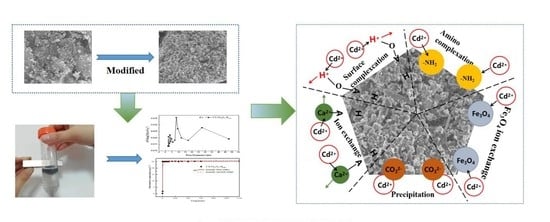
Adsorption Performance of Cd(II) by Chitosan-Fe3O4-Modified Fish Bone Char
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbent Preparation
2.1.1. Preparation of Fish Bone Char
2.1.2. Preparation of Nano Fe3O4
2.1.3. Preparation of Chitosan-Fe3O4-Modified Fish Bone Char
2.2. Characterization
2.3. Adsorption
2.4. Statistical Analysis
3. Results and Discussion
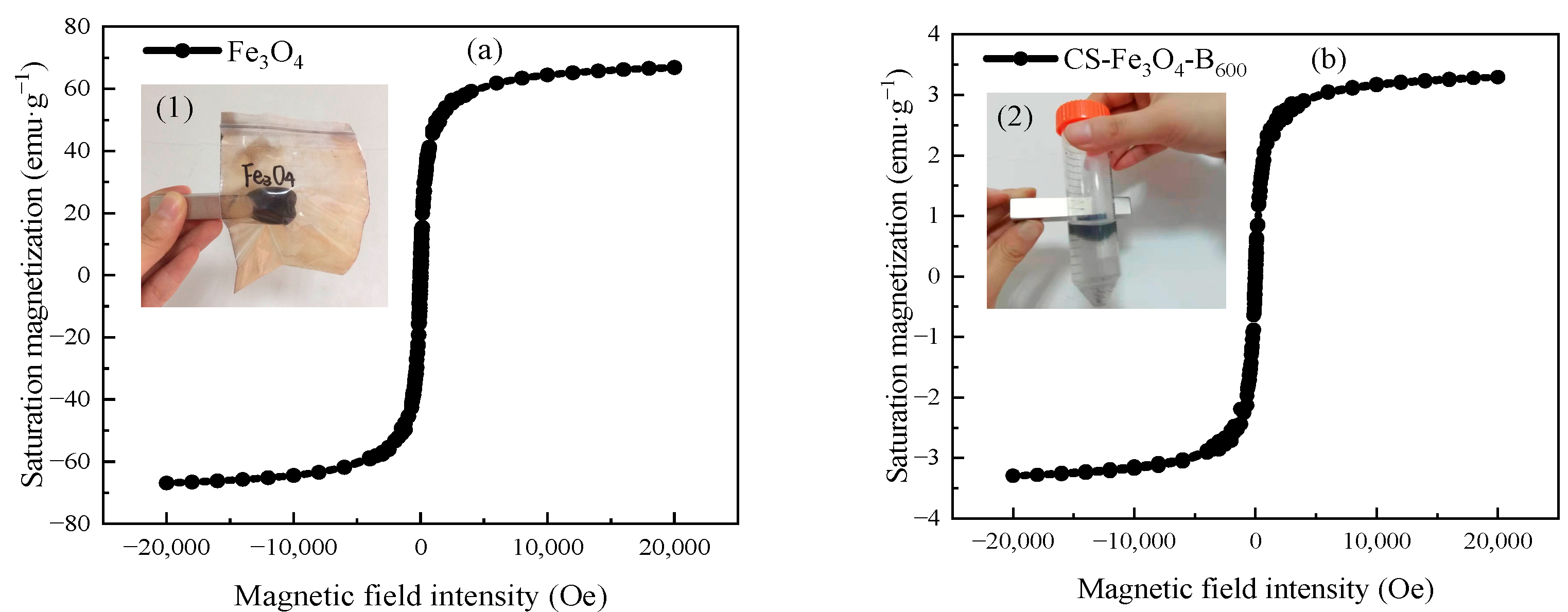
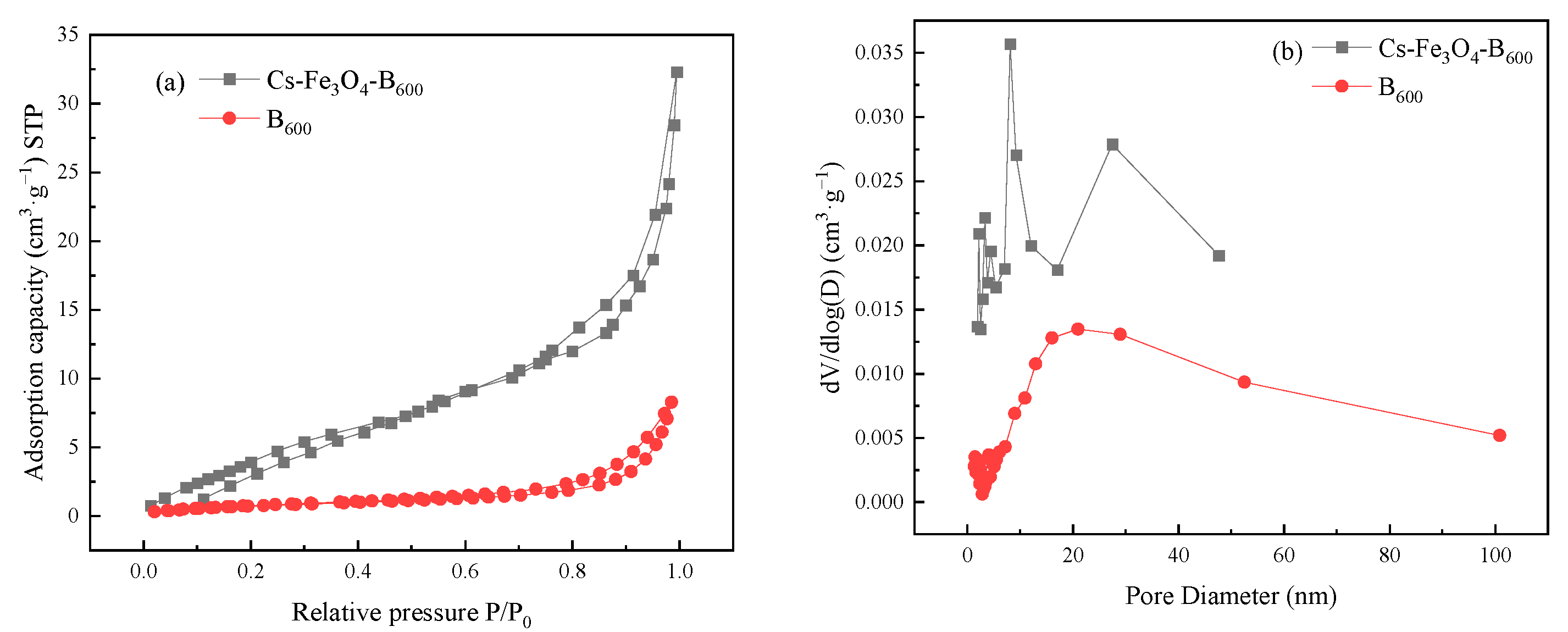
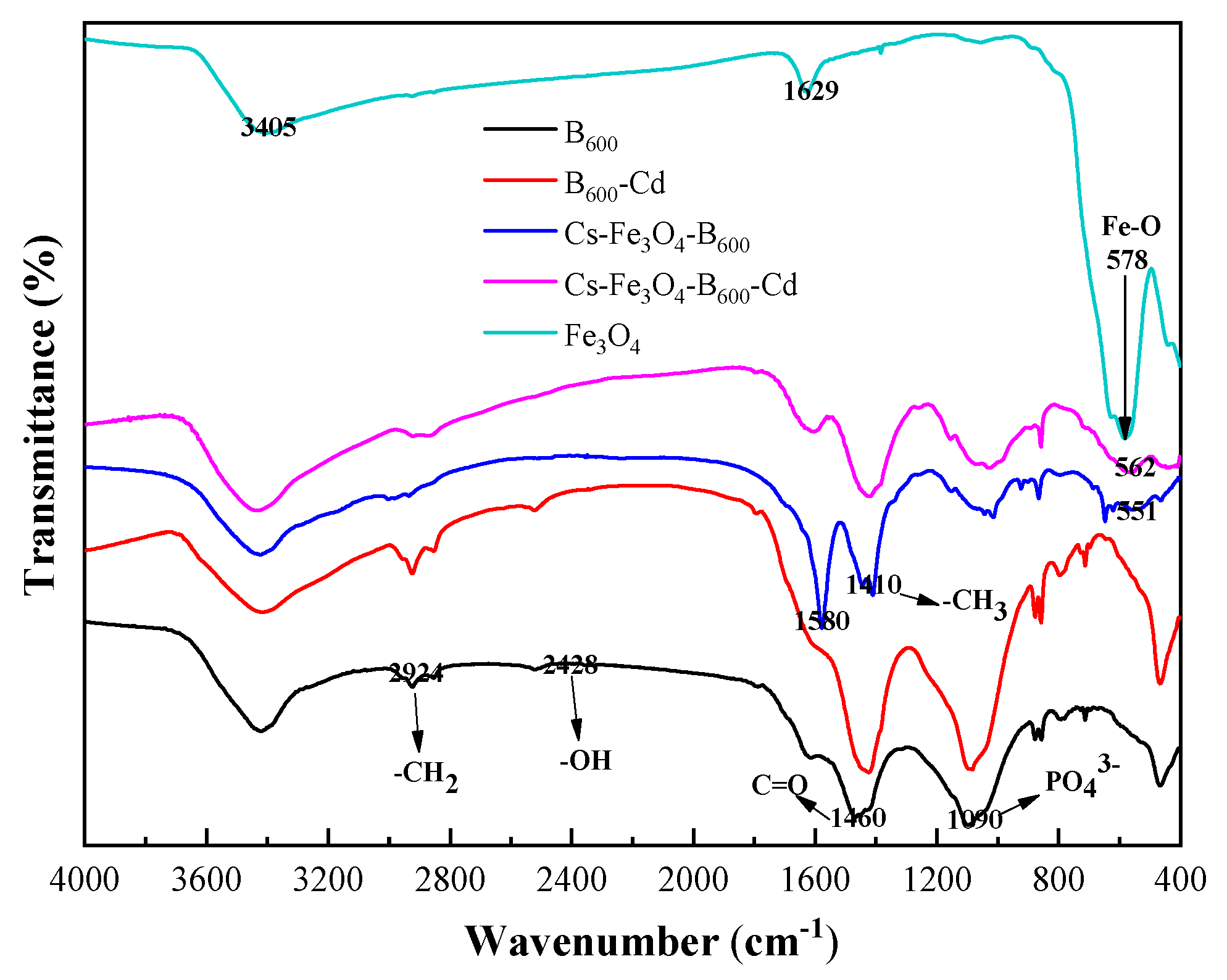
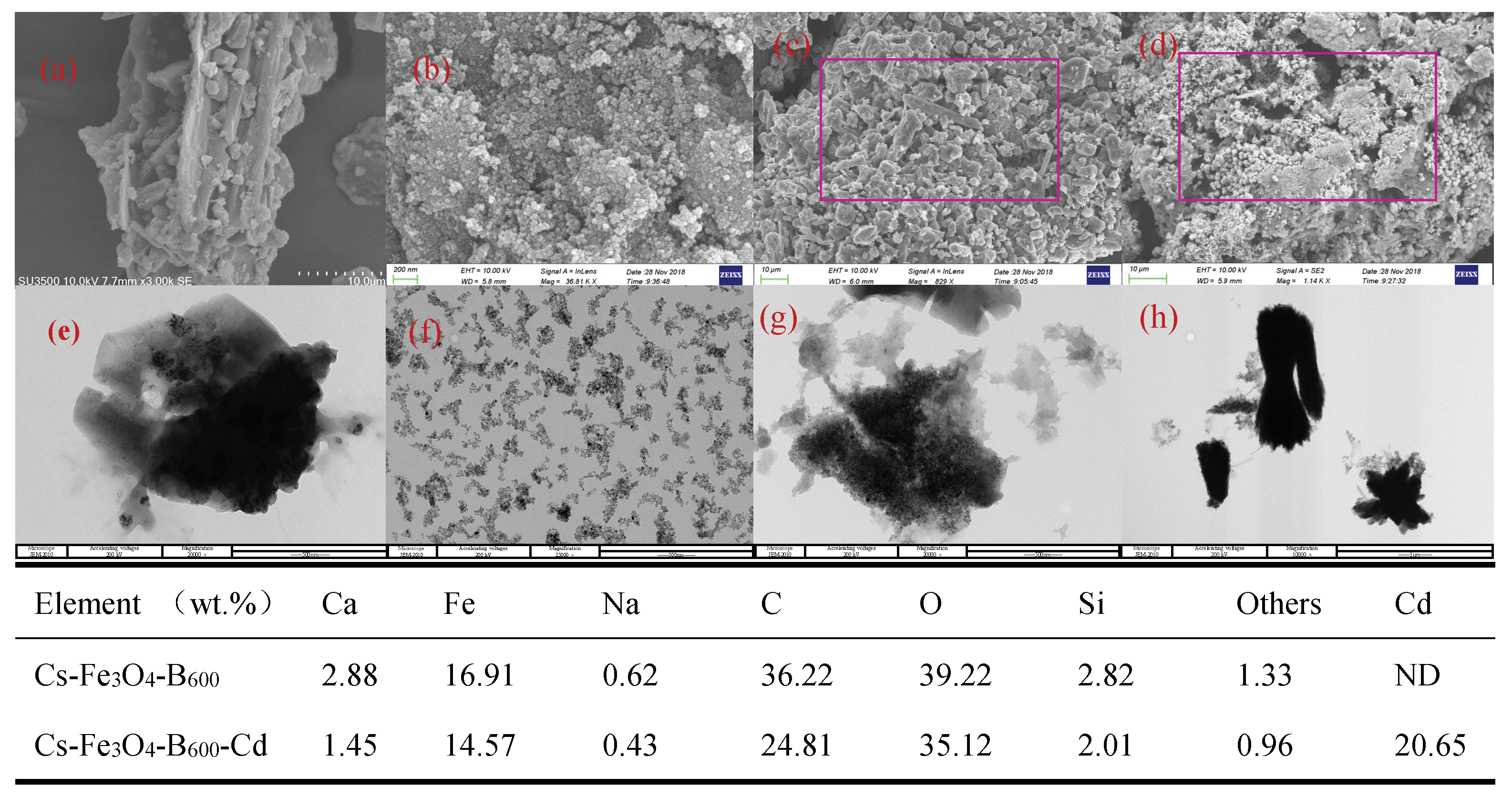
3.1. Characterization of Cs-Fe3O4-B600
3.2. The Performance of Cd(II) Adsorption on Cs-Fe3O4-B600
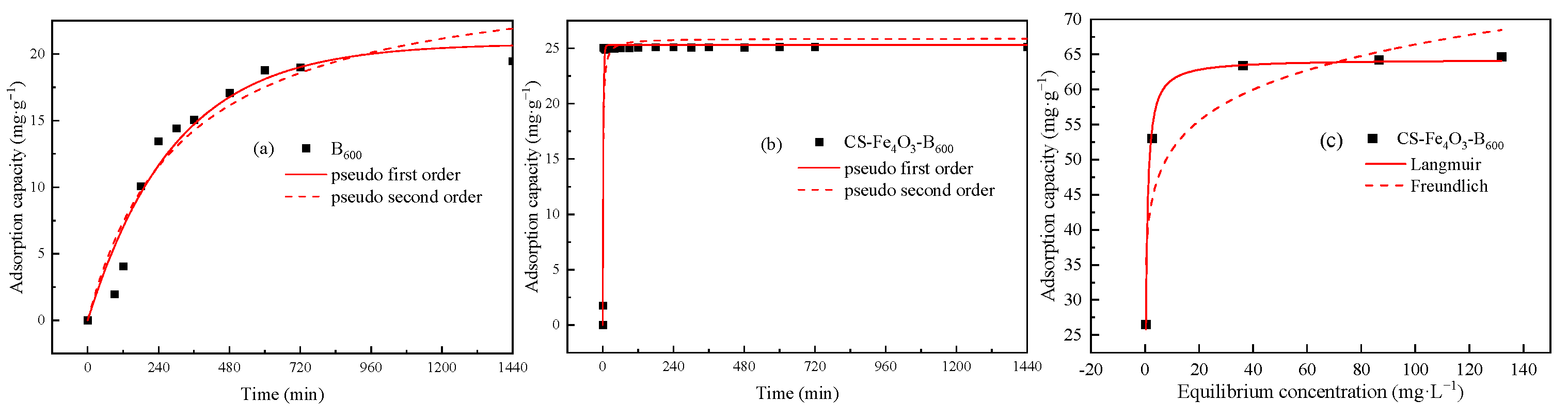
3.2.1. Adsorption Kinetic Characteristics
3.2.2. Adsorption Isotherm Characteristics
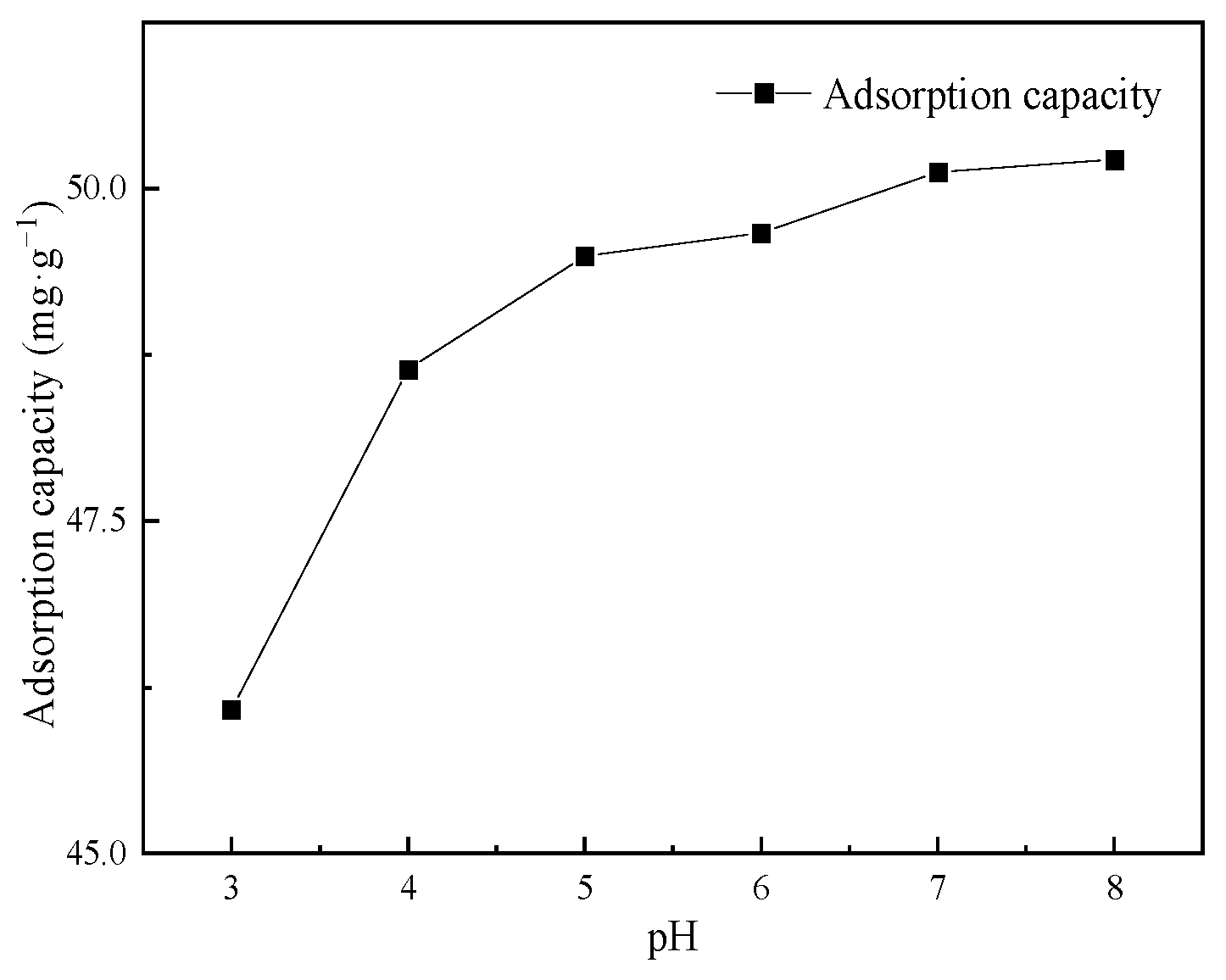
3.2.3. Influence of pH on Cd(II) Adsorption
3.3. Characterization of Cs-Fe3O4-B600 before and after Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Huang, Q.; Zhang, H.; Wang, Q.; Xue, R.; Guo, G.; Hu, J.; Li, T.; Wang, J.; Hu, S. Characterization of Biochars Produced by Co-Pyrolysis of Hami Melon (Cantaloupes) Straw Mixed with Polypropylene and Their Adsorption Properties of Cadmium. Int. J. Environ. Res. Public Health 2021, 18, 11413. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, M.; Wang, H.; Tang, D.; Huang, J.; Sun, Y. Study on the Remediation of Cd Pollution by the Biomineralization of Urease-Producing Bacteria. Int. J. Environ. Res. Public Health 2019, 16, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Yang, X.; Wang, P.; Wang, Z.; Li, M.; Zhao, F. Dietary cadmium intake from rice and vegetables and potential health risk: A case study in Xiangtan, southern China. Sci. Total Environ. 2018, 639, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Nio-Savala, A.G.; Mi, Z.D.; Wan, Y.N.; Fangmeier, A. Cadmium accumulation in wheat and maize grains from China: Interaction of soil properties, novel enrichment models and soil thresholds. Environ. Pollut. 2021, 275, 116–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, W.; Zhu, C.; Tang, X. Analysis of Heavy Metal Pollution in Cultivated Land of Different Quality Grades in Yangtze River Delta of China. Int. J. Environ. Res. Public Health 2021, 18, 9876. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, X.; Wang, X.; Feng, K.; Su, J.; Dong, J. The mechanism of cadmium sorption by sulphur-modified wheat straw biochar and its application cadmium-contaminated soil. Sci. Total Environ. 2020, 714, 136550. [Google Scholar] [CrossRef] [PubMed]
- Coutand, M.; Deydie, E.; Cyr, M.; Mouchet, F.; Gauthier, L.; Guilet, R.; Savaete, L.B.; Cren, S.; Clastres, P. Evaluation of laboratory and industrial meat and bone meal combustion residue as cadmium immobilizing material for remediation of polluted aqueous solutions: “Chemical and ecotoxicological studies”. J. Hazard. Mater. 2009, 166, 945–953. [Google Scholar] [CrossRef]
- Gupta, V.K.; Carrott, P.J.M.; Ribeiro Carrott, M.M.L.; Suhas. Low-Cost Adsorbents: Growing Approach to Wastewater Treatment—A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 783–842. [Google Scholar] [CrossRef]
- Merrikhpour, H.; Jalali, M. Waste calcite sludge as an adsorbent for the removal of cadmium, copper, lead, and zinc from aqueous solutions. Clean Technol. Environ. Policy 2012, 14, 845–855. [Google Scholar] [CrossRef]
- Nie, F.; Liu, R.; Zhou, Y.; Liu, Z. Research Advances of Lignocellulosic Wastes of the Adsorption of Metal Ions in Wastewater. Technol. Water Treat. 2016, 42, 12–19. [Google Scholar]
- Reynel-Avila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A. Relevance of anionic dye properties on water decolorization performance using bone char: Adsorption kinetics, isotherms and breakthrough curves. J. Mol. Liq. 2016, 219, 425–434. [Google Scholar] [CrossRef]
- Xu, Y.; Schwartz, F.W.; Traina, S.J. Sorption of Zn2+ and Cd2+ on hydroxyapatite surfaces. Environ. Sci. Technol. 1994, 28, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Dimović, S.; Smičiklas, I.; Plećaš, I.; Antonović, D.; Mitrić, M. Comparative study of differently treated animal bones for Co2+ removal. J. Hazard. Mater. 2009, 164, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Kizilkaya, B.; Tekinay, A.A.; Dilgin, Y. Adsorption and removal of Cu (II) ions from aqueous solution using pretreated fish bones. Desalination 2010, 264, 37–47. [Google Scholar] [CrossRef]
- Wang, D.; Iqbal, M.; Zhu, M.Q. Synthesis of Fe3O4 Magnetic Nanoparticles in a Helical Microreactor. Key Eng. Mater. 2020, 842, 174–181. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Liu, J.; Wang, W.; Zhang, W.; Dong, F. Adsorption of arsenic(V) on bone char: Batch, column and modeling studies. Environ. Earth Sci. 2014, 842, 174–181. [Google Scholar] [CrossRef]
- Moreno, J.C.; Gómez, R.; Giraldo, L. Removal of Mn, Fe, Ni and Cu Ions from Wastewater Using Cow Bone Charcoal. Materials 2010, 3, 452–466. [Google Scholar] [CrossRef] [Green Version]
- Sneddon, I.R.; Orueetxebarria, M.; Hodson, M.E.; Schofield, P.F.; Valsami-Jones, E. Use of bone meal amendments to immobilise Pb, Zn and Cd in soil: A leaching column study. Environ. Pollut. 2006, 144, 816–825. [Google Scholar] [CrossRef]
- Qin, R.; Li, F.; Chen, M.; Jiang, W. Preparation of chitosan-ethylenediaminetetraacetate-enwrapped magnetic CoFe2O4 nanoparticles via zero-length emulsion crosslinking method. Appl. Surf. Sci. 2009, 256, 27–32. [Google Scholar] [CrossRef]
- Srivastava, M.; Ojha, A.K.; Chaubey, S.; Singh, J. In-situ synthesis of magnetic (NiFe2O4/CuO/FeO) nanocomposites. J. Solid State Chem. 2010, 183, 2669–2674. [Google Scholar] [CrossRef]
- Covaliu, C.I.; Berger, D.; Matei, C.; Diamandescu, L.; Vasile, E.; Cristea, C.; Ionita, V.; Iovu, H. Magnetic nanoparticles coated with polysaccharide polymers for potential biomedical applications. J. Nanopart. Res. 2011, 13, 6169–6180. [Google Scholar] [CrossRef]
- Singh, J.; Srivastava, M.; Dutta, J.; Dutta, P.K. Preparation and properties of hybrid monodispersed magnetic α-Fe2O3 based chitosan nanocomposite film for industrial and biomedical applications. Int. J. Biol. Macromol. 2011, 48, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yao, W.; Cheng, Y.; Qian, H.; Wang, X.; Ding, Y.; Wu, W.; Jiang, X. Multifold enhanced T2 relaxation of ZnFe2O4 nanoparticles by jamming them inside chitosan nanospheres. J. Mater. Chem. 2012, 22, 5684–5693. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Varnoosfaderani, S.; Hebard, A.; Yao, Y.; Inyang, M. Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour. Technol. 2013, 130, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, M.; Zhang, L. Co-adsorption and sequential adsorption of the co-existence four heavy metal ions and three fluoroquinolones on the functionalized ferromagnetic 3D NiFe2O4 porous hollow microsphere. J. Colloid Interface Sci. 2018, 511, 135–144. [Google Scholar] [CrossRef]
- Gong, Y.; Gai, L.; Tang, J.; Fu, J.; Wang, Q.; Zeng, E.Y. Reduction of Cr(VI) in simulated groundwater by FeS-coated iron magnetic nanoparticles. Sci. Total Environ. 2017, 595, 743–751. [Google Scholar] [CrossRef]
- Shekari, H.; Sayadi, M.H.; Rezaei, M.R.; Allahresani, A. Synthesis of nickel ferrite/titanium oxide magnetic nanocomposite and its use to remove hexavalent chromium from aqueous solutions. Surf. Interfaces 2017, 8, 199–205. [Google Scholar] [CrossRef]
- Pisanic, T.R.; Blackwell, J.D.; Shubayev, V.I.; Fiñones, R.R.; Jin, S. Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials 2007, 28, 2572–2581. [Google Scholar] [CrossRef]
- Luo, L.; Li, Q.; Xu, Y.; Ding, Y.; Wang, X.; Deng, D.; Xu, Y. Amperometric glucose biosensor based on NiFe2O4 nanoparticles and chitosan. Sens. Actuat. B Chem. 2010, 145, 293–298. [Google Scholar] [CrossRef]
- Du, W.Q.; Cao, W.; Zhou, H.; Yang, W.T.; Gu, J.F.; Peng, P.Q.; Zhang, P.; Liao, B.H. Optimization and the mechanismin treatment of heavy metals wastewater with magnetic biochar. Acta Sci. Circumstantiae 2018, 38, 492–500. [Google Scholar]
- Hu, X.Y.; Chen, Y.J.; Zhang, S.S.; Wang, X.Q.; Li, C.C.; Guo, X. Cd removal from aqueous solution using magnetic biochar derived from maize straw and its recycle. Trans. Chin. Soc. Agric. Eng. 2018, 34, 208–218. [Google Scholar]
- Jiangxin, X.; Qintie, L.; Xiaosheng, Y.; Guangcai, Y. Removal of Cd from aqueous solution by chitosan coated MgO-biochar and its in-situ remediation of Cd-contaminated soil. Environ. Res. 2021, 195, 110650. [Google Scholar]
- Kumuduni, N.P.; Sok, K.; Avanthi, D.I.; Yohey, H.; Yoon, -E.C.; Raj, M.; Binoy, S.; Yong, S.O. Fe(III) loaded chitosan-biochar composite fibers for the removal of phosphate from water. J. Hazard. Mater. 2021, 415, 125464. [Google Scholar]
- Yuehui, T.; Xirui, W.; Xue, N.; Le, W.; Ting, Z.; Huimin, S.; Nong, W.; Xianqiang, Y. Efficient removal of Cd (II) from aqueous solution by chitosan modified kiwi branch biochar. Chemosphere 2022, 289, 133251. [Google Scholar]
- Reza, D.; Safari, M.; Maleki, A.; Rezaee, R.; Shahmoradi, B.; Shahmohammadi, S.; Ghahramani, E. Decontamination of arsenic (V)-contained liquid phase utilizing Fe3O4/bone char nanocomposite encapsulated in chitosan biopolymer. Environ. Sci. Pollut. Res. 2017, 24, 15157–15166. [Google Scholar]
- Mohammadi, A.; Barikani, M. Synthesis and characterization of superparamagnetic Fe3O4 nanoparticles coated with thiodiglycol. Mater. Charact. 2014, 90, 88–93. [Google Scholar] [CrossRef]
- Hu, B.; Wu, L.; Ou, M.; Wang, X.; Tang, Y. Sorption Studies of Chromium(VI) onto Cerium/Ferroferric Oxide Composites. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2627–2637. [Google Scholar] [CrossRef]
- Lan, F.; Liu, K.X.; Jiang, W.; Zeng, X.B.; Wu, Y.; Gu, Z.W. Facile synthesis of monodisperse superparamagnetic Fe3O4/PMMA composite nanospheres with high magnetization. Nanotechnology 2011, 22, 225604. [Google Scholar] [CrossRef]
- Huang, Z.; Tang, F. Preparation, structure, and magnetic properties of mesoporous magnetite hollow spheres. J. Colloid Interface Sci. 2005, 281, 432–436. [Google Scholar] [CrossRef]
- Dirgayanti, D.S.; Koesnarpadi, S.; Hindryawati, N. Synthesis and characterization of Fe3O4-Activated Carbon and it’s application to adsorb methylene blue. IOP Conf. Ser. Earth Environ. Sci. 2021, 623, 012070. [Google Scholar] [CrossRef]
- Dong, S.L.; Weng, T.; Wang, X.L.; Wang, H.X.; Liu, Y.J. Preprartion and characterization of Fe3O4/CS@CQDs bifuntional magnetic fluorescent composite. New Chem. Mater. 2018, 46, 121–124. [Google Scholar]
- Yuan, J.; Qian, Y.J.; Xue, G.; Zhang, Q.; LI, Q.; Liu, Z.H.; Li, X.Y. Removal of Cadmium and Lead in Water by Magnetic Carbon Prepared from Activated Sludge with Hydrothermal Carbonization. Environ. Eng. 2020, 38, 55–62. [Google Scholar]
- Zheng, X.Q.; Miao, T.B.; Zhang, C.; Ye, X.X.; Liu, M.H. Adsorption Behavior of Cr(VI) on Aminated Cyanoethyl Lignin Adsorbent. China Pulp Pap. 2015, 34, 37–43. [Google Scholar]
- Zhang, F.; Wang, X.; Xionghui, J.; Ma, L. Efficient arsenate removal by magnetite-modified water hyacinth biochar. Environ. Pollut. 2016, 216, 575–583. [Google Scholar] [CrossRef]
- Cao, W.; Zhou, H.; Deng, G.Y.; Du, W.Q. Effects and mechanisms of magnetic iron supported on rice husk biochar removing Pb2+ in wastewater. Chin. J. Environ. Eng. 2017, 11, 1437–1444. [Google Scholar]
- Du, C.; Song, Y.; Shi, S.; Jiang, B.; Yang, J.; Xiao, S. Preparation and characterization of a novel Fe3O4-graphene-biochar composite for crystal violet adsorption. Sci. Total Environ. 2020, 711, 134662. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.; Gu, J.; Huang, F.; Yang, W.; Wang, S.; Yuan, T.; Liao, B. Effects of nano-Fe3O4-modified biochar on iron plaque formation and Cd accumulation in rice (Oryza sativa L.). Environ. Pollut. 2020, 260, 113970. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.; Zhang, M.; Zhou, R.; Li, J.; Zhao, W.; Wang, L. Optimize the preparation of Fe3O4-modified magnetic mesoporous biochar and its removal of methyl orange in wastewater. Environ. Monit. Assess. 2021, 193, 179. [Google Scholar] [CrossRef]
- Li, Y.J.; Yang, Z.M.; Chen, Y.C.; Huang, L.; Tang, H.Y. Adsorption, Reclaim, and Regeneration of Cd by Magnetic Calcium Dihydrogen Phosphate Nanoparticles. Environ. Sci. 2019, 40, 1849–1856. [Google Scholar]
- Alqadami, A.A.; Khan, M.A.; Otero, M.; Siddiqui, M.R.; Jeon, B.; Batoo, K.M. A magnetic nanocomposite produced from camel bones for an efficient adsorption of toxic metals from water. J. Clean. Prod. 2018, 178, 293–304. [Google Scholar] [CrossRef]
- Lu, S.; Gibb, S.W. Copper removal from wastewater using spent-grain as biosorbent. Bioresour. Technol. 2008, 99, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Tekin, N.; Şafaklı, A.; Bingöl, D. Process modeling and thermodynamics and kinetics evaluation of Basic Yellow 28 adsorption onto sepiolite. Desalination Water Treat. 2015, 54, 2023–2035. [Google Scholar] [CrossRef]
- Zheng, L.; Gao, Y.; Du, J.; Zhang, W.; Huang, Y.; Zhao, Q.; Duan, L.; Liu, Y.; Naidu, R.; Pan, X. Single and Binary Adsorption Behaviour and Mechanisms of Cd2+, Cu2+ and Ni2+ onto Modified Biochar in Aqueous Solutions. Processes 2021, 9, 1829. [Google Scholar] [CrossRef]
- Yan, L.; Li, S.; Yu, H.; Shan, R.; Du, B.; Liu, T. Facile solvothermal synthesis of Fe3O4/bentonite for efficient removal of heavy metals from aqueous solution. Powder Technol. 2016, 301, 632–640. [Google Scholar] [CrossRef]
- Xiao, S.G.; Liu, Y.X.; Yu, X.J.; Jin, G.Z.; Xiao, X.X. Adsorption and Desorption of Cd2+ on Activated Carbon Modified by Glutamate Coupling Chitosan. Inn. Mong. Ind. 2015, 41, 6–8. [Google Scholar]
- Tan, Z.; Wang, Y.; Kasiulienė, A.; Huang, C.; Ai, P. Cadmium removal potential by rice straw-derived magnetic biochar. Clean Technol. Environ. Policy 2017, 19, 761–774. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitosan composites with inorganics, morphogenetic proteins and stem cells, for bone regeneration. Carbohyd. Polym. 2011, 83, 1433–1445. [Google Scholar] [CrossRef]
- Cheng, S.S.; Yang, X.H.; Zhang, C.H.; Xie, W.C.; Qin, X.M.; Lu, H.Y. Adsorption of Cd2+ and Pb2+ by Chitosan. J. Fish. China 2011, 35, 410–416. [Google Scholar]
- Yan, S.Q.; Zhang, H.C.; Fu, H.; Pan, Y.J. Preparation and adsorption properties of magnetic chitosan adsorbent for heavy metals. J. Shanghai Ocean. Univ. 2018, 27, 739–747. [Google Scholar]
- Wang, C.X.; Wang, L.X.; Yang, W.L. Preparation and characterization of functional inorganic/organic composite microspheres via electrostatic interaction. J. Colloid Interface Sci. 2009, 333, 749–756. [Google Scholar] [CrossRef]
- Ghafoor, S.; Ata, S. Synthesis of carboxyl-modified Fe3O4@SiO2 nanoparticles and their utilization for the remediation of cadmium and nickel from aqueous solution. J. Chil. Chem. Soc. 2017, 62, 3588–3592. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.L.; Peng, Q.; Wang, X.; Chen, J.P.; Li, Y.D. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 2010, 44, 2782–2785. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.X.; Liu, G.X.; Dong, X.T.; Wang, J.X.; Xu, J.; Yu, W.S. Preparation and characteristics of Fe3O4 @YVO4:Eu3+ bifunctional magnetic-luminescent nanocomposites. J. Alloys Compd. 2011, 509, 6930–6934. [Google Scholar] [CrossRef]
- Shang, J.; Zhao, J. Effect of chitosan modification on Pb(II) and Cd(II) adsorption by magnetic Fe3O4. Environ. Pollut. Control 2017, 39, 746–751. [Google Scholar]
| Materials | C0 (mg·L−1) | qe, exp (mg·g−1) | Pseudo-First-Order Kinetics Model | Pseudo-Second-Order Kinetics Model | ||||
|---|---|---|---|---|---|---|---|---|
| qe, cal (mg·g−1) | k1 (1·min−1) | R2 | qe, cal (mg·g−1) | k2 (g·mg−1·min−1) | R2 | |||
| B600 | 150 | 19.487 | 20.792 | 0.0034 | 0.9385 | 26.696 | 0.00001 | 0.9081 |
| Cs-Fe3O4-B600 | 150 | 25.134 | 25.284 | 0.4825 | 0.8989 | 25.871 | 0.0302 | 0.8305 |
| Adsorption Isotherm Model | Parameter | Adsorbent | Adsorbent |
|---|---|---|---|
| Cs-Fe3O4-B600 | B600 | ||
| Langmuir | qm (mg·g−1) | 64.310 | 37.799 |
| KL (L·mg−1) | 2.0890 | 0.0591 | |
| R2 | 0.8653 | 0.9892 | |
| Freundlich | KF (mg·g−1(L·g−1)1/n) | 39.804 | 7.4522 |
| n | 8.9967 | 3.1546 | |
| R2 | 0.7704 | 0.9362 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Luo, W.; Sun, T.; Xu, Y.; Sun, Y. Adsorption Performance of Cd(II) by Chitosan-Fe3O4-Modified Fish Bone Char. Int. J. Environ. Res. Public Health 2022, 19, 1260. https://doi.org/10.3390/ijerph19031260
Yang W, Luo W, Sun T, Xu Y, Sun Y. Adsorption Performance of Cd(II) by Chitosan-Fe3O4-Modified Fish Bone Char. International Journal of Environmental Research and Public Health. 2022; 19(3):1260. https://doi.org/10.3390/ijerph19031260
Chicago/Turabian StyleYang, Wenhao, Wenwen Luo, Tong Sun, Yingming Xu, and Yuebing Sun. 2022. "Adsorption Performance of Cd(II) by Chitosan-Fe3O4-Modified Fish Bone Char" International Journal of Environmental Research and Public Health 19, no. 3: 1260. https://doi.org/10.3390/ijerph19031260
APA StyleYang, W., Luo, W., Sun, T., Xu, Y., & Sun, Y. (2022). Adsorption Performance of Cd(II) by Chitosan-Fe3O4-Modified Fish Bone Char. International Journal of Environmental Research and Public Health, 19(3), 1260. https://doi.org/10.3390/ijerph19031260