement on physical activity and exercise intensity terminology. J. Sci. Med. Sport 2010, 13, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.H.; Oxman, A.D.; Schünemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef]
- Jedel, E.; Labrie, F.; Odén, A.; Holm, G.; Nilsson, L.; Janson, P.O.; Lind, A.-K.; Ohlsson, C.; Stener-Victorin, E. Impact of electro-acupuncture and physical exercise on hyperandrogenism and oligo/amenorrhea in women with polycystic ovary syndrome: A randomized controlled trial. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E37–E45. [Google Scholar] [CrossRef] [Green Version]
- Stener-Victorin, E.; Baghaei, F.; Holm, G.; Janson, P.O.; Olivecrona, G.; Lönn, M.; Mannerås-Holm, L. Effects of acupuncture and exercise on insulin sensitivity, adipose tissue characteristics, and markers of coagulation and fibrinolysis in women with polycystic ovary syndrome: Secondary analyses of a randomized controlled trial. Fertil. Steril. 2012, 97, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Setji, T.L.; Sanders, L.L.; Lowry, K.P.; Otvos, J.D.; E Kraus, W.; Svetkey, P.L. Effects of exercise on lipoprotein particles in women with polycystic ovary syndrome. Med. Sci. Sports Exerc. 2009, 41, 497–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turan, V.; Mutlu, E.K.; Solmaz, U.; Ekin, A.; Tosun, O.; Tosun, G.; Mat, E.; Gezer, C.; Malkoç, M. Benefits of short-term structured exercise in non-overweight women with polycystic ovary syndrome: A prospective randomized controlled study. J. Phys. Ther. Sci. 2015, 27, 2293–2297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizza, L.; Smith, C.A.; Swaraj, S.; Agho, K.; Cheema, B.S. The feasibility of progressive resistance training in women with polycystic ovary syndrome: A pilot randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2016, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almenning, I.; Rieber-Mohn, A.; Lundgren, K.M.; Shetelig Løvvik, T.; Garnæs, K.K.; Moholdt, T. Effects of High Intensity Interval Training and Strength Training on Metabolic, Cardiovascular and Hormonal Outcomes in Women with Polycystic Ovary Syndrome: A Pilot Study. PLoS ONE 2015, 10, e0138793. [Google Scholar] [CrossRef] [Green Version]
- Benham, J.L.; Booth, J.E.; Corenblum, B.; Doucette, S.; Friedenreich, C.M.; Rabi, D.M.; Sigal, R.J. Exercise training and reproductive outcomes in women with polycystic ovary syndrome: A pilot randomized controlled trial. Clin. Endocrinol. (Oxf). 2021, 95, 332–343. [Google Scholar] [CrossRef]
- Bruner, B.; Chad, K.; Chizen, D. Effects of exercise and nutritional counseling in women with polycystic ovary syndrome. Appl Physiol. Nutr. Metab. 2006, 31, 384–391. [Google Scholar] [CrossRef] [Green Version]
- Costa, E.C.; DE Sá, J.C.F.; Stepto, N.K.; Costa, I.B.B.; Farias Junior, L.F.; Moreira, S.D.N.T.; Soares, E.M.M.; Lemos, T.M.A.M.; Browne, R.A.V.; De Azevedo, G.D. Aerobic Training Improves Quality of Life in Women with Polycystic Ovary Syndrome. Med. Sci. Sports Exerc. 2018, 50, 1357–1366. [Google Scholar] [CrossRef]
- Ribeiro, V.B.; Kogure, G.S.; Lopes, I.P.; Silva, R.C.; Pedroso, D.C.C.; De Melo, A.S.; de Souza, H.C.D.; Ferriani, R.A.; Miranda Furtado, C.L.; Dos Reis, R.M. Effects of continuous and intermittent aerobic physical training on hormonal and metabolic profile, and body composition in women with polycystic ovary syndrome: A randomized controlled trial. Clin. Endocrinol. (Oxf). 2020, 93, 173–186. [Google Scholar] [CrossRef]
- Thomson, R.L.; Buckley, J.D.; Noakes, M.; Clifton, P.M.; Norman, R.J.; Brinkworth, G.D. The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 3373–3380. [Google Scholar] [CrossRef] [Green Version]
- Konopka, A.R.; Asante, A.; Lanza, I.R.; Robinson, M.M.; Johnson, M.L.; Man, C.D.; Cobelli, C.; Amols, M.H.; Irving, B.A.; Nair, K. Defects in mitochondrial efficiency and H2O2 emissions in obese women are restored to a lean phenotype with aerobic exercise training. Diabetes 2015, 64, 2104–2115. [Google Scholar] [CrossRef] [Green Version]
- Samadi, Z.; Bambaeichi, E.; Valiani, M.; Shahshahan, Z. Evaluation of Changes in Levels of Hyperandrogenism, Hirsutism and Menstrual Regulation After a Period of Aquatic High Intensity Interval Training in Women with Polycystic Ovary Syndrome. Int. J. Prev. Med. 2019, 10, 187. [Google Scholar] [PubMed]
- Lionett, S.; Kiel, I.A.; Røsbjørgen, R.; Lydersen, S.; Larsen, S.; Moholdt, T. Absent Exercise-Induced Improvements in Fat Oxidation in Women With Polycystic Ovary Syndrome After High-Intensity Interval Training. Front. Physiol. 2021, 12, 649794. [Google Scholar] [CrossRef] [PubMed]
- Nybacka, Å.; Carlström, K.; Ståhle, A.; Nyrén, S.; Hellström, P.M.; Hirschberg, A.L. Randomized comparison of the influence of dietary management and/or physical exercise on ovarian function and metabolic parameters in overweight women with polycystic ovary syndrome. Fertil. Steril. 2011, 96, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Roessler, K.K.; Birkebaek, C.; Ravn, P.; Andersen, M.S.; Glintborg, D. Effects of exercise and group counselling on body composition and VO2max in overweight women with polycystic ovary syndrome. Acta Obstet. Gynecol. Scand. 2013, 92, 272–277. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Jedel, E.; Janson, P.O.; Sverrisdottir, Y.B. Low-frequency electroacupuncture and physical exercise decrease high muscle sympathetic nerve activity in polycystic ovary syndrome. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R387–R395. [Google Scholar] [CrossRef] [Green Version]
- Vigorito, C.; Giallauria, F.; Palomba, S.; Cascella, T.; Manguso, F.; Lucci, R.; De Lorenzo, A.; Tafuri, D.; Lombardi, G.; Colao, A.; et al. Beneficial effects of a three-month structured exercise training program on cardiopulmonary functional capacity in young women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2007, 92, 1379–1384. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Wu, H.; Sun, W.; Wang, C. Improvement of anti-Müllerian hormone and oxidative stress through regular exercise in Chinese women with polycystic ovary syndrome. Hormones (Athens) 2021, 20, 339–345. [Google Scholar] [CrossRef]
- Knaeps, S.; Lefevre, J.; Wijtzes, A.; Charlier, R.; Mertens, E.; Bourgois, J.G. Independent Associations between Sedentary Time, Moderate-To-Vigorous Physical Activity, Cardiorespiratory Fitness and Cardio-Metabolic Health: A Cross-Sectional Study. PLoS ONE 2016, 11, e0160166. [Google Scholar] [CrossRef]
- Lyerly, G.W.; Sui, X.; Lavie, C.J.; Church, T.S.; Hand, G.A.; Blair, S.N. The association between cardiorespiratory fitness and risk of all-cause mortality among women with impaired fasting glucose or undiagnosed diabetes mellitus. Mayo. Clin. Proc. 2009, 84, 780–786. [Google Scholar] [CrossRef] [Green Version]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA 2009, 301, 2024–2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kite, C.; Lahart, I.M.; Afzal, I.; Broom, D.R.; Randeva, H.; Kyrou, I.; Brown, J.E. Exercise, or exercise and diet for the management of polycystic ovary syndrome: A systematic review and meta-analysis. Syst. Rev. 2019, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- American Heart Association; Heart, L.N.; Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; A Franklin, B.; Gordon, D.J.; Krauss, R.M.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar]
- E Staiano, A.; A Reeder, B.; Elliott, S.; Joffres, M.R.; Pahwa, P.; A Kirkland, S.; Paradis, G.; Katzmarzyk, P.T. Body mass index versus waist circumference as predictors of mortality in Canadian adults. Int. J. Obes. 2012, 36, 1450–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janiszewski, P.M.; Janssen, I.; Ross, R. Does waist circumference predict diabetes and cardiovascular disease beyond commonly evaluated cardiometabolic risk factors? Diabetes Care 2007, 30, 3105–3109. [Google Scholar] [CrossRef] [Green Version]
- De Koning, L.; Merchant, A.T.; Pogue, J.; Anand, S.S. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: Meta-regression analysis of prospective studies. Eur. Heart J. 2007, 28, 850–856. [Google Scholar] [CrossRef]
- Joham, A.E.; Boyle, J.A.; Zoungas, S.; Teede, H.J. Hypertension in Reproductive-Aged Women With Polycystic Ovary Syndrome and Association With Obesity. Am. J. Hypertens. 2015, 28, 847–851. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.W.; Whelton, P.K.; Allen, N.; Clark, D.; Gidding, S.S.; Muntner, P.; Nesbitt, S.; Mitchell, N.S.; Townsend, R.; Falkner, B. Management of Stage 1 Hypertension in Adults With a Low 10-Year Risk for Cardiovascular Disease: Filling a Guidance Gap: A Scientific Statement From the American Heart Association. Hypertension 2021, 77, e58–e67. [Google Scholar] [CrossRef]
- Boudreaux, M.Y.; Talbott, E.O.; Kip, K.E.; Brooks, M.M.; Witchel, S.F. Risk of T2DM and impaired fasting glucose among PCOS subjects: Results of an 8-year follow-up. Curr. Diab. Rep. 2006, 6, 77–83. [Google Scholar] [CrossRef]
- Umpierre, D.; Ribeiro, P.A.; Kramer, C.K.; Leitão, C.B.; Zucatti, A.T.; Azevedo, M.J.; Gross, J.L.; Ribeiro, J.P.; Schaan, B.D. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: A systematic review and meta-analysis. JAMA 2011, 305, 1790–1799. [Google Scholar] [CrossRef] [Green Version]
- Schofield, J.D.; Liu, Y.; Rao-Balakrishna, P.; Malik, R.A.; Soran, H. Diabetes Dyslipidemia. Diabetes Ther. 2016, 7, 203–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Qin, H.; Qiu, S.; Chen, G.; Chen, Y. Correlation of triglyceride to high-density lipoprotein cholesterol ratio with nonalcoholic fatty liver disease among the non-obese Chinese population with normal blood lipid levels: A retrospective cohort research. Lipids Health Dis. 2019, 18, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.J.; Choi, Y.M. Dyslipidemia in women with polycystic ovary syndrome. Obstet. Gynecol. Sci. 2013, 56, 137–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mika, A.; Macaluso, F.; Barone, R.; Di Felice, V.; Sledzinski, T. Effect of Exercise on Fatty Acid Metabolism and Adipokine Secretion in Adipose Tissue. Front. Physiol. 2019, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Benham, J.L.; Yamamoto, J.M.; Friedenreich, C.M.; Rabi, D.M.; Sigal, R.J. Role of exercise training in polycystic ovary syndrome: A systematic review and meta-analysis. Clin. Obes. 2018, 8, 275–284. [Google Scholar] [CrossRef]
- Dos Santos, I.K.; Ashe, M.C.; Cobucci, R.N.; Soares, G.M.; De Oliveira Maranhão, T.M.; Dantas, P.M.S. The effect of exercise as an intervention for women with polycystic ovary syndrome: A systematic review and meta-analysis. Medicine (Baltim.) 2020, 99, e19644. [Google Scholar] [CrossRef]
- Sabag, A.; Keating, S.E.; Way, K.L.; Sultana, R.N.; Lanting, S.M.; Twigg, S.M.; Johnson, N.A. The association between cardiorespiratory fitness, liver fat and insulin resistance in adults with or without type 2 diabetes: A cross-sectional analysis. BMC Sports Sci. Med. Rehabil. 2021, 13, 40. [Google Scholar] [CrossRef]
- Fennell, C.; Peroutky, K.; Glickman, E. Effects of supervised training compared to unsupervised training on physical activity, muscular endurance, and cardiovascular parameters. MOJ Orthop. Rheumatol. 2016, 5, 00184. [Google Scholar] [CrossRef] [Green Version]
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef]

| Study | Groups | Subjects | Age (Years) Mean ± SD | BMI (kg/m2) Mean ± SD | Diagnostic Criteria Used | Other Characteristics |
|---|---|---|---|---|---|---|
| Almenning et al., 2015 [25] | HIIT | 8 | NR | 26.1 ± 6.5 | Rotterdam 2003 or confirmation via general practitioner | Inactive adults |
| RT | 8 | NR | 27.4 ± 6.9 | |||
| CON | 9 | NR | 26.5 ± 5.0 | |||
| Benham et al., 2021 [26] | HIIT | 12 | 29.1 ± 3.1 | 31.4 ± 8.6 | Rotterdam 2003 | Inactive adults |
| MICT | 12 | 29.5 ± 4.6 | 31.3 ± 9.0 | |||
| CON | 15 | 29.1 ± 5.4 | 31.6 ± 8.2 | |||
| Brown et al., 2009 [22] | MICT | 8 | NR | NR | ≤8 menses per year and clinical or biochemical evidence of hyperandrogenism | Inactive, pre-menopausal adults aged 18–50 |
| CON | 12 | NR | NR | |||
| Bruner et al., 2006 [27] | MICT + RT | 7 | 32.3 ± 2.6 | 36.2 ± 5.3 | Rotterdam 2003 | Inactive adults with moderate and central obesity |
| CON | 5 | 28.4 ± 6 | 37.1 ± 7.6 | |||
| Costa et al., 2018 [28] | MICT | 14 | 27.6 ± 4.5 | 32 ± 4.2 | Rotterdam 2003 | Inactive adults aged 18–34 with a BMI of 28–39.9 kg/m2 |
| CON | 13 | 24.4 ± 5.0 | 33.6 ± 5.1 | |||
| Jedel et al., 2011 [20] | MICT | 30 | 30.2 ± 4.7 | 27.7 ± 6.44 | Ultrasound-verified polycystic ovaries, together with either oligo/amenorrhea and/or clinical signs of hyperandrogenism | Adults aged 18–37 with no pharmacological treatment 12 weeks before intervention |
| CON | 15 | 30.1 ± 4.2 | 26.8 ± 5.56 | |||
| Konopka et al., 2015 [31] | MICT | 12 | 35 ± 5 | 33 ± 5 | Rotterdam 2003 | Inactive adults with insulin resistance and a BMI of 28–40 kg/m2 |
| CON | 13 | |||||
| Lionett et al., 2021 [33] | LV-HIIT | 13 | 30 ± 7 | 29.8 ± 6.5 | Rotterdam 2003 | Adults aged 18–45, undertaking <2 weekly moderate-to-vigorous intensity endurance exercise sessions |
| HIIT | 14 | |||||
| CON | 15 | |||||
| Nybacka et al., 2011 [34] | MICT | 12 | 31.1 ± 4.7 | 38.8 ± 7.9 | Rotterdam 2003 | Adults between 18 to 40 with a BMI > 27 kg/m2 |
| CON | 14 | 29.3 ± 5.9 | 34.7 ± 5.0 | |||
| Ribeiro et al., 2020 [29] | HIIT | 29 | 29.0 ± 4.3 | 28.7 ± 4.8 | Rotterdam 2003 | Inactive adultsaged 18–39 |
| MICT | 28 | 29.1 ± 5.3 | 28.4 ± 5.6 | |||
| CON | 30 | 28.5 ± 5.8 | 29.1 ± 5.2 | |||
| Roessler et al., 2013 [35] | HIIT | 8 | 31.0 ± 8.5 | 32.3 ± 7.4 | Rotterdam 2003 | Adults with a BMI of 25–40 kg/m2 |
| CON | 9 | 36.7 ± 8.4 | 36.0 ± 6.9 | |||
| Samadi et al., 2019 [32] | HIIT | 15 | 29.25 ± 2.80 | 32.8 ± 4.49 | Rotterdam 2003 | Adults aged 20–35 with insulin resistance and a BMI ≥ 30kg/m2 |
| CON | 15 | 26.0 ± 4.38 | 34.06 ± 4.45 | |||
| Stener-Victorin et al. 2009 [36] | MICT | 5 | 30.4 ± 5.5 | 26.8 ± 4.8 | Rotterdam 2003 | Adults aged 18–37 |
| CON | 6 | 31.0 ± 3.2 | 28.0 ± 6.2 | |||
| Stener-Victorin et al. 2012 [21] | MICT | 30 | NR | NR | Rotterdam 2003 | Adults aged 18–37 |
| CON | 15 | NR | NR | |||
| Thomson et al., 2008 [30] | MICT | 18 | 29.3 ± 6.8 | 36.1 ± 4.8 | Rotterdam 2003 | Inactive adults aged 18–41 with a BMI of 25–55 kg/m2 |
| MICT + RT | 20 | |||||
| CON | 14 | |||||
| Turan et al., 2015 [23] | MICT + RT | 14 | 24.45 ± 10.8 | 21.8 ± 3.7 | Rotterdam 2003 | Inactive adults aged 17–34 with BMI < 25 kg/m2 |
| CON | 16 | 21.9 ± 4.4 | ||||
| Vigorito et al., 2007 [37] | MICT | 45 | 21.7 ± 2.3 | 29.3 ± 2.9 | Rotterdam 2003 | Adults with overweight or obesity |
| CON | 45 | 21.9 ± 1.9 | 29.4 ± 3.5 | |||
| Vizza et al., 2016 [24] | RT | 7 | 26 ± 7 | 41.3 ± 12.5 | None used. Diagnosis confirmed via the participant’s physician. | Adults aged 18–42 not participating in RT at time of recruitment |
| CON | 6 | 29 ± 3 | 34.0 ± 9.4 | |||
| Wu et al., 2021 [38] | MICT | 19 | 32.7 ± 3.2 | 23.8 ± 3 | Rotterdam 2003 | Adults aged 18–40, undertaking physical exercise <3 times per week |
| CON | 19 | 33.2 ± 2.9 | 24.1 ± 3.2 |
| Study | Groups | Mode | Frequency (Days) | Intensity | Session Duration (Minutes) | Intervention Duration (Weeks) | Additional Intervention |
|---|---|---|---|---|---|---|---|
| Almenning et al., 2015 [25] | HIIT | Treadmill or outdoor walking/running and/or cycling (self-selected) | 2/7 | WU: 10 min at 70% HRmax HIIT: 4 × 4 min at 90–95% HRmax and 3 min at 70% HRmax CD: 5 min at 70% HRmax | 38 | 10 | Participants in all groups advised to maintain usual diets |
| RT | 8 dynamic strength drills | 3/7 | 75% 1RM for 3 sets of 10 repetitions, with 1 min rest between sets | NR | |||
| CON | Advised to adhere to ≥150 min of weekly moderate-intensity exercise without any follow-up during the ten-week intervention period | ||||||
| Benham et al., 2021 [26] | HIIT | Aerobic exercise equipment of choice (e.g., treadmill, cycle ergometer, etc.) | 3/7 | WU: 5 min HIIT: 10 × 30 s at 90% HRR and 90 s of low-intensity aerobic exercise CD: 5 min | 30 | 26 | |
| MICT | Aerobic exercise equipment of choice (e.g., treadmill, cycle ergometer, etc.) | 3/7 | WU: 5 min MICT: 40 min at 50–60% HRR CD: 5 min | 50 | |||
| CON | Participants in CON instructed to maintain usual level of physical activity | ||||||
| Brown et al., 2009 [22] | MICT | Aerobic exercise equipment of choice (e.g., treadmill, cycle ergometer, etc.) | Dependent on bodyweight and VO2peak | 14 kcal/kg/week at 50% VO2peak | Dependent on bodyweight and VO2peak, capped at 60 min every 24 h | 12 | Participants in both groups advised to maintain usual diets |
| CON | No intervention | ||||||
| Bruner et al., 2006 [27] | MICT + RT | Treadmill walking or stationary cycling | 3/7 | WU: 10 min MICT: 30 min at 70–85% HRmax CD: 10 min | 40 | 12 | Participants in both groups were encouraged to attend 1 h weekly seminars regarding long-term nutritional strategies |
| Biceps curl, lat pulldown, leg curl, leg extension, shoulder press, chest press, leg press, hip abduction, hip adduction, hip flexion, hip extension, back extension | 2 → 3 sets of 10 → 15 repetitions, with weight increasing by 5% or 2.2 kg. Encouraged to participate in physical activity (i.e., walking) on non-supervised days, and given an activity log to document this | 90 | |||||
| CON | No exercise intervention | ||||||
| Costa et al., 2018 [28] | MICT | Walking and/or jogging | 3/7 | WU: 5 min 40 min at: Weeks 1–4: 60–70% HRmax Weeks 5–8: 70–75% HRmax Weeks 9–12: 75–80% HRmax Weeks 13–16: 80–85% HRmax CD: 5 min | 50 | 16 | Participants in both groups advised to maintain usual diets |
| CON | No intervention | ||||||
| Jedel et al., 2011 [20] | MICT | Self-selected aerobic exercise, e.g., brisk walking, cycling | ≥ 3/7 | Self-selected pace faster than normal walking with HR of >120 bpm | 30–45 | 16 | Participants in both groups were given information regarding the importance of physical activity and healthy diet |
| CON | No exercise intervention | ||||||
| Konopka et al., 2015 [31] | MICT | Stationary cycling | 5/7 | 60 min at 65% VO2peak | 60 | 12 | Participants were provided a standardised diet (50% carbohydrate, 30% fat, and 20% protein) three days prior to and for the duration of the study |
| CON | No intervention | ||||||
| Lionett et al., 2021 [33] | LV-HIIT | Treadmill or outdoor walking/running | 3/7 | WU: 10 min HIIT: 10 × 1 min at a maximally sustainable intensity, interspersed with 1 min of passive recovery or low-intensity walking CD: 3 min | 32 | 16 | |
| HIIT | Treadmill or outdoor walking/running | 3/7 | WU: 10 min HIIT: 4 × 4 min at 90–95% HRmax, separated by 3 min of active recovery at ∼70% of HRmax CD: 3 min | 38 | |||
| CON | Participants in CON instructed to maintain usual level of physical activity, and informed about current recommendations for physical activity in adults | ||||||
| Nybacka et al., 2011 [34] | Varied | Designed to enhance both the type and the level of physical activity to a level conforming to each individual patient’s capacity, goals, and interest at the beginning of the intervention | NR | NR | NR | 17 | Participants in both groups were asked to reduce daily energy intake by −600 kcal and maintain practices in accordance with Swedish nutritional recommendations |
| CON | |||||||
| Ribeiro et al., 2020 [29] | HIIT | Treadmill | 3/7 | WU: 5 min at 50–60% HRmax HIIT: 6 → 10 bouts of 2 min at 70–90% HRmax then 3 min at 60–70% HRmax, with HR target increasing every 2–4 weeks CD: 5 min at 50–60% HRmax | Weeks 1–3: 30 Weeks 4–6: 35 Weeks 7–10: 40 Weeks 11–13: 45 Weeks 14–16: 50 | 16 | Participants in all groups advised to maintain usual diets |
| MICT | Treadmill | 3/7 | WU: 5 min at 50–60% HRmax MICT: 65–80% HRmax, gradually increasing every 2–4 weeks CD: 5 min at 50–60% HRmax | ||||
| CON | Advised to maintain daily physical activity profile | ||||||
| Roessler et al., 2013 [35] | HIIT | Cycling and walking/running | 3/7 | WU: 15 min at 70–75% HRmax. HIIT: repeated intervals of 0.5–5 min at 80–100% HRmax interspersed with 0.5 to 3 min rest at 45–65% HRmax. CD: 5 min. | 45 | 8 | |
| CON | Physical activity counselling | 1/7 | |||||
| Samadi et al., 2019 [32] | HIIT | Aquatic | 3/7 | WU: 5 min jogging and stretching HIIT: 4 × 4 min bouts of 8 × 20 s at maximal intensity followed by 10 s of rest at 80–95% HRmax. 1 min of jogging at 75% HRmax was performed between each 4 min bout. CD: 5 min stretching | 30 | 12 | Participants in both groups took 3 pills of metformin (1500 mg) daily from the beginning of the intervention, and were advised to maintain usual diets |
| CON | No regular exercises were performed | ||||||
| Stener-Victorin et al., 2009 [36] | MICT | Self-selected aerobic exercise, e.g., brisk walking, cycling | ≥3/7 | Self-selected pace faster than normal walking with HR of >120 bpm | 30–45 | 16 | Participants in both groups were given information regarding the importance of physical activity and healthy diet |
| CON | No exercise intervention | ||||||
| Stener-Victorin et al., 2012 [21] | MICT | Self-selected aerobic exercise, e.g., brisk walking, cycling | ≥3/7 | Self-selected pace faster than normal walking with HR of >120 bpm | 30–45 | 16 | Participants in both groups were given information regarding the importance of physical activity and healthy diet |
| CON | No exercise intervention | ||||||
| Thomson et al., 2008 [30] | MICT | Walking/jogging | 5/7 | 60–65% HRmax progressing to 75–80% HRmax over 20 weeks | 25–30 progressing to 45 over 20 weeks | 20 | Participants were prescribed a diet of 5000–6000 kJ/d, with 30% protein, 40% carbohydrate, and 30% fat (<8% saturated fat) |
| MICT + RT | Walking/jogging | 3/7 | 60–65% HRmax progressing to 75–80% HRmax over 20 weeks | 25–30 progressing to 45 over 20 weeks | |||
| Bench press, lat pulldown, leg press, knee extension, and sit-ups | 2/7 on non-consecutive days | Weeks 1–2: 3 x 12 repetitions at 50–60% 1RM Weeks 3–20: 3 x 12 repetitions at 65–75% 1RM | 3 x 12 repetitions of each exercise | ||||
| CON | Dietary intervention only | ||||||
| Turan et al., 2015 [23] | MICT + RT | Stepping | 3/7 | WU: 5 min walking on a treadmill at a low pace + static stretching MICT: 5–7 min → 20 min of stepping on a 10 cm-20 cm step at 10–15/20 RPE or 65–70% HRmax. CD: 5 min walking on a treadmill at a low pace | 50–60 | 8 | Participants in both groups were given general dietary and behavioural advice, and prescribed a diet of 50% carbohydrates, 25% protein, and 25% fat |
| Resistance band exercises targeting the back, trunk, and lower-body muscles | 3/7 | 1 × 15 repetitions at 5–6/10 RPE with 30–60 s of rest between each exercise. | |||||
| CON | Dietary intervention only | ||||||
| Vigorito et al., 2007 [37] | MICT | Stationary cycling | 3/7 | WU: 5 min MICT: 30 min at 60–70% VO2max CD: 5 min | 40 | 12 | Participants in both groups were counselled to achieve a healthy balanced meal plan with a nutritional composition in which 50% of the calories were from carbohydrate, 25% from protein, and 25% from fat |
| CON | No intervention | ||||||
| Vizza et al., 2016 [24] | RT | Lat pulldown, leg curl, seated row, leg press, calf raise, chest press, split squat, shoulder press, biceps curl, triceps extension and abdominal curl | 2/7 non-consecutively | WU: 5 min on bicycle ergometer or treadmill RT: Performed to neuromuscular fatigue i.e., 8–12 RM; absolute loads increased with strength gains CD: 5 min on bicycle ergometer or treadmill | Weeks 1–2: 2 sets of each exercise Weeks 3–12: 3 sets of each exercise except spilt squats and shoulder press | 12 | |
| Home-based calisthenics: hip rotations, side leg raises, push-ups on knees, wall squats, oblique curls, core stabilisation exercises | 2/7 on days without supervised RT | NR | 3 × 10 repetitions of each exercise | ||||
| CON | Advised to continue current lifestyle | ||||||
| Wu et al., 2021 [38] | MICT | Stationary cycling | 4/7 | WU: 15 min MICT: 30 min at VO2AT CD: 15 min | 60 | 12 | Participants in both groups advised to maintain usual diets |
| CON | |||||||
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | /29 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Almenning et al., 2015 [25] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 22 |
| Benham et al., 2021 [26] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 25 |
| Brown et al., 2009 [22] | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 17 |
| Bruner et al., 2006 [27] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 16 |
| Costa et al., 2018 [28] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 23 |
| Jedel et al., 2010 [20] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 20 |
| Konopka et al., 2015 [31] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 18 |
| Lionett et al., 2021 [33] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 22 |
| Nybacka et al., 2011 [34] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 20 |
| Ribeiro et al., 2020 [29] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 21 |
| Roessler et al., 2013 [35] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 22 |
| Samadi et al., 2019 [32] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 16 |
| Stener-Victorin et al., 2009 [36] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 22 |
| Stener-Victorin et al., 2012 [21] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 23 |
| Thomson et al., 2008 [30] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 22 |
| Turan et al., 2015 [23] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 21 |
| Vigorito et al., 2007 [37] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 21 |
| Vizza et al., 2016 [24] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 23 |
| Wu et al., 2021 [38] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 21 |
| Exercise compared to non-exercise control for women with PCOS | |||||
| Patient or population: women with PCOS Setting: Intervention: exercise Comparison: non-exercise control | |||||
| Outcomes | Anticipated absolute effects * (95% CI) | No. of participants (studies) | Certainty of evidence (GRADE) | Comments | |
| Score with control | Score with exercise | ||||
| Cardiorespiratory fitness (reported in ml/kg/min) | Mean VO2max = 29.50 mL/kg/min | MD 4.00 mL/kg/min higher (2.61 higher to 5.40 higher) | 343 (9) | ⊕⊕◯◯ a Low | Exercise may increase cardiorespiratory fitness in women with PCOS. |
| Waist circumference (reported in cm) | Mean waist circumference = 95.93 cm | MD 1.48 cm lower (2.35 lower to 0.62 lower) | 462 (12) | ⊕⊕◯◯ b Low | Exercise may elicit modest reductions in waist circumference in women with PCOS. |
| Systolic blood pressure (reported in mmHg) | Mean blood pressure = 116.24 mmHg | MD 1.88 mmHg lower (5.09 lower to 1.34 higher) | 282 (6) | ⊕◯◯◯ c,d Very low | It is unlikely that exercise elicits meaningful changes in systolic blood pressure in women with PCOS (and normal blood pressure) but we are very uncertain. |
| HOMA-IR | Mean HOMA-IR index = 2.69 | MD 0.17 lower (0.44 lower to 0.09 higher) | 337 (10) | ⊕⊕◯◯ e Low | It is unlikely that exercise elicits meaningful changes in HOMA-IR in women with PCOS. |
| Fasting blood glucose (reported in mmol/L) | Mean fasting blood glucose = 4.93 mmol/L | MD 0.08 mmol/L higher (0.03 lower to 0.18 higher) | 424 (11) | ⊕⊕◯◯ f Low | It is unlikely that exercise elicits meaningful changes in fasting blood glucose in women with PCOS (and normal blood glucose). |
| Triglycerides (reported in mmol/L) | Mean blood triglycerides = 1.24 mmol/L | MD 0.03 mmol/L lower (0.07 lower to 0.01 higher) | 360 (8) | ⊕⊕◯◯ g Low | It is unlikely that exercise elicits meaningful changes in blood triglycerides in women with PCOS (and normal blood triglyceride levels). |
| HDL-C (reported in mmol/L) | Mean HDL-C = 1.30 mmol/L | MD 0.02 mmol/L higher (0.02 lower to 0.06 higher) | 360 (8) | ⊕⊕◯◯ g Low | It is unlikely that exercise elicits meaningful changes in HDL-C in women with PCOS (and normal HDL-C). |
| * The score in the intervention group (and its 95% CI) is based on the assumed score in the comparison group. PCOS: Polycystic Ovary Syndrome, CI: confidence interval, GRADE: Grading of Recommendations, Assessment, Development, and Evaluation, VO2max: maximal oxygen uptake, MD: mean difference, HOMA-IR: homeostatic model assessment of insulin resistance, HDL-C: high-density lipoprotein cholesterol. | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
| Explanations a Downgraded two levels for serious risk of bias: 7 of 9 included studies had an unclear or high risk of bias for blinding of outcome assessment, and 5 of 9 included studies had an unclear or high risk of bias for allocation concealment and incomplete outcome data, respectively. b Downgraded three levels for serious risk of bias: 10 of 12 included studies had an unclear or high risk of bias for blinding of outcome assessment, 6 of 12 included studies had an unclear or high risk of bias for allocation concealment, 7 of 12 studies had an unclear or high risk of bias for selective reporting, and 6 of 12 included studies did not report intervention adherence. c Downgraded two levels for serious risk of bias: 4 of 6 included studies had an unclear or high risk of bias for blinding of outcome assessment, 4 of 6 included studies had an unclear or high risk of bias for allocation concealment, and 4 of 6 included studies had an unclear or high risk of bias for selective reporting. d Downgraded one level for serious imprecision: small sample size. e Downgraded two levels for serious risk of bias: 8 of 10 included studies had an unclear or high risk of bias for blinding of outcome assessment, 6 of 10 included studies had an unclear or high risk of bias for incomplete outcome data, and 5 of 10 studies did not report intervention adherence. f Downgraded two levels for serious risk of bias: 8 of 11 included studies had an unclear or high risk of bias for blinding of outcome assessment, 6 of 11 included studies had an unclear or high risk of bias for allocation concealment and selective reporting, respectively. g Downgraded two levels for serious risk of bias: 5 of 8 included studies had an unclear or high risk of bias for blinding of outcome assessment, 4 of 8 included studies had an unclear or high risk of bias for allocation concealment and selective reporting, respectively. | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breyley-Smith, A.; Mousa, A.; Teede, H.J.; Johnson, N.A.; Sabag, A. The Effect of Exercise on Cardiometabolic Risk Factors in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 1386. https://doi.org/10.3390/ijerph19031386
Breyley-Smith A, Mousa A, Teede HJ, Johnson NA, Sabag A. The Effect of Exercise on Cardiometabolic Risk Factors in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(3):1386. https://doi.org/10.3390/ijerph19031386
Chicago/Turabian StyleBreyley-Smith, Annabelle, Aya Mousa, Helena J. Teede, Nathan A. Johnson, and Angelo Sabag. 2022. "The Effect of Exercise on Cardiometabolic Risk Factors in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 3: 1386. https://doi.org/10.3390/ijerph19031386
APA StyleBreyley-Smith, A., Mousa, A., Teede, H. J., Johnson, N. A., & Sabag, A. (2022). The Effect of Exercise on Cardiometabolic Risk Factors in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(3), 1386. https://doi.org/10.3390/ijerph19031386







