The Association between Health-Enhancing Physical Activity and Quality of Life in Patients with Chronic Kidney Disease: Propensity Score Matching Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Measurements
2.3. Health-Related Quality of Life
2.4. Variables
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Participants
3.2. Linear Regression with Ordinary Least Squares Method
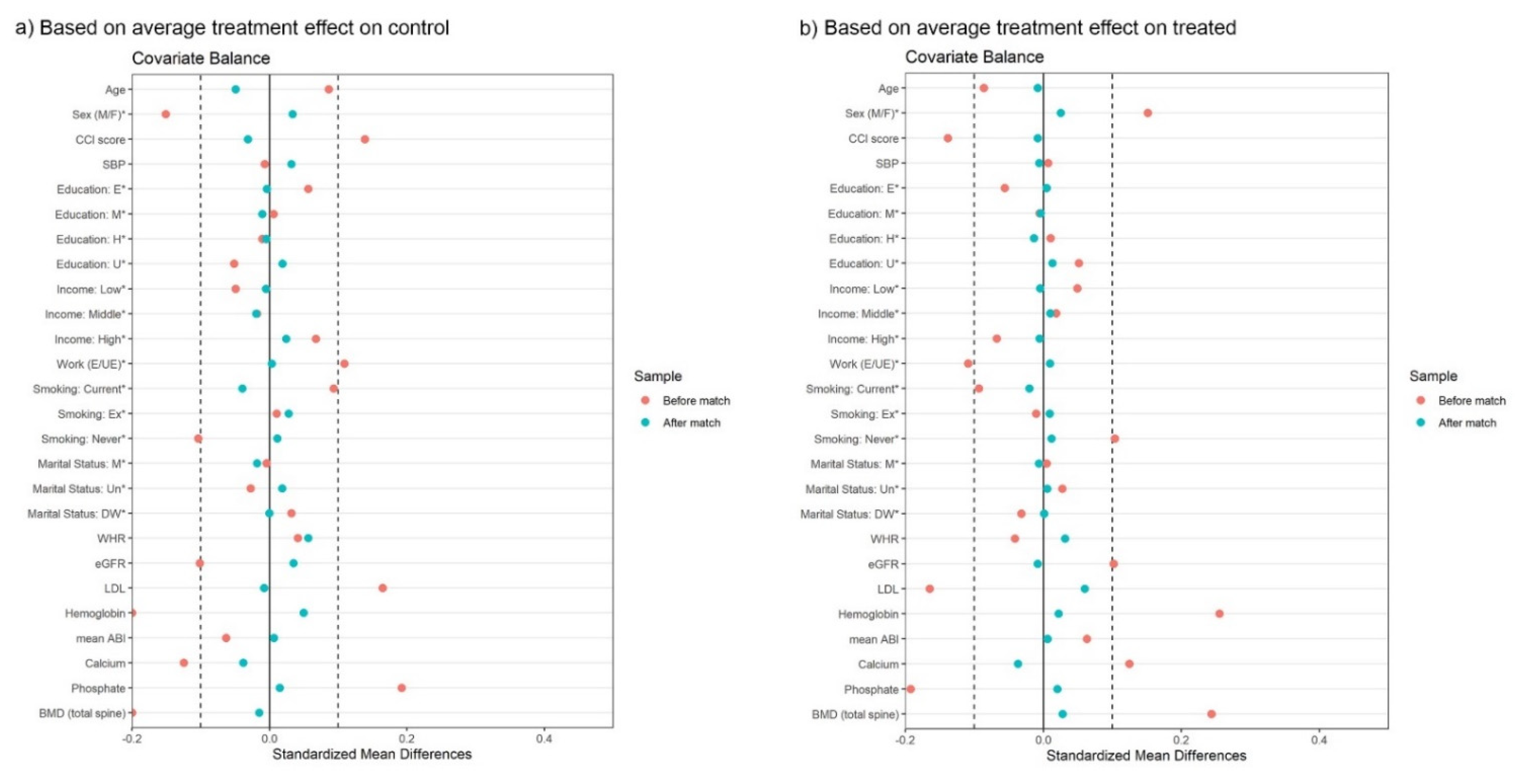
3.3. Propensity Score Matching Analysis
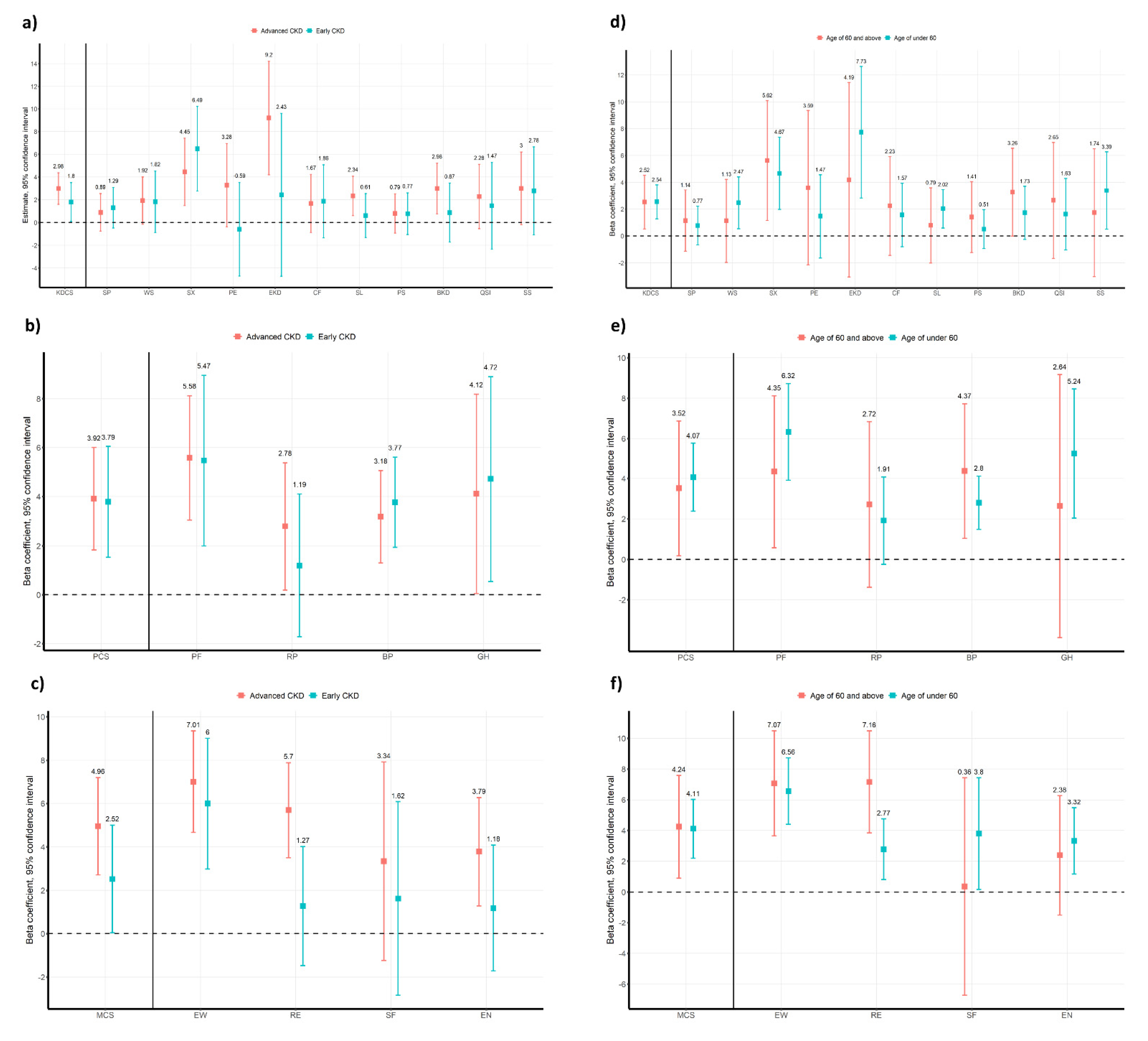
3.4. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.M.; Oh, H.J.; Choi, H.Y.; Lee, H.; Ryu, D.R. Impact of chronic kidney disease on mortality: A nationwide cohort study. Kidney Res. Clin. Pract. 2019, 38, 382–390. [Google Scholar] [CrossRef]
- Perlman, R.L.; Finkelstein, F.O.; Liu, L.; Roys, E.; Kiser, M.; Eisele, G.; Burrows-Hudson, S.; Messana, J.M.; Levin, N.; Rajagopalan, S.; et al. Quality of life in chronic kidney disease (CKD): A cross-sectional analysis in the Renal Research Institute-CKD study. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2005, 45, 658–666. [Google Scholar] [CrossRef]
- Unruh, M.; Benz, R.; Greene, T.; Yan, G.; Beddhu, S.; DeVita, M.; Dwyer, J.T.; Kimmel, P.L.; Kusek, J.W.; Martin, A.; et al. Effects of hemodialysis dose and membrane flux on health-related quality of life in the HEMO Study. Kidney Int. 2004, 66, 355–366. [Google Scholar] [CrossRef][Green Version]
- Group, W. Study protocol for the World Health Organization project to develop a Quality of Life assessment instrument (WHOQOL). Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 1993, 2, 153–159. [Google Scholar] [CrossRef]
- Phyo, A.Z.Z.; Freak-Poli, R.; Craig, H.; Gasevic, D.; Stocks, N.P.; Gonzalez-Chica, D.A.; Ryan, J. Quality of life and mortality in the general population: A systematic review and meta-analysis. BMC Public Health 2020, 20, 1596. [Google Scholar] [CrossRef]
- Ko, H.Y.; Lee, J.K.; Shin, J.Y.; Jo, E. Health-Related Quality of Life and Cardiovascular Disease Risk in Korean Adults. Korean J. Fam. Med. 2015, 36, 349–356. [Google Scholar] [CrossRef][Green Version]
- Oh, T.R.; Choi, H.S.; Kim, C.S.; Bae, E.H.; Oh, Y.K.; Kim, Y.S.; Choi, K.H.; Kim, S.W.; Ma, S.K. Association between health related quality of life and progression of chronic kidney disease. Sci. Rep. 2019, 9, 19595. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Cheung, A.K.; Chang, T.I.; Cushman, W.C.; Furth, S.L.; Hou, F.F.; Ix, J.H.; Knoll, G.A.; Muntner, P.; Pecoits-Filho, R.; Sarnak, M.J.; et al. Executive summary of the KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021, 99, 559–569. [Google Scholar] [CrossRef]
- Oh, K.H.; Park, S.K.; Park, H.C.; Chin, H.J.; Chae, D.W.; Choi, K.H.; Han, S.H.; Yoo, T.H.; Lee, K.; Kim, Y.S.; et al. KNOW-CKD (KoreaN cohort study for Outcome in patients with Chronic Kidney Disease): Design and methods. BMC Nephrol. 2014, 15, 80. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Buuren, S.; Groothuis-Oudshoorn, C. MICE: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 1, 45–67. [Google Scholar] [CrossRef]
- Ho, D.; Imai, K.; King, G.; Stuart, E. Matching as Nonparametric Preprocessing for Reducing Model Dependence in Parametric Causal Inference. Political Anal. 2007, 15, 199–236. [Google Scholar] [CrossRef]
- Imai, K.; King, G.; Lau, O. Toward A Common Framework for Statistical Analysis and Development. J Comput Graph Stat. 2008, 17, 892–913. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2021. Available online: https://www.r-project.org/ (accessed on 6 December 2021).
- Walters, B.A.; Hays, R.D.; Spritzer, K.L.; Fridman, M.; Carter, W.B. Health-related quality of life, depressive symptoms, anemia, and malnutrition at hemodialysis initiation. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2002, 40, 1185–1194. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Bolton, K.; Culleton, B.; Harvey, K.S.; Ikizler, T.A.; Johnson, C.A.; Kausz, A.; Kimmel, P.L.; Kusek, J.; et al. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2002, 39 (Suppl. 1), S1–S266. [Google Scholar]
- Brazier, J.E.; Harper, R.; Jones, N.M.; O’Cathain, A.; Thomas, K.J.; Usherwood, T.; Westlake, L. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. BMJ (Clin. Res. Ed.) 1992, 305, 160–164. [Google Scholar] [CrossRef]
- Kilgour-Christie, J.; Watt, A.H. Measuring patients’ views of their health. The term outcome is ambiguous. BMJ (Clin. Res. Ed.) 1993, 307, 126. [Google Scholar] [CrossRef][Green Version]
- Perneger, T.V.; Leplège, A.; Etter, J.F.; Rougemont, A. Validation of a French-language version of the MOS 36-Item Short Form Health Survey (SF-36) in young healthy adults. J. Clin. Epidemiol. 1995, 48, 1051–1060. [Google Scholar] [CrossRef]
- Mok, A.; Khaw, K.T.; Luben, R.; Wareham, N.; Brage, S. Physical activity trajectories and mortality: Population based cohort study. BMJ (Clin. Res. Ed.) 2019, 365, l2323. [Google Scholar] [CrossRef]
- Oja, P.; Kelly, P.; Pedisic, Z.; Titze, S.; Bauman, A.; Foster, C.; Hamer, M.; Hillsdon, M.; Stamatakis, E. Associations of specific types of sports and exercise with all-cause and cardiovascular-disease mortality: A cohort study of 80 306 British adults. Br. J. Sports Med. 2017, 51, 812–817. [Google Scholar] [CrossRef]
- Diaz, K.M.; Shimbo, D. Physical activity and the prevention of hypertension. Curr. Hypertens. Rep. 2013, 15, 659–668. [Google Scholar] [CrossRef]
- Sato, T.; Kohzuki, M.; Ono, M.; Muto, M.; Osugi, T.; Kawamura, K.; Naganuma, W.; Sato, M.; Shishito, N. Association between physical activity and change in renal function in patients after acute myocardial infarction. PLoS ONE 2019, 14, e0212100. [Google Scholar] [CrossRef]
- Chen, Y.; Hicks, A.; While, A.E. Quality of life and related factors: A questionnaire survey of older people living alone in Mainland China. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2014, 23, 1593–1602. [Google Scholar] [CrossRef]
- Han, K.T.; Park, E.C.; Kim, J.H.; Kim, S.J.; Park, S. Is marital status associated with quality of life? Health Qual. Life Outcomes 2014, 12, 109. [Google Scholar] [CrossRef]
- Gil-Lacruz, M.; Gil-Lacruz, A.I.; Gracia-Pérez, M.L. Health-related quality of life in young people: The importance of education. Health Qual. Life Outcomes 2020, 18, 187. [Google Scholar] [CrossRef]
- Klompstra, L.; Ekdahl, A.W.; Krevers, B.; Milberg, A.; Eckerblad, J. Factors related to health-related quality of life in older people with multimorbidity and high health care consumption over a two-year period. BMC Geriatr. 2019, 19, 187. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- D’Agostino, R.B., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 1998, 17, 2265–2281. [Google Scholar] [CrossRef]
- Taft, C.; Karlsson, J.; Sullivan, M. Do SF-36 summary component scores accurately summarize subscale scores? Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2001, 10, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Hackney, A.C. Stress and the neuroendocrine system: The role of exercise as a stressor and modifier of stress. Expert Rev. Endocrinol. Metab. 2006, 1, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Madaan, V.; Petty, F.D. Exercise for mental health. Prim. Care Companion J. Clin. Psychiatry 2006, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Pinckard, K.; Baskin, K.K.; Stanford, K.I. Effects of Exercise to Improve Cardiovascular Health. Front. Cardiovasc. Med. 2019, 6, 69. [Google Scholar] [CrossRef]
- Greene, T. Randomized and observational studies in nephrology: How strong is the evidence? Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2009, 53, 377–388. [Google Scholar] [CrossRef]


| Variables | Total Subjects (n = 1618) | Health-Enhancing Physical Activity | p-Value | |
|---|---|---|---|---|
| No (906) | Yes (712) | |||
| Age (years) | 52.3 ± 12.3 | 52.8 ± 12.4 | 51.7 ± 12.1 | 0.085 |
| Female | 605 (37.4%) | 399 (44.0%) | 206 (28.9%) | <0.001 |
| Income | 0.002 | |||
| Low | 405 (25.0%) | 205 (22.6%) | 200 (28.1%) | |
| Middle | 847 (52.3%) | 470 (51.9%) | 377 (52.9%) | |
| High | 366 (22.6%) | 231 (25.5%) | 135 (19.0%) | |
| Educational status | 0.002 | |||
| Below elementary school | 169 (10.4%) | 117 (12.9%) | 52 (7.3%) | |
| Middle school | 162 (10.0%) | 93 (10.3%) | 69 (9.7%) | |
| High school | 561 (34.7%) | 310 (34.2%) | 251 (35.3%) | |
| Above university | 726 (44.9%) | 386 (42.6%) | 340 (47.8%) | |
| Marital status | 0.009 | |||
| Married | 1339 (82.8%) | 748 (82.6%) | 591 (83.0%) | |
| Unmarried | 182 (11.2%) | 91 (10.0%) | 91 (12.8%) | |
| Divorced or windowed | 97 (6.0%) | 67 (7.4%) | 30 (4.2%) | |
| Employed | 992 (61.3%) | 512 (56.5%) | 480 (67.4%) | <0.001 |
| Smoking history | <0.001 | |||
| Current | 823 (50.9%) | 498 (55.0%) | 325 (45.6%) | |
| Never | 273 (16.9%) | 157 (17.3%) | 116 (16.3%) | |
| Ex-smoker | 522 (32.3%) | 251 (27.7%) | 271 (38.1%) | |
| Diabetes mellitus | 509 (31.5%) | 310 (34.2%) | 199 (27.9%) | 0.021 |
| Hypertension | 1548 (95.7%) | 856 (94.5%) | 692 (97.2%) | 0.011 |
| Charlson comorbidity index | 3.0 [2.0; 5.0] | 3.0 [2.0; 5.0] | 3.0 [2.0; 4.0] | 0.01 |
| Body mass index (kg/m2) | 24.5 ± 3.4 | 24.4 ± 3.5 | 24.6 ± 3.3 | 0.239 |
| Waist-hip ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.179 |
| Hemoglobin (g/dL) | 12.9 ± 2.0 | 12.7 ± 2.0 | 13.2 ± 2.0 | <0.001 |
| Serum albumin (g/dL) | 4.2 ± 0.4 | 4.2 ± 0.5 | 4.2 ± 0.4 | 0.054 |
| Serum uric acid (mg/dL) | 7.0 ± 1.9 | 7.0 ± 1.9 | 7.0 ± 1.8 | 0.732 |
| Fasting glucose (mg/dL) | 99.0 [91.0; 111.0] | 99.0 [91.0; 112.0] | 98.5 [92.0; 110.5] | 0.807 |
| C-reactive protein | 0.6 [0.2; 1.6] | 0.6 [0.3; 1.6] | 0.6 [0.2; 1.6] | 0.6 |
| Calcium (mg/dL) | 9.1 ± 0.5 | 9.1 ± 0.6 | 9.2 ± 0.5 | 0.013 |
| Phosphate (mg/dL) | 3.7 ± 0.7 | 3.7 ± 0.7 | 3.6 ± 0.6 | <0.001 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 48.0 [29.1; 76.3] | 44.2 [27.0; 73.1] | 51.5 [32.1; 77.1] | 0.004 |
| CKD stages | 0.002 | |||
| 1 | 280 (17.3%) | 156 (17.2%) | 124 (17.4%) | |
| 2 | 306 (18.9%) | 161 (17.8%) | 145 (20.4%) | |
| 3a | 273 (16.9%) | 130 (14.3%) | 143 (20.1%) | |
| 3b | 339 (21.0%) | 192 (21.2%) | 147 (20.6%) | |
| 4 | 320 (19.8%) | 203 (22.4%) | 117 (16.4%) | |
| 5 | 100 (6.2%) | 64 (7.1%) | 36 (5.1%) | |
| Protein to creatinine ratio (g/g Creatinine) | 0.5 [0.1; 1.5] | 0.5 [0.1; 1.6] | 0.5 [0.1; 1.4] | 0.177 |
| High density lipoprotein (mg/dL) | 47.0 [38.0; 58.0] | 47.0 [38.0; 57.0] | 47.0 [39.0; 58.0] | 0.377 |
| Low density lipoprotein (mg/dL) | 93.0 [73.0; 115.0] | 95.5 [75.0; 118.0] | 91.0 [71.5; 112.0] | 0.002 |
| Triglyceride (mg/dL) | 134.0 [94.0; 195.0] | 136.0 [95.0; 198.0] | 130.0 [92.5; 190.0] | 0.38 |
| Mean ankle brachial pressure index | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 | 0.131 |
| Bone mineral density (total spine, g/cm2) | −0.1 [−1.0; 0.9] | −0.2 [−1.2; 0.7] | 0.2 [−0.8; 1.1] | <0.001 |
| Physical component summary score | 73.5 ± 17.9 | 70.2 ± 18.7 | 77.7 ± 15.7 | <0.001 |
| Mental component summary score | 70.5 ± 18.0 | 67.6 ± 18.5 | 74.1 ± 16.6 | <0.001 |
| Kidney disease component summary score | 73.2 ± 12.8 | 71.1 ± 12.9 | 76.0 ± 12.2 | <0.001 |
| Beta Coefficients | Confidence Interval | p-Value | |
|---|---|---|---|
| Kidney disease component summary score | 2.56 | 1.496–3.624 | <0.001 |
| Symptoms/problem lists | 1.043 | −0.173–2.258 | 0.093 |
| Work status | 2.108 | −0.63–4.846 | 0.131 |
| Sexual function | 6.577 | 2.526–10.628 | 0.001 |
| Physician encouragement | 1.61 | −0.37–3.59 | 0.111 |
| Effects of kidney disease | 1.812 | 0.521–3.103 | 0.006 |
| Cognitive function | 0.861 | −0.406–2.127 | 0.183 |
| Sleep | 2.042 | 0.359–3.725 | 0.017 |
| Patient satisfaction | 1.929 | −0.311–4.17 | 0.091 |
| Burden of kidney disease | 3.111 | 0.669–5.552 | 0.013 |
| Quality of social interaction | 2.046 | 0.418–3.675 | 0.014 |
| Social support | 5.022 | 2.731–7.313 | <0.001 |
| Physical component summary score | 4.058 | 2.517–5.599 | <0.001 |
| Physical function | 3.538 | 2.182–4.895 | <0.001 |
| Role-physical | 4.634 | 1.674–7.593 | 0.002 |
| Bodily pain | 2.344 | 0.408–4.28 | 0.018 |
| General health | 5.717 | 3.695–7.739 | <0.001 |
| Mental component summary score | 4.193 | 2.529–5.857 | <0.001 |
| Emotional well-being | 4.045 | 2.353–5.736 | <0.001 |
| Role-emotional | 2.846 | −0.446–6.139 | 0.09 |
| Social function | 3.262 | 1.376–5.148 | 0.001 |
| Energy/fatigue | 6.62 | 4.803–8.437 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, T.R.; Choi, H.S.; Suh, S.H.; Kim, C.S.; Bae, E.H.; Sung, S.; Han, S.H.; Oh, K.H.; Ma, S.K.; Kim, S.W. The Association between Health-Enhancing Physical Activity and Quality of Life in Patients with Chronic Kidney Disease: Propensity Score Matching Analysis. Int. J. Environ. Res. Public Health 2022, 19, 1318. https://doi.org/10.3390/ijerph19031318
Oh TR, Choi HS, Suh SH, Kim CS, Bae EH, Sung S, Han SH, Oh KH, Ma SK, Kim SW. The Association between Health-Enhancing Physical Activity and Quality of Life in Patients with Chronic Kidney Disease: Propensity Score Matching Analysis. International Journal of Environmental Research and Public Health. 2022; 19(3):1318. https://doi.org/10.3390/ijerph19031318
Chicago/Turabian StyleOh, Tae Ryom, Hong Sang Choi, Sang Heon Suh, Chang Seong Kim, Eun Hui Bae, Suah Sung, Seung Hyeok Han, Kook Hwan Oh, Seong Kwon Ma, and Soo Wan Kim. 2022. "The Association between Health-Enhancing Physical Activity and Quality of Life in Patients with Chronic Kidney Disease: Propensity Score Matching Analysis" International Journal of Environmental Research and Public Health 19, no. 3: 1318. https://doi.org/10.3390/ijerph19031318
APA StyleOh, T. R., Choi, H. S., Suh, S. H., Kim, C. S., Bae, E. H., Sung, S., Han, S. H., Oh, K. H., Ma, S. K., & Kim, S. W. (2022). The Association between Health-Enhancing Physical Activity and Quality of Life in Patients with Chronic Kidney Disease: Propensity Score Matching Analysis. International Journal of Environmental Research and Public Health, 19(3), 1318. https://doi.org/10.3390/ijerph19031318






