Review of Novel Potential Insulin Resistance Biomarkers in PCOS Patients—The Debate Is Still Open
Abstract
1. Introduction
2. Materials and Methods
3. Nesfatin-1
4. Preptin
5. Myonectin
6. Omentin-1
7. Gremlin
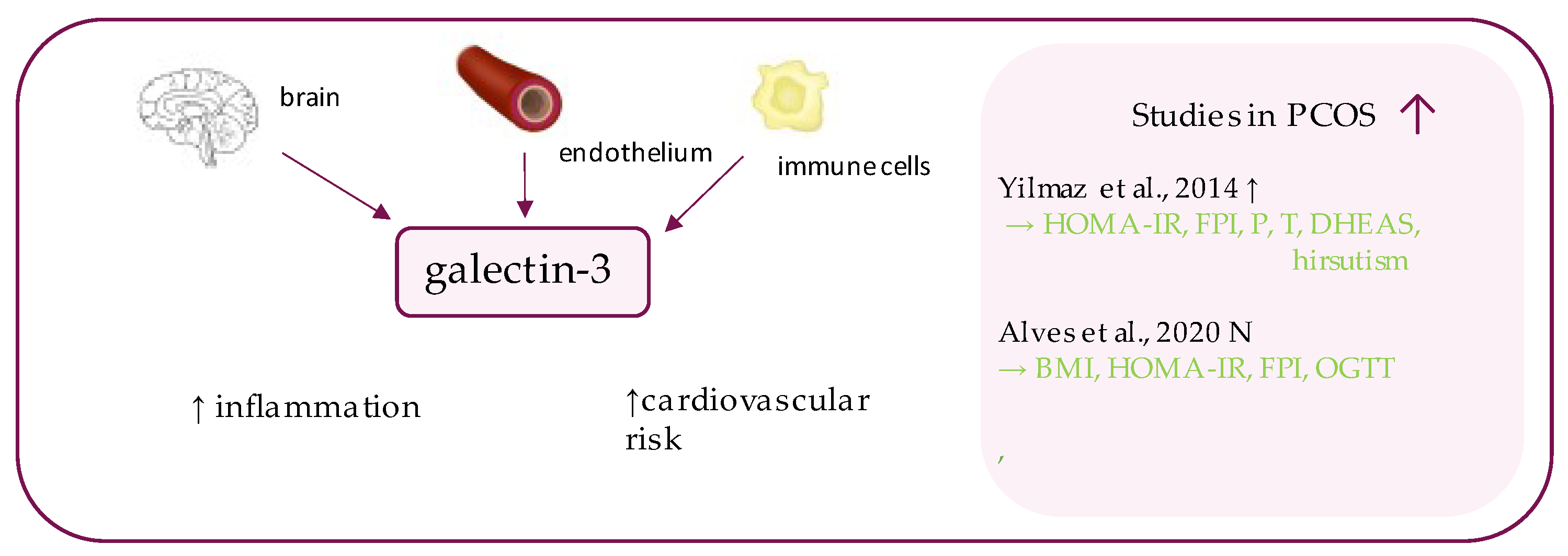
8. Galectin-3
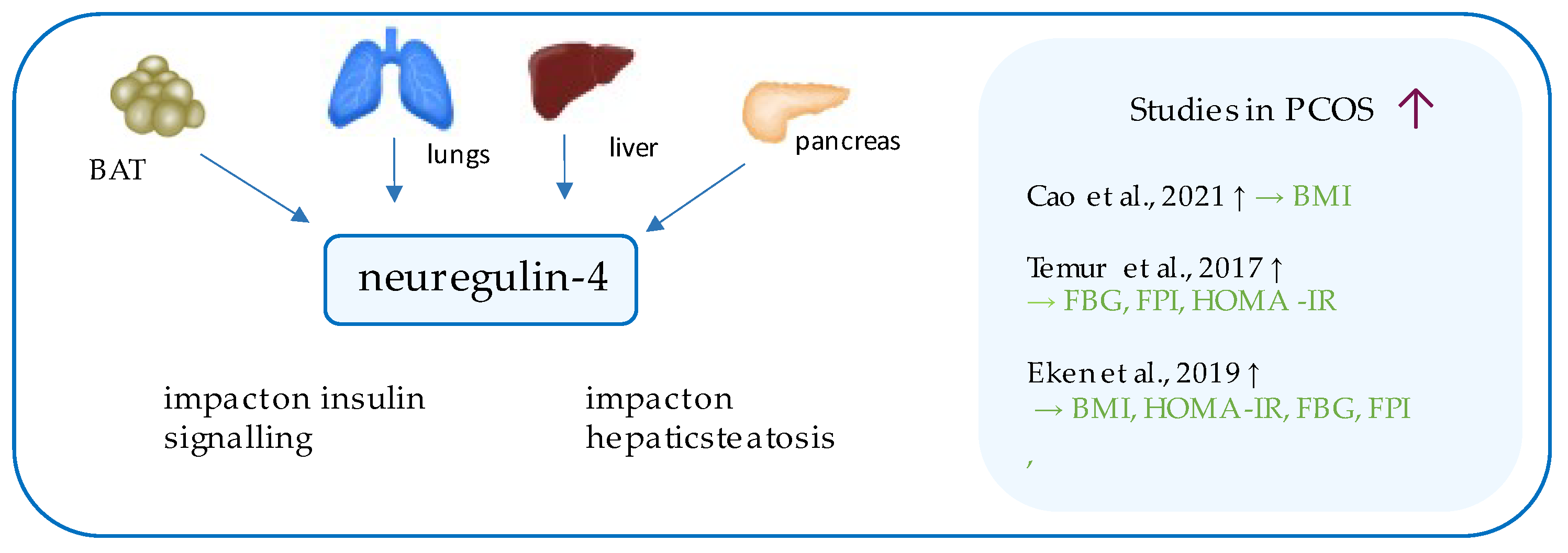
9. Neuregulin-4
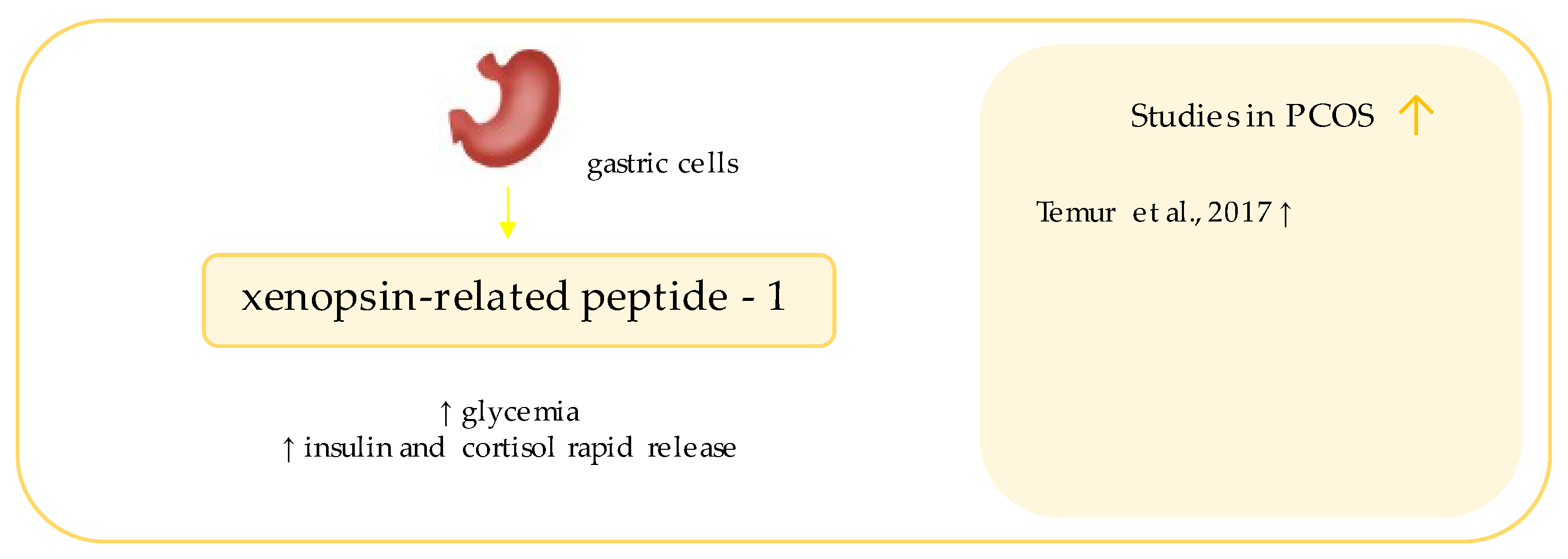
10. Xenopsin-Related Peptide
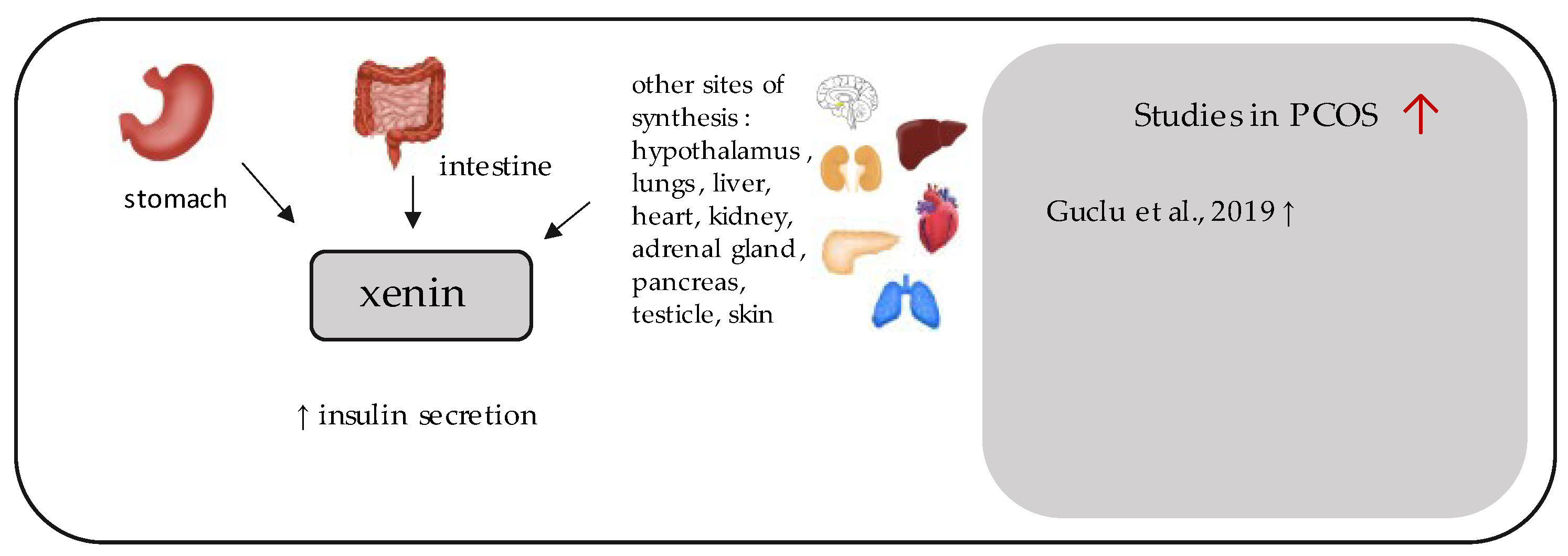
11. Xenin
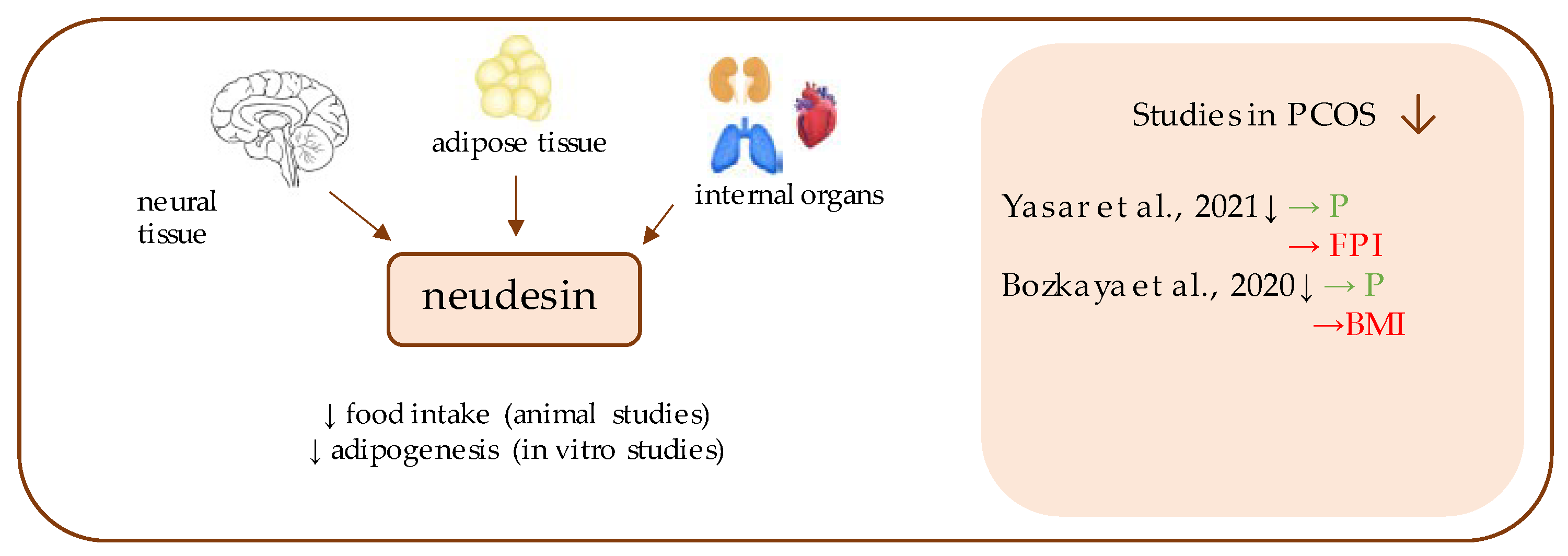
12. Neudesin
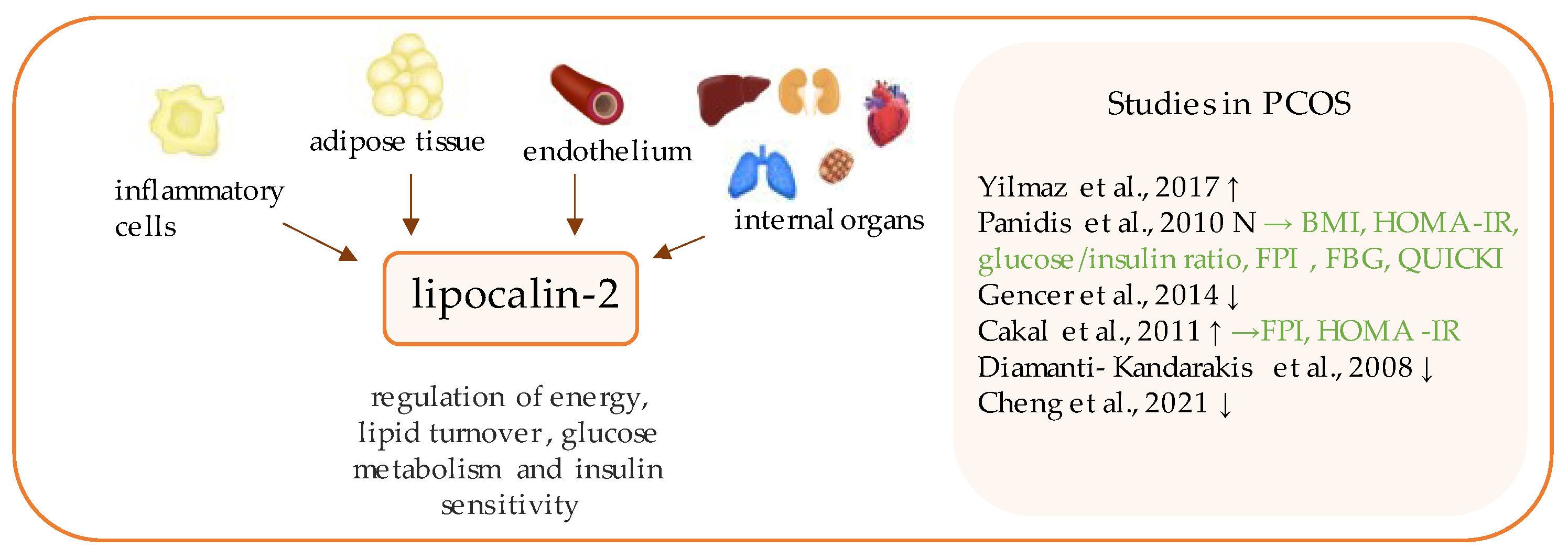
13. Lipocalin-2
14. Discussion
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mierzwicka, A.; Bolanowski, M. New peptides players in metabolic disorders. Postępy Higieny i Medycyny Doświadczalnej 2016, 70, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Czerwińska, M.; Czarzasta, K.; Cudnoch-Jędrzejewska, A. New Peptides as Potential Players in the Crosstalk Between the Brain and Obesity, Metabolic and Cardiovascular Diseases. Front. Physiol. 2021, 12, 692642. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S. Three new players in energy regulation: Preptin, adropin and irisin. Peptides 2014, 56, 94–110. [Google Scholar] [CrossRef] [PubMed]
- Polak, K.; Czyzyk, A.; Simoncini, T.; Meczekalski, B. New markers of insulin resistance in polycystic ovary syndrome. J. Endocrinol. Investig. 2017, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.; Lehrke, M.; Parhofer, K.G.; Broedl, U.C. Adipokines and Insulin Resistance. Mol. Med. 2008, 14, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Zorena, K.; Jachimowicz-Duda, O.; Ślęzak, D.; Robakowska, M.; Mrugacz, M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef]
- Landecho, M.F.; Tuero, C.; Valentí, V.; Bilbao, I.; De La Higuera, M.; Frühbeck, G. Relevance of Leptin and Other Adipokines in Obesity-Associated Cardiovascular Risk. Nutrients 2019, 11, 2664. [Google Scholar] [CrossRef]
- Chen, X.; Jia, X.; Qiao, J.; Guan, Y.; Kang, J. Adipokines in reproductive function: A link between obesity and polycystic ovary syndrome. J. Mol. Endocrinol. 2013, 50, R21–R37. [Google Scholar] [CrossRef][Green Version]
- Ozegowska, K.; Pawelczyk, L. The role of insulin and selected adipocytokines in patients with polycystic ovary syndrome (PCOS)—A literature review. Ginekol. Polska 2015, 86, 300–304. [Google Scholar] [CrossRef]
- De Leo, V.; Musacchio, M.C.; Cappelli, V.; Massaro, M.G.; Morgante, G.; Petraglia, F. Genetic, hormonal and metabolic aspects of PCOS: An update. Reprod. Biol. Endocrinol. 2016, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Sirmans, S.; Pate, K. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin. Epidemiol. 2013, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef] [PubMed]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Sharif, E. New markers for the detection of polycystic ovary syndrome. Obstet. Gynecol. Int. J. 2019, 10, 62–65. [Google Scholar] [CrossRef][Green Version]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Pang, Y. Metabolic Syndrome and PCOS: Pathogenesis and the Role of Metabolites. Metabolites 2021, 11, 869. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Ye, W.; Xie, T.; Song, Y.; Zhou, L. The role of androgen and its related signals in PCOS. J. Cell. Mol. Med. 2021, 25, 1825–1837. [Google Scholar] [CrossRef]
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol. Metab. 2020, 35, 100937. [Google Scholar] [CrossRef]
- Schiattarella, A.; Riemma, G.; La Verde, M.; Franci, G.; Chianese, A.; Fasulo, D.; Fichera, M.; Gallo, P.; De Franciscis, P. Polycystic Ovary Syndrome and Probiotics: A Natural Approach to an Inflammatory Disease. Curr. Womens Heal. Rev. 2021, 17, 14–20. [Google Scholar] [CrossRef]
- Parker, J.; O’Brien, C.; Hawrelak, J. A narrative review of the role of gastrointestinal dysbiosis in the pathogenesis of polycystic ovary syndrome. Obstet. Gynecol. Sci. 2022, 65, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Giampaolino, P.; Foreste, V.; Di Filippo, C.; Gallo, A.; Mercorio, A.; Serafino, P.; Improda, F.; Verrazzo, P.; Zara, G.; Buonfantino, C.; et al. Microbiome and PCOS: State-Of-Art and Future Aspects. Int. J. Mol. Sci. 2021, 22, 2048. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xu, P.; Wang, J.; Zhang, C. The role of MiRNA in polycystic ovary syndrome (PCOS). Gene 2019, 706, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Çaltekin, M.D.; Caniklioğlu, A.; Yalçın, S.E.; Kırmızı, D.A.; Baser, E.; Yalvaç, E.S. DLK1 and Nesfatin-1 levels and the relationship with metabolic parameters in polycystic ovary syndrome: Prospective, controlled study. J. Turk. Soc. Obstet. Gynecol. 2021, 18, 124–130. [Google Scholar] [CrossRef]
- Mierzwicka, A.; Kuliczkowska-Plaksej, J.; Kolačkov, K.; Bolanowski, M. Preptin in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2018, 34, 470–475. [Google Scholar] [CrossRef]
- Demir, I.; Guler, A. Association of decreased myonectin levels with metabolic and hormonal disturbance in polycystic ovary syndrome. Gynecol. Endocrinol. 2020, 36, 947–950. [Google Scholar] [CrossRef]
- Franik, G.; Sadlocha, M.; Madej, P.; Owczarek, A.; Skrzypulec-Plinta, V.; Plinta, R.; Chudek, J.; Olszanecka-Glinianowicz, M. Circulating omentin-1 levels and inflammation in polycystic ovary syndrome. Ginekol. Polska 2020, 91, 308–312. [Google Scholar] [CrossRef]
- Koroglu, N.; Mathyk, B.A.; Tola, E.N.; Cetin, B.A.; Yuksel, I.T.; Dag, I.; Yıldırım, G.Y. Gremlin-1 and gremlin-2 levels in polycystic ovary syndrome and their clinical correlations. Gynecol. Endocrinol. 2019, 35, 604–607. [Google Scholar] [CrossRef]
- Yilmaz, H.; Celik, H.T.; Özdemir, Ö.; Kalkan, D.; Namuslu, M.; Abusoglu, S.; Atalay, C.R.; Yigitoglu, R. Serum galectin-3 levels in women with PCOS. J. Endocrinol. Investig. 2014, 37, 181–187. [Google Scholar] [CrossRef]
- Cao, S.; Hu, Y. Effects of serum irisin, neuregulin 4, and weight management on obese adolescent girls with polycystic ovary syndrome. Biosci. Rep. 2021, 41. [Google Scholar] [CrossRef] [PubMed]
- Temur, M.; Özün Özbay, P.; Aksun, S.; Yilmaz, Ö.; Çift, T.; Üstünel, S.; Calan, M. Elevated circulating levels of xenopsin-related peptide-1 are associated with polycystic ovary syndrome. Arch. Gynecol. Obstet. 2017, 296, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Guclu, Y.A.; Sahin, E.; Aksit, M. The relationship between elevated serum xenin and insulin resistance in women with polycystic ovary syndrome: A case-control study. Gynecol. Endocrinol. 2019, 35, 960–964. [Google Scholar] [CrossRef]
- Yasar, H.Y.; Demirpence, M.; Colak, A.; Zeytinli, M.; Yasar, E.; Taylan, A. Serum neudesin levels in patients with polycystic ovary syndrome. Ginekol. Polska 2021. [Google Scholar] [CrossRef]
- Yilmaz, Ö.; Temur, M.; Calan, M.; Kume, T.; Özbay, P.Ö.; Karakulak, M.; Yapucu, S. Związek między stężeniami lipokaliny-2 i testosteronu wolnego w zespole policystycznych jajników. Endokrynol. Polska 2017, 68, 7–12. [Google Scholar] [CrossRef][Green Version]
- Cao, X.; Liu, X.M.; Zhou, L.H. Recent Progress in Research on the Distribution and Function of NUCB2/Nesfatin-1 in Peripheral Tissues. Endocr. J. 2013, 60. [Google Scholar] [CrossRef]
- Oh-I, S.; Shimizu, H.; Satoh, T.; Okada, S.; Adachi, S.; Inoue, K.; Eguchi, H.; Yamamoto, M.; Imaki, T.; Hashimoto, K.; et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 2006, 443, 709–712. [Google Scholar] [CrossRef]
- Ademoglu, E.N.; Gorar, S.; Carlioglu, A.; Yazıcı, H.; Dellal, F.D.; Berberoglu, Z.; Akdeniz, D.; Uysal, S.; Karakurt, F.; Carlıoglu, A. Plasma nesfatin-1 levels are increased in patients with polycystic ovary syndrome. J. Endocrinol. Investig. 2014, 37, 715–719. [Google Scholar] [CrossRef]
- Stengel, A.; Taché, Y. Regulation of Food Intake: The Gastric X/A-like Endocrine Cell in the Spotlight. Curr. Gastroenterol. Rep. 2009, 11, 448–454. [Google Scholar] [CrossRef]
- Stengel, A.; Goebel, M.; Yakubov, I.; Wang, L.; Witcher, D.; Coskun, T.; Taché, Y.; Sachs, G.; Lambrecht, N.W.G. Identification and Characterization of Nesfatin-1 Immunoreactivity in Endocrine Cell Types of the Rat Gastric Oxyntic Mucosa. Endocrinol. 2009, 150, 232–238. [Google Scholar] [CrossRef]
- Gonzalez, R.; Tiwari, A.; Unniappan, S. Pancreatic beta cells colocalize insulin and pronesfatin immunoreactivity in rodents. Biochem. Biophys. Res. Commun. 2009, 381, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, J.; Tang, Y.; Bi, F.; Liu, J.-N. The novel function of nesfatin-1: Anti-hyperglycemia. Biochem. Biophys. Res. Commun. 2010, 391, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Nakata, M.; Manaka, K.; Yamamoto, S.; Mori, M.; Yada, T. Nesfatin-1 enhances glucose-induced insulin secretion by promoting Ca2+ influx through L-type channels in mouse islet.BETA.-cells. Endocr. J. 2011, 58, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Galiano, D.G. Emerging Roles of NUCB2/Nesfatin-1 in the Metabolic Control of Reproduction. Curr. Pharm. Des. 2013, 19, 6966–6972. [Google Scholar] [CrossRef]
- Garcia-Galiano, D.; Navarro, V.M.; Roa, J.; Ruiz-Pino, F.; Sánchez-Garrido, M.A.; Pineda, R.; Castellano, J.M.; Romero, M.; Aguilar, E.; Gaytán, F.; et al. The Anorexigenic Neuropeptide, Nesfatin-1, Is Indispensable for Normal Puberty Onset in the Female Rat. J. Neurosci. 2010, 30, 7783–7792. [Google Scholar] [CrossRef]
- Zhang, N.; Zhuang, L.; Gai, S.; Shan, Y.; Wang, S.; Li, F.; Chen, L.; Zhao, D.; Liu, X. Beneficial phytoestrogenic effects of resveratrol on polycystic ovary syndromein rat model. Gynecol. Endocrinol. 2021, 37, 337–341. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, H.; Li, Q.; Lao, K.; Wang, Y. The role of nesfatin-1 expression in letrozole-induced polycystic ovaries in the rat. Gynecol. Endocrinol. 2017, 33, 438–441. [Google Scholar] [CrossRef]
- Ullah, K.; Rahman, T.U.; Wu, D.-D.; Lin, X.-H.; Liu, Y.; Guo, X.-Y.; Leung, P.; Zhang, R.-J.; Huang, H.-F.; Sheng, J.-Z. Phoenixin-14 concentrations are increased in association with luteinizing hormone and nesfatin-1 concentrations in women with polycystic ovary syndrome. Clin. Chim. Acta 2017, 471, 243–247. [Google Scholar] [CrossRef]
- Deniz, R.; Gurates, B.; Aydin, S.; Celik, H.; Sahin, I.; Baykus, Y.; Catak, Z.; Aksoy, A.; Citil, C.; Gungor, S. Nesfatin-1 and other hormone alterations in polycystic ovary syndrome. Endocr. 2012, 42, 694–699. [Google Scholar] [CrossRef]
- Catak, Z.; Yavuzkir, S.; Kocdemir, E.; Ugur, K.; Yardim, M.; Sahin, İ.; Agirbas, E.P.; Aydin, S. NUCB2/Nesfatin-1 in the Blood and Follicular Fluid in Patients with Polycystic Ovary Syndrome and Poor Ovarian Response. Journal of Reproduction and Infertility 2019, 20. [Google Scholar]
- Alp, E.; Görmüş, U.; Güdücü, N.; Bozkurt, S. Nesfatin-1 levels and metabolic markers in polycystic ovary syndrome. Gynecol. Endocrinol. 2015, 31, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Taskin, M.I.; Eser, B.; Adali, E.; Kara, H.; Cuce, C.; Hismiogulları, A.A. NUCB2 gene polymorphism and its relationship with nesfatin-1 levels in polycystic ovary syndrome. Gynecol. Endocrinol. 2016, 32, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Sahin, F.K.; Sahin, S.B.; Ural, U.M.; Cure, M.C.; Senturk, S.; Tekin, Y.B.; Balik, G.; Cüre, E.; Yuce, S.; Kirbas, A. Nesfatin-1 and Vitamin D levels may be associated with systolic and diastolic blood pressure values and hearth rate in polycystic ovary syndrome. Bosn. J. Basic Med Sci. 2015, 15, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, L.; Yang, M.; Liu, H.; Boden, G.; Yang, G. Increased Plasma Levels of Nesfatin-1 in Patients with Newly Diagnosed Type 2 Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2011, 120, 91–95. [Google Scholar] [CrossRef]
- Li, Q.-C.; Wang, H.-Y.; Chen, X.; Guan, H.-Z.; Jiang, Z.-Y. Fasting plasma levels of nesfatin-1 in patients with type 1 and type 2 diabetes mellitus and the nutrient-related fluctuation of nesfatin-1 level in normal humans. Regul. Pept. 2010, 159, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, C.M.; Phillips, A.R.; Cooper, G. Preptin derived from proinsulin-like growth factor II (proIGF-II) is secreted from pancreatic islet β-cells and enhances insulin secretion. Biochem. J. 2001, 360, 431–439. [Google Scholar] [CrossRef]
- Van Doorn, J. Insulin-like Growth Factor-II and Bioactive Proteins Containing a Part of the E-Domain of pro-Insulin-like Growth Factor-II. BioFactors 2020, 46, 563–578. [Google Scholar] [CrossRef]
- Celik, O.; Celik, N.; Hascalik, S.; Sahin, I.; Aydin, S.; Ozerol, E. An appraisal of serum preptin levels in PCOS. Fertil. Steril. 2011, 95, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.A.; Abbas, H.J.; Naser, N.A. Preptin and Adropin Levels as New Predictor in Women with Polycystic Ovary Syndrome. J. Pharm. Sci. Res. 2018, 10, 3005–3008. [Google Scholar]
- Celik, N.; Aydin, S.; Ugur, K.; Yardim, M.; Acet, M.; Yavuzkir, S.; Sahin, İ.; Celik, O. Patatin-like phospholipase domain containing 3-gene (adiponutrin), preptin, kisspeptin and amylin regulates oocyte developmental capacity in PCOS. Cell. Mol. Biol. 2018, 64, 7–12. [Google Scholar] [CrossRef]
- Şentürk, Ş.; Hatirnaz, S.; Kanat-Pektaş, M. Serum Preptin and Amylin Levels with Respect to Body Mass Index in Polycystic Ovary Syndrome Patients. Med. Sci. Monit. 2018, 24, 7517–7523. [Google Scholar] [CrossRef] [PubMed]
- Bu, Z.; Kuok, K.; Meng, J.; Wang, R.; Xu, B.; Zhang, H. The relationship between polycystic ovary syndrome, glucose tolerance status and serum preptin level. Reprod. Biol. Endocrinol. 2012, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, L.; Chen, W.; Liu, H.; Boden, G.; Li, K. Circulating preptin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Ann. Med. 2009, 41, 52–56. [Google Scholar] [CrossRef]
- Aslan, M.; Celik, O.; Karsavuran, N.; Celik, N.; Dogan, D.G.; Botan, E.; Kafkasli, A. Maternal serum and cord blood preptin levels in gestational diabetes mellitus. J. Perinatol. 2010, 31, 350–355. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seldin, M.M.; Peterson, J.M.; Byerly, M.S.; Wei, Z.; Wong, G.W. Myonectin (CTRP15), a Novel Myokine That Links Skeletal Muscle to Systemic Lipid Homeostasis. J. Biol. Chem. 2012, 287, 11968–11980. [Google Scholar] [CrossRef]
- Seldin, M.M.; Tan, S.Y.; Wong, G.W. Metabolic function of the CTRP family of hormones. Rev. Endocr. Metab. Disord. 2013, 15, 111–123. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar]
- Li, S.; Huang, X.; Zhong, H.; Peng, Q.; Chen, S.; Xie, Y.; Qin, X.; Qin, A. Low circulating adiponectin levels in women with polycystic ovary syndrome: An updated meta-analysis. Tumor Biol. 2014, 35, 3961–3973. [Google Scholar] [CrossRef]
- Toulis, K.; Goulis, D.; Farmakiotis, D.; Georgopoulos, N.; Katsikis, I.; Tarlatzis, B.; Papadimas, I.; Panidis, D. Adiponectin levels in women with polycystic ovary syndrome: A systematic review and a meta-analysis. Hum. Reprod. Updat. 2009, 15, 297–307. [Google Scholar] [CrossRef]
- Little, H.C.; Rodriguez, S.; Lei, X.; Tan, S.Y.; Stewart, A.N.; Sahagun, A.; Sarver, D.C.; Wong, G.W. Myonectin deletion promotes adipose fat storage and reduces liver steatosis. FASEB J. 2019, 33, 8666–8687. [Google Scholar] [CrossRef]
- Berezin, A.E.; Lichtenauer, M. Myokines and Heart Failure: Challenging Role in Adverse Cardiac Remodeling, Myopathy, and Clinical Outcomes. Dis. Markers 2021, 2021, 6644631. [Google Scholar] [CrossRef] [PubMed]
- Otaka, N.; Shibata, R.; Ohashi, K.; Uemura, Y.; Kambara, T.; Enomoto, T.; Ogawa, H.; Ito, M.; Kawanishi, H.; Maruyama, S.; et al. Myonectin Is an Exercise-Induced Myokine That Protects the Heart From Ischemia-Reperfusion Injury. Circ. Res. 2018, 123, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich-Nikitin, I.; Kirshenbaum, L.A. Exercise-Induced Myonectin Protects Against Ischemia-Reperfusion Injury. Circ. Res. 2018, 123, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, C.-L.; Xiang, R.-L.; Wu, L.-L.; Li, L. CTRP15 derived from cardiac myocytes attenuates TGFβ1-induced fibrotic response in cardiac fibroblasts. Cardiovasc. Drugs Ther. 2020, 34, 591–604. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Ye, J. Correlation analysis of myonectin levels with metabolic and hormonal disorders in patients with polycystic ovary syndrome. Ann. Palliat. Med. 2021, 10, 3404–3409. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.-L.; Zhu, Y.-J.; Li, C.-G.; Tang, Y.-Z.; Ni, C.-L.; Chen, L.-M.; Niu, W.-Y. Circulating Serum Myonectin Levels in Obesity and Type 2 Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2021, 129, 528–534. [Google Scholar] [CrossRef]
- Pourranjbar, M.; Arabnejad, N.; Naderipour, K.; Rafie, F. Effects of Aerobic Exercises on Serum Levels of Myonectin and Insulin Resistance in Obese and Overweight Women. J. Med. Life 2019, 11, 381–386. [Google Scholar] [CrossRef]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 2014, 46, 678–684. [Google Scholar] [CrossRef]
- Li, K.; Liao, X.; Wang, K.; Mi, Q.; Zhang, T.; Jia, Y.; Xu, X.; Luo, X.; Zhang, C.; Liu, H.; et al. Response to Letter to the Editor: "Myonectin Predicts the Development of Type 2 Diabetes". J. Clin. Endocrinol. Metab. 2018, 103, 4040–4041. [Google Scholar] [CrossRef]
- Lawen, A. Is erythroferrone finally the long sought-after systemic erythroid regulator of iron? World J. Biol. Chem. 2015, 6, 78–82. [Google Scholar] [CrossRef]
- Anithasri, A.; Ananthanarayanan, P.H.; Veena, P. A Study on Omentin-1 and Prostate Specific Antigen in Women on Treatment for Polycystic Ovary Syndrome. Indian J. Clin. Biochem. 2017, 34, 108–114. [Google Scholar] [CrossRef]
- Yang, R.-Z.; Lee, M.-J.; Hu, H.; Pray, J.; Wu, H.-B.; Hansen, B.C.; Shuldiner, A.R.; Fried, S.K.; McLenithan, J.C.; Gong, D.-W. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1253–E1261. [Google Scholar] [CrossRef]
- Fain, J.N.; Sacks, H.S.; Buehrer, B.; Bahouth, S.W.; Garrett, E.; Wolf, R.Y.; Carter, R.A.; Tichansky, D.S.; Madan, A.K. Identification of omentin mRNA in human epicardial adipose tissue: Comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int. J. Obes. 2008, 32, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Cloix, L.; Reverchon, M.; Cornuau, M.; Froment, P.; Ramé, C.; Costa, C.; Froment, G.; LeComte, P.; Chen, W.; Royère, D.; et al. Expression and Regulation of INTELECTIN1 in Human Granulosa-Lutein Cells: Role in IGF-1-Induced Steroidogenesis through NAMPT. Biol. Reprod. 2014, 91, 50. [Google Scholar] [CrossRef]
- De Souza Batista, C.M.; Yang, R.-Z.; Lee, M.-J.; Glynn, N.M.; Yu, D.-Z.; Pray, J.; Ndubuizu, K.; Patil, S.; Schwartz, A.; Kligman, M.; et al. Omentin Plasma Levels and Gene Expression Are Decreased in Obesity. Diabetes 2007, 56, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Adya, R.; Farhatullah, S.; Chen, J.; Lehnert, H.; Randeva, H.S. Metformin Treatment May Increase Omentin-1 Levels in Women With Polycystic Ovary Syndrome. Diabetes 2010, 59, 3023–3031. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Catalán, V.; Ortega, F.; Gómez-Ambrosi, J.; Ricart, W.; Frühbeck, G.; Fernández-Real, J.M. Circulating omentin concentration increases after weight loss. Nutr. Metab. 2010, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, H.; Kuramoto, J.; Kameshima, S.; Usui, T.; Okada, M.; Hara, Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem. Biophys. Res. Commun. 2011, 408, 339–343. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Chan, L.; Zhou, S.-W. Omentin: Linking metabolic syndrome and cardiovascular disease. Curr. Vasc. Pharmacol. 2014, 12, 136–143. [Google Scholar] [CrossRef]
- Kazama, K.; Usui, T.; Okada, M.; Hara, Y.; Yamawaki, H. Omentin plays an anti-inflammatory role through inhibition of TNF-α-induced superoxide production in vascular smooth muscle cells. Eur. J. Pharmacol. 2012, 686, 116–123. [Google Scholar] [CrossRef]
- Guzel, E.C.; Celik, C.; Abali, R.; Kucukyalcin, V.; Celik, E.; Guzel, M.; Yilmaz, M. Omentin and chemerin and their association with obesity in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2014, 30, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Mahde, A.; Shaker, M.; Al-Mashhadani, Z. Study of Omentin1 and Other Adipokines and Hormones in PCOS Patients. Oman Med. J. 2009, 24, 108–118. [Google Scholar] [CrossRef]
- Choi, J.-H.; Rhee, E.-J.; Kim, K.-H.; Woo, H.-Y.; Lee, W.-Y.; Sung, K.-C. Plasma omentin-1 levels are reduced in non-obese women with normal glucose tolerance and polycystic ovary syndrome. Eur. J. Endocrinol. 2011, 165, 789–796. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Orlik, B.; Madej, P.; Owczarek, A.; Skałba, P.; Chudek, J.; Olszanecka-Glinianowicz, M. Plasma omentin and adiponectin levels as markers of adipose tissue dysfunction in normal weight and obese women with polycystic ovary syndrome. Clin. Endocrinol. 2013, 81, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Guvenc, Y.; Var, A.; Goker, A.; Kuscu, N.K. Assessment of serum chemerin, vaspin and omentin-1 levels in patients with polycystic ovary syndrome. J. Int. Med Res. 2016, 44, 796–805. [Google Scholar] [CrossRef]
- Tan, B.K.; Adya, R.; Farhatullah, S.; Lewandowski, K.C.; O’hare, P.; Lehnert, H.; Randeva, H.S. Omentin-1, a Novel Adipokine, Is Decreased in Overweight Insulin-Resistant Women With Polycystic Ovary Syndrome. Diabetes 2008, 57, 801–808. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Ghasemi, S.; Kalantarhormozi, M.; Nabipour, I.; Abbasi, F.; Aminfar, A.; Jaffari, S.M.; Motamed, N.; Movahed, A.; Mirzaie, M.; et al. Relationship among plasma adipokines, insulin and androgens level as well as biochemical glycemic and lipidemic markers with incidence of PCOS in women with normal BMI. Gynecol. Endocrinol. 2012, 28, 521–524. [Google Scholar] [CrossRef]
- Güneş, M.; Bukan, N. Examination of angiopoietin-like protein 4, neuropeptide Y, omentin-1 levels of obese and non-obese patients with polycystic ovary syndrome. Gynecol. Endocrinol. 2015, 31. [Google Scholar] [CrossRef]
- Tang, Y.-L.; Yu, J.; Zeng, Z.-G.; Liu, Y.; Liu, J.-Y.; Xu, J.-X. Circulating omentin-1 levels in women with polycystic ovary syndrome: A meta-analysis. Gynecol. Endocrinol. 2016, 33, 244–249. [Google Scholar] [CrossRef]
- Bongrani, A.; Mellouk, N.; Rame, C.; Cornuau, M.; Guérif, F.; Froment, P.; Dupont, J. Ovarian Expression of Adipokines in Polycystic Ovary Syndrome: A Role for Chemerin, Omentin, and Apelin in Follicular Growth Arrest and Ovulatory Dysfunction? Int. J. Mol. Sci. 2019, 20, 3778. [Google Scholar] [CrossRef]
- Nolan, K.; Kattamuri, C.; Rankin, S.A.; Read, R.J.; Zorn, A.M.; Thompson, T.B. Structure of Gremlin-2 in Complex with GDF5 Gives Insight into DAN-Family-Mediated BMP Antagonism. Cell Rep. 2016, 16, 2077–2086. [Google Scholar] [CrossRef]
- Kisonaite, M.; Wang, X.; Hyvönen, M. Structure of Gremlin-1 and analysis of its interaction with BMP. Biochem. J. 2016, 473, 1593–1604. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone Morphogenetic Proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Hedjazifar, S.; Shahidi, R.K.; Hammarstedt, A.; Bonnet, L.; Church, C.; Boucher, J.; Blüher, M.; Smith, U. The Novel Adipokine Gremlin 1 Antagonizes Insulin Action and Is Increased in Type 2 Diabetes and NAFLD/NASH. Diabetes 2020, 69, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Hammarstedt, A.; Gogg, S.; Hedjazifar, S.; Nerstedt, A.; Smith, U. Impaired Adipogenesis and Dysfunctional Adipose Tissue in Human Hypertrophic Obesity. Physiol. Rev. 2018, 98, 1911–1941. [Google Scholar] [CrossRef]
- Qin, N.; Tyasi, T.L.; Sun, X.; Chen, X.; Zhu, H.; Zhao, J.; Xu, R. Determination of the roles of GREM1 gene in granulosa cell proliferation and steroidogenesis of hen ovarian prehierarchical follicles. Theriogenology 2020, 151, 28–40. [Google Scholar] [CrossRef]
- Sudo, S.; Avsian-Kretchmer, O.; Wang, L.S.; Hsueh, A.J.W. Protein Related to DAN and Cerberus Is a Bone Morphogenetic Protein Antagonist That Participates in Ovarian Paracrine Regulation. J. Biol. Chem. 2004, 279, 23134–23141. [Google Scholar] [CrossRef]
- Nilsson, E.E.; Larsen, G.; Skinner, M.K. Roles of Gremlin 1 and Gremlin 2 in regulating ovarian primordial to primary follicle transition. Reprod. 2014, 147, 865–874. [Google Scholar] [CrossRef]
- Glister, C.; Regan, S.L.; Samir, M.; Knight, P.G. Gremlin, noggin, chordin and follistatin differentially modulate BMP-induced suppression of androgen secretion by bovine ovarian theca cells. J. Mol. Endocrinol. 2019, 62, 15–25. [Google Scholar] [CrossRef]
- Dumont, A.; Robin, G.; Dewailly, D. Anti-müllerian hormone in the pathophysiology and diagnosis of polycystic ovarian syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 377–384. [Google Scholar] [CrossRef]
- Pellatt, L.; Hanna, L.; Brincat, M.; Galea, R.; Brain, H.; Whitehead, S.; Mason, H. Granulosa Cell Production of Anti-Müllerian Hormone Is Increased in Polycystic Ovaries. J. Clin. Endocrinol. Metab. 2007, 92, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Jindal, S.; Greenseid, K.; Berger, D.; Santoro, N.; Pal, L. Impaired Gremlin 1 (GREM1) Expression in Cumulus Cells in Young Women with Diminished Ovarian Reserve (DOR). J. Assist. Reprod. Genet. 2012, 29, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Hasegawa, A.; Tsubamoto, H.; Wakimoto, Y.; Kumamoto, K.; Shibahara, H. Effects of gremlin-2 on the transition of primordial follicles during early folliculogenesis in the human ovary. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 203, 72–77. [Google Scholar] [CrossRef]
- McKenzie, L.J.; Pangas, S.A.; Carson, S.A.; Kovanci, E.; Cisneros, P.; Buster, J.E.; Amato, P.; Matzuk, M.M. Human cumulus granulosa cell gene expression: A predictor of fertilization and embryo selection in women undergoing IVF. Hum. Reprod. 2004, 19, 2869–2874. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.-N.; Liang, X.-Y.; Fang, C.; Zhang, M.-F. Abnormal expression of growth differentiation factor 9 and bone morphogenetic protein 15 in stimulated oocytes during maturation from women with polycystic ovary syndrome. Fertil. Steril. 2011, 96, 464–468. [Google Scholar] [CrossRef]
- Ho, M.K.; Springer, T.A. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J. Immunol. 1982, 128, 1221–1228. [Google Scholar]
- Roff, C.F.; Wang, J.L. Endogenous lectins from cultured cells. Isolation and characterization of carbohydrate-binding proteins from 3T3 fibroblasts. J. Biol. Chem. 1983, 258, 10657–10663. [Google Scholar] [CrossRef]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef]
- Sullivan, A.L.; Grimley, P.M.; Metzger, H. Electron Microscopic Localization of Immunoglobulin E on the Surface Membrane of Human Basophils. J. Exp. Med. 1971, 134, 1403–1416. [Google Scholar] [CrossRef]
- Liu, F.T.; Patterson, R.J.; Wang, J.L. Intracellular Functions of Galectins. Biochim. Biophys. Acta-Gen. Sub. 2002, 1572, 263–273. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M.F.; Hou, S.; Li, P. Inflammation and Insulin Resistance: New Targets Encourage New Thinking: Galectin-3 and LTB4 Are pro-Inflammatory Molecules That Can Be Targeted to Restore Insulin Sensitivity. BioEssays 2017, 39, 1700036. [Google Scholar] [CrossRef]
- Siwicki, M.; Engblom, C.; Pittet, M.J. Gal3 Links Inflammation and Insulin Resistance. Cell Metab. 2016, 24, 655–656. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.; Cakmak, M.; Inan, O.; Darçın, T.; Akçay, A. Increased levels of galectin-3 were associated with prediabetes and diabetes: New risk factor? J. Endocrinol. Investig. 2015, 38, 527–533. [Google Scholar] [CrossRef]
- Li, J.; Mao, Y.-S.; Chen, F.; Xia, D.-X.; Zhao, T.-Q. Palmitic acid up regulates Gal-3 and induces insulin resistance in macrophages by mediating the balance between KLF4 and NF-κB. Exp. Ther. Med. 2021, 22, 1028. [Google Scholar] [CrossRef]
- Alves, M.T.; De Souza, I.D.P.; Ferreira, C.N.; Cândido, A.L.; Bizzi, M.F.; Oliveira, F.R.; Reis, F.M.; Gomes, K.B. Galectin-3 is a potential biomarker to insulin resistance and obesity in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2020, 36, 760–763. [Google Scholar] [CrossRef]
- Yavuz, I.H.; Ozaydin-Yavuz, G.; Çokluk, E.; Kurtoğlu, Z.; Bilgili, S.G. Investigation of galectin-3, lipocalin 2, retinol binding protein (RBP), small dense low-density lipoprotein (sdLDL) in patients with hirsutism. Adv. Dermatol. Allergol. 2019, 36, 177–183. [Google Scholar] [CrossRef]
- Ilhan, G.A.; Kanlioglu, C.; Arslan, G.; Yildizhan, B.; Pekin, T. Galectin-3 as a novel biomarker in women with PCOS. Arch. Gynecol. Obstet. 2018, 298, 821–825. [Google Scholar] [CrossRef]
- Gavaldà-Navarro, A.; Villarroya, J.; Cereijo, R.; Giralt, M.; Villarroya, F. The endocrine role of brown adipose tissue: An update on actors and actions. Rev. Endocr. Metab. Disord. 2021. [Google Scholar] [CrossRef]
- Wang, G.-X.; Zhao, X.-Y.; Meng, Z.-X.; Kern, M.; Dietrich, A.; Chen, Z.; Cozacov, Z.; Zhou, D.; Okunade, A.L.; Su, X.; et al. The brown fat–enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat. Med. 2014, 20, 1436–1443. [Google Scholar] [CrossRef]
- Gumà, A.; Díaz-Sáez, F.; Camps, M.; Zorzano, A. Neuregulin, an Effector on Mitochondria Metabolism That Preserves Insulin Sensitivity. Front. Physiol. 2020, 11, 696. [Google Scholar] [CrossRef]
- Harari, D.; Tzahar, E.; Romano, J.; Shelly, M.; Pierce, J.H.; Andrews, G.C.; Yarden, Y. Neuregulin-4: A novel growth factor that acts through the ErbB-4 receptor tyrosine kinase. Oncogene 1999, 18, 2681–2689. [Google Scholar] [CrossRef]
- Yang, F.; Li, X.-N. Research progress of neuregulin 4 biological function. Sheng Li Xue Bao Acta Physiol. Sin. 2017, 69, 351–356. [Google Scholar]
- Kim, J.-E.; Kim, J.-S.; Jo, M.-J.; Cho, E.; Ahn, S.-Y.; Kwon, Y.-J.; Ko, G.-J. The Roles and Associated Mechanisms of Adipokines in Development of Metabolic Syndrome. Molecules 2022, 27, 334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, B.; Wang, W.; Miao, S.; Zhou, S.; Cheng, X.; Zhu, J.; Liu, C. Alteration of serum neuregulin 4 and neuregulin 1 in gestational diabetes mellitus. Ther. Adv. Endocrinol. Metab. 2021, 12, 20420188211049614. [Google Scholar] [CrossRef] [PubMed]
- Temur, M.; Calan, M.; Akşit, M.; Yilmaz, Ö.; Çift, T.; Akselim, B.; Dinçgez Çakmak, B.; Üstünyurt, E. Increased serum neuregulin 4 levels in women with polycystic ovary syndrome: A case-control study. Ginekol. Polska 2017, 88, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Eken, M.K.; Ersoy, G.S.; Abide, C.Y.; Sanverdi, I.; Devranoglu, B.; Kutlu, T.; Cevik, O. Association between circulating neuregulin 4 levels and metabolic, aterogenic, and AMH profile of polycystic ovary syndrome. J. Obstet. Gynaecol. 2019, 39, 975–980. [Google Scholar] [CrossRef]
- Araki, K.; Tachibana, S.; Uchiyama, M.; Nakajima, T.; Yasuhara, T. Isolation and structure of a new active peptide xenopsin on rat stomach strip and some biogenic amines in the skin of Xenopus laevis. Chem. Pharm. Bull. 1975, 23, 3132–3140. [Google Scholar] [CrossRef]
- Zahid, O.K.; Mechkarska, M.; Ojo, O.O.; Abdel-Wahab, Y.H.; Flatt, P.R.; Meetani, M.A.; Conlon, J.M. Caerulein-and xenopsin-related peptides with insulin-releasing activities from skin secretions of the clawed frogs, Xenopus borealis and Xenopus amieti (Pipidae). Gen. Comp. Endocrinol. 2011, 172, 314–320. [Google Scholar] [CrossRef]
- Feurle, G.E.; Carraway, R.E.; Rix, E.; Knauf, W. Evidence for the presence of xenopsin-related peptide(s) in the gastric mucosa of mammals. J. Clin. Investig. 1985, 76, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.; Stöckmann, F.; Conlon, J.M. Xenopsin- and neurotensin-like peptides in gastric juice from patients with duodenal ulcers. Eur. J. Clin. Investig. 1987, 17, 306–312. [Google Scholar] [CrossRef]
- Kawanishi, K.; Goto, A.; Ishida, T.; Kawamura, K.; Nishina, Y.; Machida, S.; Yamamoto, S.; Ofuji, T. The Effects of Xenopsin on Endocrine Pancreas and Gastric Antrum in Dogs. Horm. Metab. Res. 1978, 10, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Zinner, M.J.; Kasher, F.; Modlin, I.M.; Jaffe, B.M. Effect of xenopsin on blood flow, hormone release, and acid secretion. Am. J. Physiol. Liver Physiol. 1982, 243, G195–G199. [Google Scholar] [CrossRef]
- Dunaif, A. Insulin Resistance and the Polycystic Ovary Syndrome: Mechanism and Implications for Pathogenesis*. Endocr. Rev. 1997, 18, 774–800. [Google Scholar] [CrossRef]
- Feurle, G.; Hamscher, G.; Kusiek, R.; Meyer, H.; Metzger, J. Identification of xenin, a xenopsin-related peptide, in the human gastric mucosa and its effect on exocrine pancreatic secretion. J. Biol. Chem. 1992, 267, 22305–22309. [Google Scholar] [CrossRef]
- Craig, S.L.; Gault, V.A.; Irwin, N. Emerging therapeutic potential for xenin and related peptides in obesity and diabetes. Diabetes/Metabolism Res. Rev. 2018, 34, e3006. [Google Scholar] [CrossRef] [PubMed]
- Hamscher, G.; Meyer, H.E.; Metzger, J.W.; Feurle, G.E. Distribution, formation, and molecular forms of the peptide xenin in various mammals. Peptides 1995, 16, 791–797. [Google Scholar] [CrossRef]
- Kuwahara, A.; Kuwahara, Y.; Kato, I.; Kawaguchi, K.; Harata, D.; Asano, S.; Inui, T.; Marunaka, Y. Xenin-25 induces anion secretion by activating noncholinergic secretomotor neurons in the rat ileum. Am. J. Physiol. Liver Physiol. 2019, 316, G785–G796. [Google Scholar] [CrossRef] [PubMed]
- Feurle, G. Xenin—A Review. Peptides 1998, 19, 609–615. [Google Scholar] [CrossRef]
- Wice, B.M.; Reeds, D.N.; Tran, H.D.; Crimmins, D.L.; Patterson, B.W.; Dunai, J.; Wallendorf, M.J.; Ladenson, J.H.; Villareal, D.T.; Polonsky, K.S. Xenin-25 Amplifies GIP-Mediated Insulin Secretion in Humans With Normal and Impaired Glucose Tolerance but Not Type 2 Diabetes. Diabetes 2012, 61, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Anlauf, M.; Weihe, E.; Hartschuh, W.; Hamscher, G.; Feurle, G.E. Localization of xenin-immunoreactive cells in the duodenal mucosa of humans and various mammals. J. Histochem. Cytochem. 2000, 48, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.I.; Irwin, N.; McKillop, A.M.; Patterson, S.; Flatt, P.R.; Gault, V.A. Evaluation of the degradation and metabolic effects of the gut peptide xenin on insulin secretion, glycaemic control and satiety. J. Endocrinol. 2010, 207, 87–93. [Google Scholar] [CrossRef]
- Martin, C.M.A.; Parthsarathy, V.; Pathak, V.; Gault, V.A.; Flatt, P.R.; Irwin, N. Characterisation of the biological activity of xenin-25 degradation fragment peptides. J. Endocrinol. 2014, 221, 193–200. [Google Scholar] [CrossRef][Green Version]
- Wice, B.M.; Wang, S.; Crimmins, D.L.; Diggs-Andrews, K.A.; Althage, M.C.; Ford, E.L.; Tran, H.; Ohlendorf, M.; Griest, T.A.; Wang, Q.; et al. Xenin-25 Potentiates Glucose-dependent Insulinotropic Polypeptide Action via a Novel Cholinergic Relay Mechanism. J. Biol. Chem. 2010, 285, 19842–19853. [Google Scholar] [CrossRef] [PubMed]
- Parthsarathy, V.; Irwin, N.; Hasib, A.; Martin, C.M.; McClean, S.; Bhat, V.K.; Ng, M.T.; Flatt, P.R.; Gault, V.A. A novel chemically modified analogue of xenin-25 exhibits improved glucose-lowering and insulin-releasing properties. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 757–764. [Google Scholar] [CrossRef]
- Holst, J.J.; Knop, F.K.; Vilsbøll, T.; Krarup, T.; Madsbad, S. Loss of Incretin Effect Is a Specific, Important, and Early Characteristic of Type 2 Diabetes. Diabetes Care 2011, 34, S251–S257. [Google Scholar] [CrossRef]
- Bhavya, S.; Lew, P.S.; Mizuno, T.M. Stimulation of white adipose tissue lipolysis by xenin, a neurotensin-related peptide. Biochem. Biophys. Res. Commun. 2018, 498, 842–848. [Google Scholar] [CrossRef]
- Kerbel, B.; Badal, K.; Sundarrajan, L.; Blanco, A.M.; Unniappan, S. Xenin is a novel anorexigen in goldfish (Carassius auratus). PLoS ONE 2018, 13, e0197817. [Google Scholar] [CrossRef]
- Byerly, M.S.; Swanson, R.D.; Semsarzadeh, N.N.; McCulloh, P.S.; Kwon, K.; Aja, S.; Moran, T.H.; Wong, G.W.; Blackshaw, S. Identification of hypothalamic neuron-derived neurotrophic factor as a novel factor modulating appetite. Am. J. Physiol. Integr. Comp. Physiol. 2013, 304, R1085–R1095. [Google Scholar] [CrossRef] [PubMed]
- Han, K.-H.; Lee, S.-H.; Ha, S.-A.; Kim, H.K.; Lee, C.; Kim, D.-H.; Gong, K.H.; Yoo, J.; Kim, S.; Kim, J.W. The functional and structural characterization of a novel oncogene GIG47 involved in the breast tumorigenesis. BMC Cancer 2012, 12, 274. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, W.; Bateman, A. Membrane-bound progesterone receptors contain a cytochrome b5-like ligand-binding domain. Genome Biol. 2002, 3. [Google Scholar] [CrossRef]
- Kimura, I.; Yoshioka, M.; Konishi, M.; Miyake, A.; Itoh, N. Neudesin, a novel secreted protein with a unique primary structure and neurotrophic activity. J. Neurosci. Res. 2005, 79, 287–294. [Google Scholar] [CrossRef]
- Kimura, I.; Konishi, M.; Miyake, A.; Fujimoto, M.; Itoh, N. Neudesin, a secreted factor, promotes neural cell proliferation and neuronal differentiation in mouse neural precursor cells. J. Neurosci. Res. 2006, 83, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Konishi, M.; Asaki, T.; Furukawa, N.; Ukai, K.; Mori, M.; Hirasawa, A.; Tsujimoto, G.; Ohta, M.; Itoh, N.; et al. Neudesin, an extracellular heme-binding protein, suppresses adipogenesis in 3T3-L1 cells via the MAPK cascade. Biochem. Biophys. Res. Commun. 2009, 381, 75–80. [Google Scholar] [CrossRef]
- Ohta, H.; Kimura, I.; Konishi, M.; Itoh, N. Neudesin as a unique secreted protein with multi-functional roles in neural functions, energy metabolism, and tumorigenesis. Front. Mol. Biosci. 2015, 2, 24. [Google Scholar] [CrossRef]
- Bozkaya, G.; Fenercioglu, O.; Demir, I.; Guler, A.; Aslanipour, B.; Calan, M. Neudesin: A neuropeptide hormone decreased in subjects with polycystic ovary syndrome. Gynecol. Endocrinol. 2020, 36, 849–853. [Google Scholar] [CrossRef]
- Wang-Eckhardt, L.; Eckhardt, M. A progesterone receptor membrane component 1 antagonist induces large vesicles independent of progesterone receptor membrane component 1 expression. Biol. Chem. 2020, 401, 1093–1099. [Google Scholar] [CrossRef]
- Polkowska, A.; Pasierowska, I.E.; Pasławska, M.; Pawluczuk, E.; Bossowski, A. Assessment of Serum Concentrations of Adropin, Afamin, and Neudesin in Children with Type 1 Diabetes. BioMed Res. Int. 2019, 2019, 6128410. [Google Scholar] [CrossRef]
- Kratochvilova, H.; Lacinova, Z.; Klouckova, J.; Kavalkova, P.; Cinkajzlova, A.; Trachta, P.; Krizova, J.; Benes, M.; Dolezalova, K.; Fried, M.; et al. Neudesin in obesity and type 2 diabetes mellitus: The effect of acute fasting and weight reducing interventions. Diabetes, Metab. Syndr. Obesity: Targets Ther. 2019, 12, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Karatas, O.; Calan, M.; Yuksel, A.; Chousein, R.; Bozkaya, G.; Karatas, M.; Uslu, A.; Tatar, E. The level of the neudesin in Type-2 Diabetic patients and the relationship between the metabolic parameters and carotid intima-media thickness. Minerva Endocrinol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Unfer, V.; Casini, M.L.; Marelli, G.; Costabile, L.; Gerli, S.; Di Renzo, G.C. Different routes of progesterone administration and polycystic ovary syndrome: A review of the literature. Gynecol. Endocrinol. 2005, 21, 119–127. [Google Scholar] [CrossRef]
- Moschen, A.R.; Adolph, T.E.; Gerner, R.R.; Wieser, V.; Tilg, H. Lipocalin-2: A Master Mediator of Intestinal and Metabolic Inflammation. Trends Endocrinol. Metab. 2017, 28, 388–397. [Google Scholar] [CrossRef]
- Kjeldsen, L.; Cowland, J.B.; Borregaard, N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzym. 2000, 1482, 272–283. [Google Scholar] [CrossRef]
- Lippi, G.; Meschi, T.; Nouvenne, A.; Mattiuzzi, C.; Borghi, L. Neutrophil Gelatinase-Associated Lipocalin in Cancer. In International Review of Cytology; Elsevier BV: Amsterdam, The Netherlands, 2014; Volume 64, pp. 179–219. [Google Scholar]
- Panidis, D.; Tziomalos, K.; Koiou, E.; Kandaraki, E.A.; Tsourdi, E.; Delkos, D.; Kalaitzakis, E.; Katsikis, I. The effects of obesity and polycystic ovary syndrome on serum lipocalin-2 levels: A cross-sectional study. Reprod. Biol. Endocrinol. 2010, 8, 151. [Google Scholar] [CrossRef][Green Version]
- Al Jaberi, S.; Cohen, A.; D’souza, C.; Abdulrazzaq, Y.M.; Ojha, S.; Bastaki, S.; Adeghate, E.A. Lipocalin-2: Structure, function, distribution and role in metabolic disorders. Biomed. Pharmacother. 2021, 142, 112002. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Yang, K.; Zang, S.; Lin, Z.; Chau, H.T.; Wang, Y.; Wang, Y. Lipocalin-2 mediates non-alcoholic steatohepatitis by promoting neutrophil-macrophage crosstalk via the induction of CXCR2. J. Hepatol. 2016, 65, 988–997. [Google Scholar] [CrossRef]
- Law, I.K.; Xu, A.; Lam, K.S.; Berger, T.; Mak, T.W.; Vanhoutte, P.M.; Liu, J.T.; Sweeney, G.; Zhou, M.; Yang, B.; et al. Lipocalin-2 Deficiency Attenuates Insulin Resistance Associated With Aging and Obesity. Diabetes 2010, 59, 872–882. [Google Scholar] [CrossRef]
- Gencer, M.; Gazi, E.; Hacıvelioğlu, S.; Binnetoğlu, E.; Barutçu, A.; Türkön, H.; Temiz, A.; Altun, B.; Vural, A.; Cevizci, S.; et al. The relationship between subclinical cardiovascular disease and lipocalin-2 levels in women with PCOS. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 99–103. [Google Scholar] [CrossRef]
- Sahin, S.B.; Cure, M.C.; Uğurlu, Y.; Ergül, E.; Gur, E.U.; Alyildiz, N.; Bostan, M. Epicardial adipose tissue thickness and NGAL levels in women with polycystic ovary syndrome. J. Ovarian Res. 2014, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.Á.; Montes-Nieto, R.; Fernández-Durán, E.; Insenser, M.; Luque-Ramírez, M.; Escobar-Morreale, H.F. Evidence for Masculinization of Adipokine Gene Expression in Visceral and Subcutaneous Adipose Tissue of Obese Women With Polycystic Ovary Syndrome (PCOS). J. Clin. Endocrinol. Metab. 2013, 98, E388–E396. [Google Scholar] [CrossRef] [PubMed]
- Koiou, E.; Tziomalos, K.; Katsikis, I.; Kandaraki, E.A.; Kalaitzakis, E.; Delkos, D.; Vosnakis, C.; Panidis, D. Weight loss significantly reduces serum lipocalin-2 levels in overweight and obese women with polycystic ovary syndrome. Gynecol. Endocrinol. 2011, 28, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Çakal, E.; Ozkaya, M.; Engin-Ustun, Y.; Ustun, Y. Serum Lipocalin-2 as an Insulin Resistance Marker in Patients with Polycystic Ovary Syndrome. J. Endocrinol. Investig. 2011, 34, 97–100. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Livadas, S.; Kandarakis, S.A.; Margeli, A.; Papassotiriou, I. Serum concentrations of atherogenic proteins neutrophil gelatinase-associated lipocalin and its complex with matrix metalloproteinase-9 are significantly lower in women with polycystic ovary syndrome: Hint of a protective mechanism? Eur. J. Endocrinol. 2008, 158, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Ng, N.Y.H.; Tam, C.H.T.; Zhang, Y.; Lim, C.K.P.; Jiang, G.; Ng, A.C.W.; Yau, T.T.L.; Cheung, L.P.; Xu, A.; et al. Association between FGF19, FGF21 and lipocalin-2, and diabetes progression in PCOS. Endocr. Connect. 2021, 10, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Sun, X.; Wang, X.; Wang, H.; Chen, X. Circulating Adipokine Levels in Nonobese Women With Polycystic Ovary Syndrome and in Nonobese Control Women: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 11, 537809. [Google Scholar] [CrossRef] [PubMed]
- Catalina, M.O.-S.; Redondo, P.C.; Granados, M.P.; Cantonero, C.; Sanchez-Collado, J.; Albarran, L.; Lopez, J.J. New Insights into Adipokines as Potential Biomarkers for Type-2 Diabetes Mellitus. Curr. Med. Chem. 2019, 26, 4119–4144. [Google Scholar] [CrossRef]
- Gnanadass, S.A.; Prabhu, Y.D.; Gopalakrishnan, A.V. Association of metabolic and inflammatory markers with polycystic ovarian syndrome (PCOS): An update. Arch. Gynecol. Obstet. 2021, 303, 631–643. [Google Scholar] [CrossRef]
- Karakas, S.E. New biomarkers for diagnosis and management of polycystic ovary syndrome. Clin. Chim. Acta 2017, 471, 248–253. [Google Scholar] [CrossRef]
- Park, S.E.; Park, C.-Y.; Sweeney, G. Biomarkers of insulin sensitivity and insulin resistance: Past, present and future. Crit. Rev. Clin. Lab. Sci. 2015, 52, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Teede, H.; Harrison, C.L.; Joham, A.E.; Moran, L.; Stepto, N.K. Biomarkers and insulin sensitivity in women with Polycystic Ovary Syndrome: Characteristics and predictive capacity. Clin. Endocrinol. 2015, 83, 50–58. [Google Scholar] [CrossRef] [PubMed]
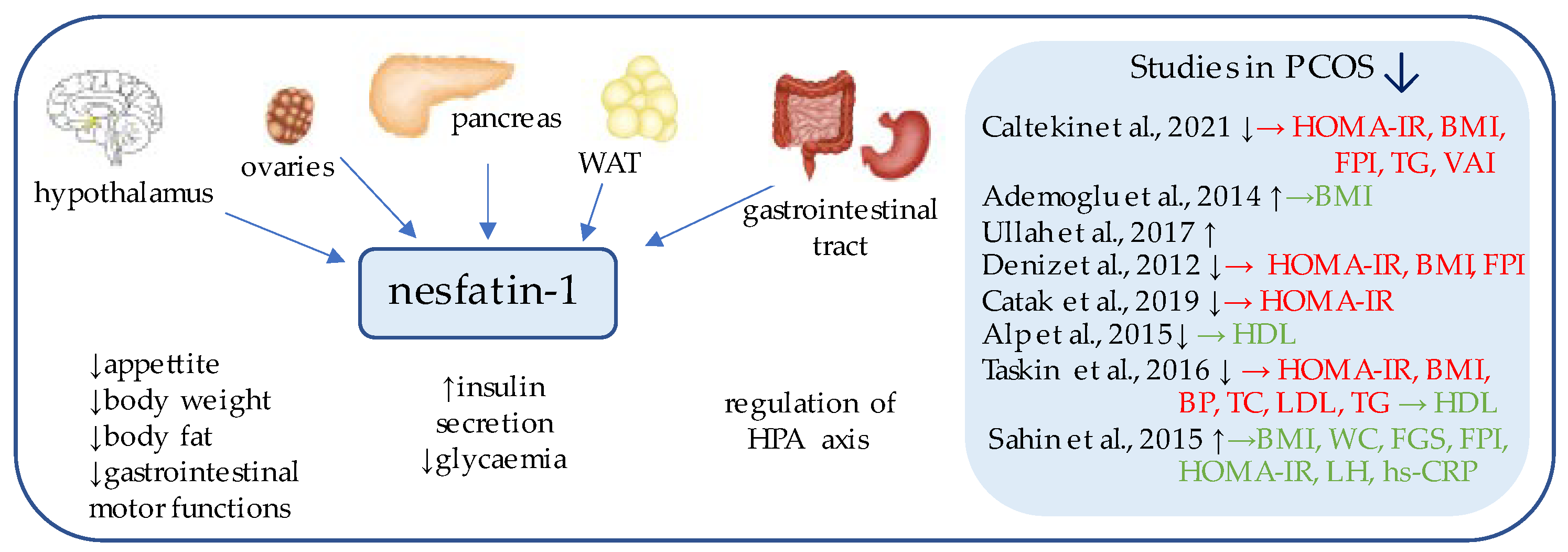
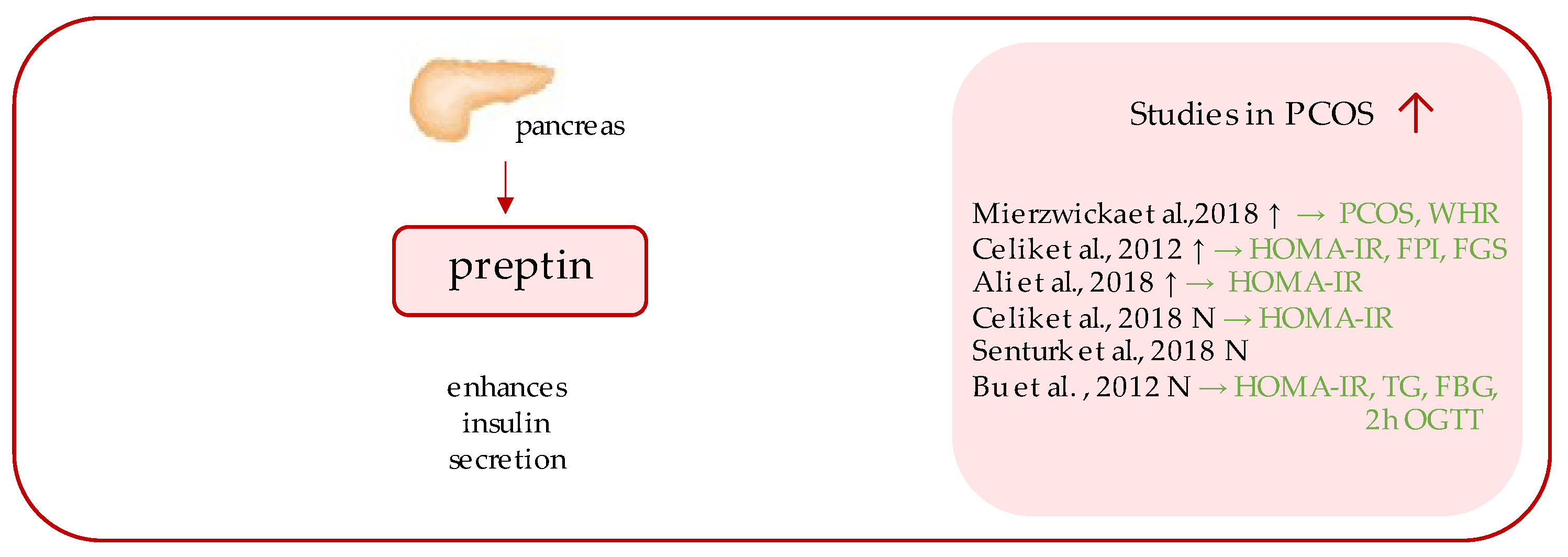
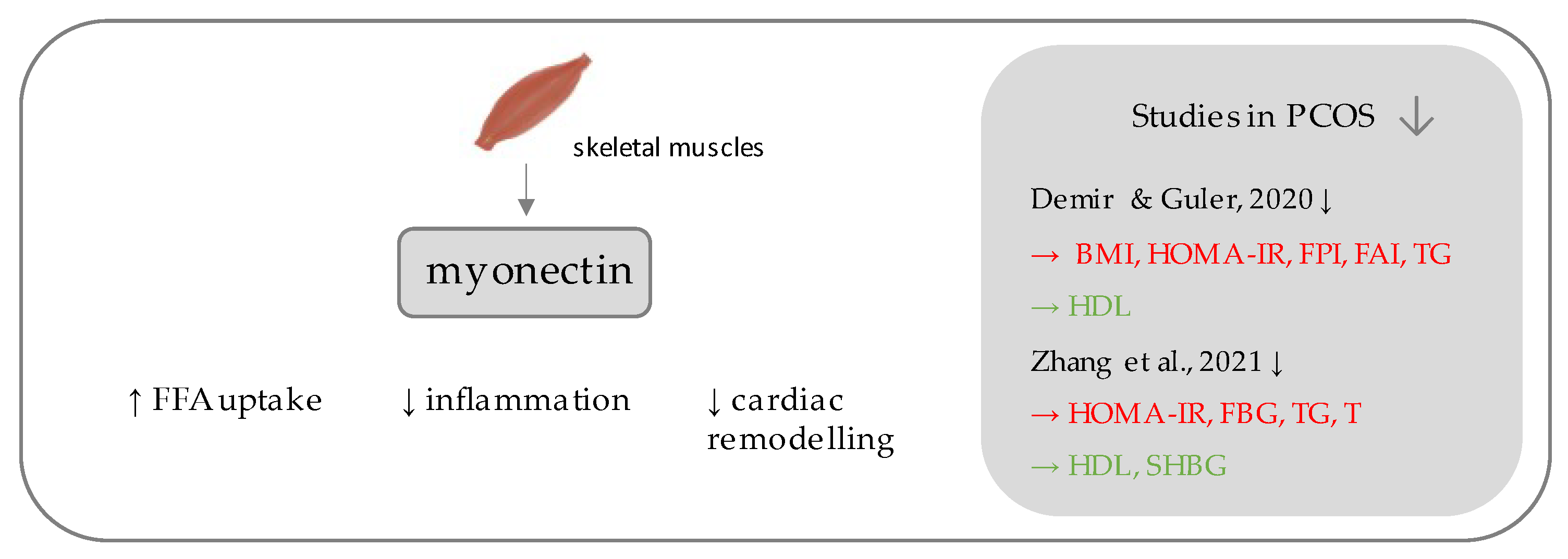
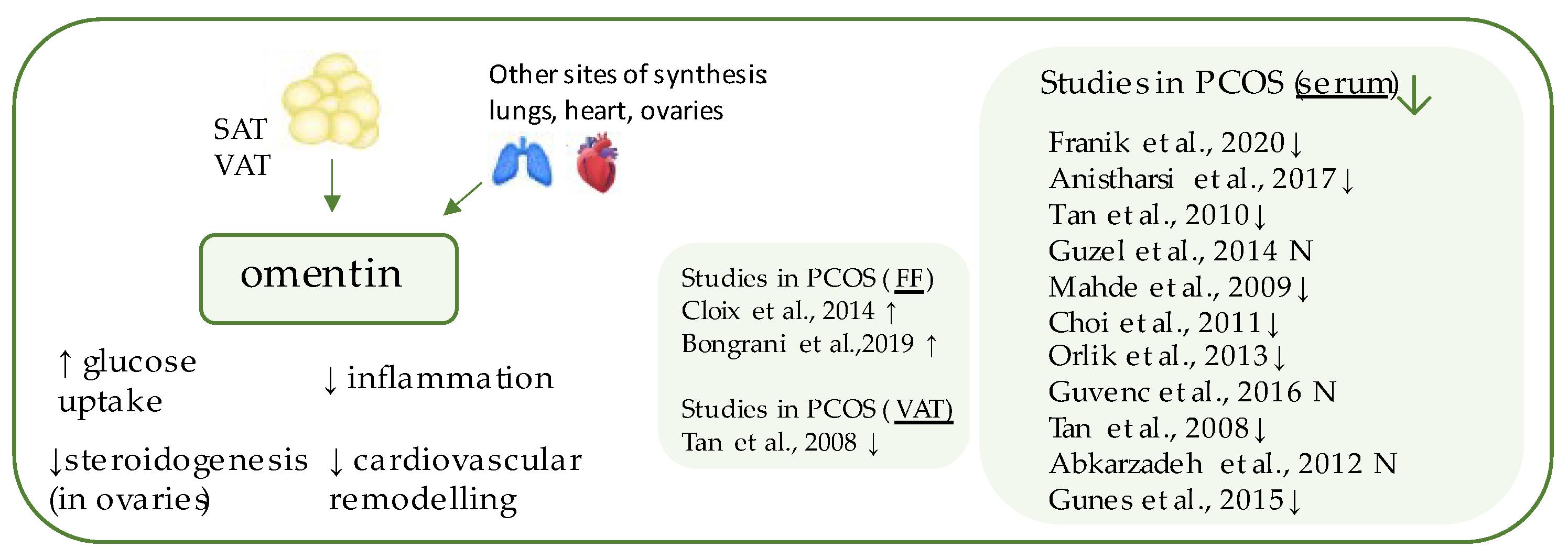

| Marker | Author | PCOS Patients (n) | Control Patients (n) | Level of the Marker (PCOS) 1 | Level of the Marker (Control) 1 | Insulin Resistance Indicators (p-Value) 2 |
|---|---|---|---|---|---|---|
| Nesfatin-1 | Demir Çaltekin et al., 2021 [25] | 44 | 40 | 17.08 ± 13.8 ng/mL (p < 0.001) | 36.8 ± 20.7 ng/mL | FPG (p = 0.999); FPI (p < 0.001); HOMA-IR (p = 0.001); VAI (p = 0.144) |
| Preptin | Mierzwicka et al., 2018 [26] | 73 | 61 | 8.88 ± 3.89 ng/mL (p = 0.0255) | 7.53 ± 2.53 ng/mL | FPG (p = 0.244); FPI (p = 0.0076); OGTT (p = 0.0665); HOMA-IR (p = 0.0009); QUICKI (p = 0.00834); Matsuda Index (p = 0.0006); 120 min Ins (p = 0.0007) |
| Myonectin | Demir & Guler 2020 [27] | 72 | 72 | 6.77 ± 1.96 ng/mL (p < 0.001) | 9.14 ± 2.87 ng/mL | FPG (p = 0.044); FPI (p < 0.001) OGTT (p = 0.218); HOMA-IR (p < 0.0001); HbA1c (p = 0.265) |
| Omentin | Franik et al., 2020 [28] | 86 | 72 | 210.5 ng/mL (149–302.7) (p < 0.001) | 515.9 ng/mL (256.3–779.0) | FPG (p < 0.001); FPI (p < 0.01); HOMA-IR (p < 0.01) |
| Gremlin-1 | Koroglu et al., 2019 [29] | 50 | 30 | 1.89 ng/mL (1.36–2.57) (p = 0.001) | 1.36 ng/mL (0.64–1.92) | FPG (p = 0.190); FPI (p = 0.056); HOMA-IR (p = 0.000) |
| Galectin-3 | Yilmaz et al., 2014 [30] | 56 | 41 | 3588.77 ± 1566.94 ng/dL (p < 0.001) | 2491.33 ± 812.04 ng/dL | FBG (p = 0.144); HOMA (p = 0.508) |
| Neuregulin-4 | Cao &Hu, 2021 [31] | 52 | 43 | 8.12 ± 3.03 ng/mL (p = 0.031) | 4.22 ± 1.25 ng/mL | Serum C peptide (p = 0.012); FPI (p = 0.026) |
| Xenopsin-related peptide | Temur et al., 2017 [32] | 40 | 38 | 6.49 ± 1.57 ng/mL (p = 0.001) | 5.29 ±1.45 ng/mL | FBG (p = 0.134); FPI (p = 0.002); HOMA-IR (p = 0.003) |
| Xenin-25 | Guclu et al., 2019 [33] | 31 | 30 | 220.79 ± 259.4 pg/mL (p = 0.007) | 68.58 ± 152.78 pg/mL | FPG (p = 0.437); FPI (p = 0.345); HOMA-IR (p = 0.478) |
| Neudesin | Yasar et al., 2021 [34] | 180 | 100 | 1.19 ± 1.08 ng/mL (p = 0.015) | 2.12 ± 1.04 ng/mL | FBG (p = 0.170); FPI (p = 0.004); HOMA-IR (p = 0.004); HbA1C (p = 0.231) |
| Lipocalin-2 | Yilmaz et al., 2017 [35] | 44 | 47 | 55.74 ± 17.54 ng/mL (p < 0.011) | 36.46 ± 19.62 ng/mL | FBG (NS); FPI (p = 0.02); HOMA-IR (p = 0.014) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruszewska, J.; Laudy-Wiaderny, H.; Kunicki, M. Review of Novel Potential Insulin Resistance Biomarkers in PCOS Patients—The Debate Is Still Open. Int. J. Environ. Res. Public Health 2022, 19, 2099. https://doi.org/10.3390/ijerph19042099
Kruszewska J, Laudy-Wiaderny H, Kunicki M. Review of Novel Potential Insulin Resistance Biomarkers in PCOS Patients—The Debate Is Still Open. International Journal of Environmental Research and Public Health. 2022; 19(4):2099. https://doi.org/10.3390/ijerph19042099
Chicago/Turabian StyleKruszewska, Jagoda, Hanna Laudy-Wiaderny, and Michał Kunicki. 2022. "Review of Novel Potential Insulin Resistance Biomarkers in PCOS Patients—The Debate Is Still Open" International Journal of Environmental Research and Public Health 19, no. 4: 2099. https://doi.org/10.3390/ijerph19042099
APA StyleKruszewska, J., Laudy-Wiaderny, H., & Kunicki, M. (2022). Review of Novel Potential Insulin Resistance Biomarkers in PCOS Patients—The Debate Is Still Open. International Journal of Environmental Research and Public Health, 19(4), 2099. https://doi.org/10.3390/ijerph19042099







