Stabilization of Zinc in Agricultural Soil Originated from Commercial Organic Fertilizer by Natural Zeolite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Soil, Organic Fertilizer and Natural Zeolite
2.2. Experimental Design
2.3. Soil and Plant Analysis
2.3.1. Soil pH and CEC Value
2.3.2. Zn Concentration in Plants
2.3.3. Chemical Speciation of Zn in Soil
2.3.4. Diffusive Gradients in Thin Films (DGT) Extractable Zn in Soil
2.4. Data Analysis
3. Result
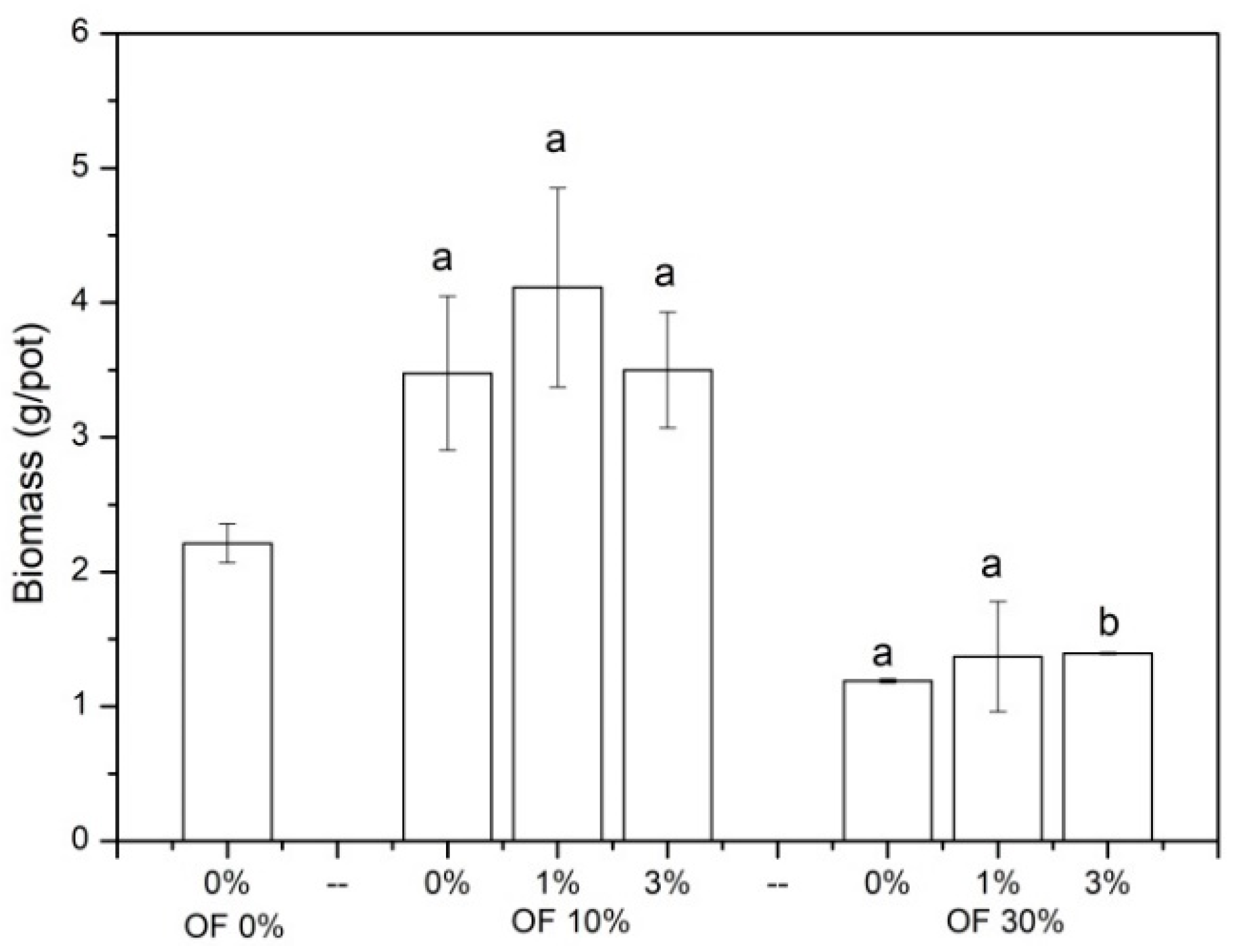
3.1. The Growth of Chinese Cabbage
3.2. Soil Properties
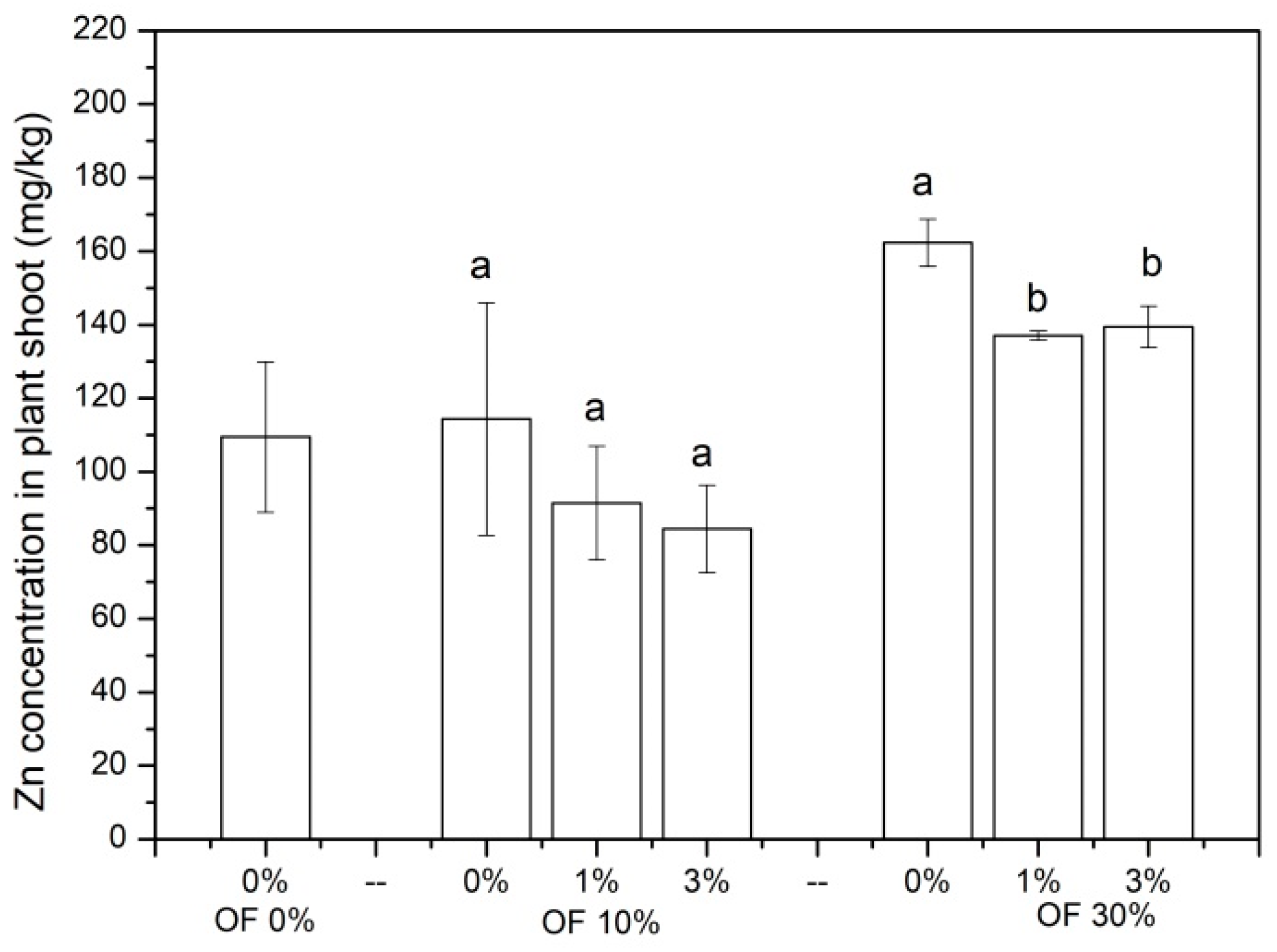
3.3. Zn Concentration in Chinese Cabbage Shoots
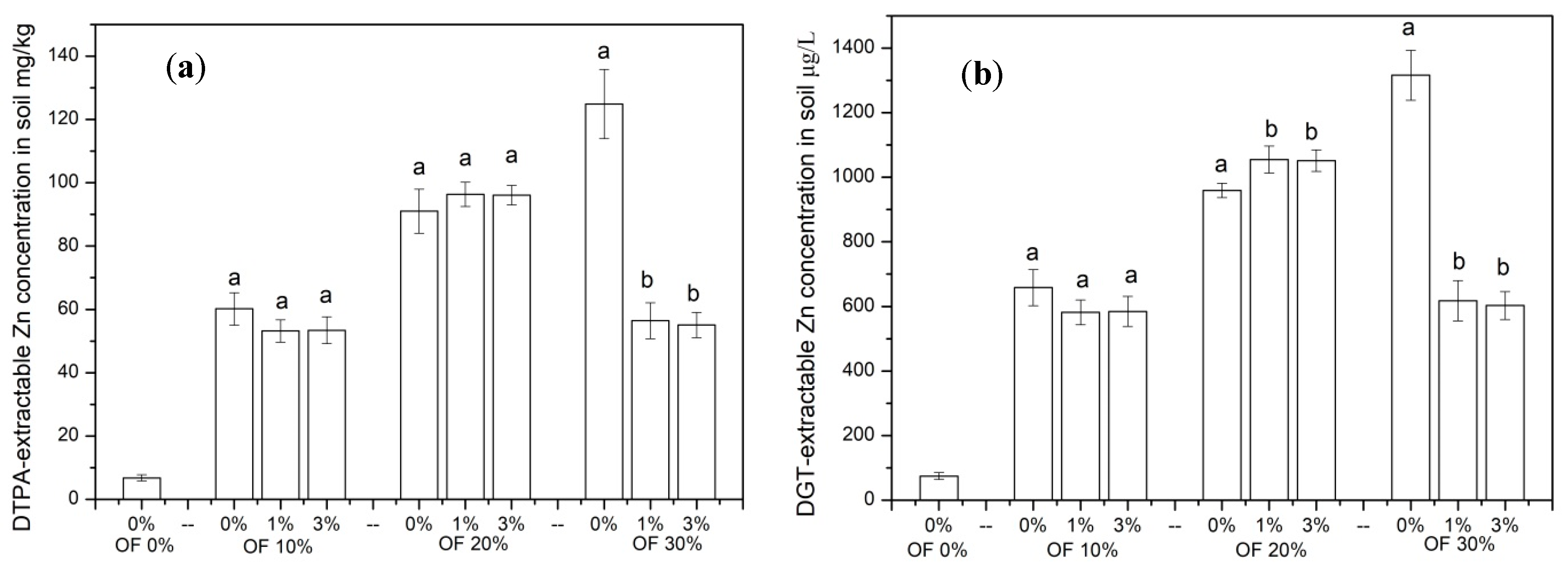
3.4. The Concentration of Zn in the Soil Extracted by Single-Step Chemical Extraction and DGT
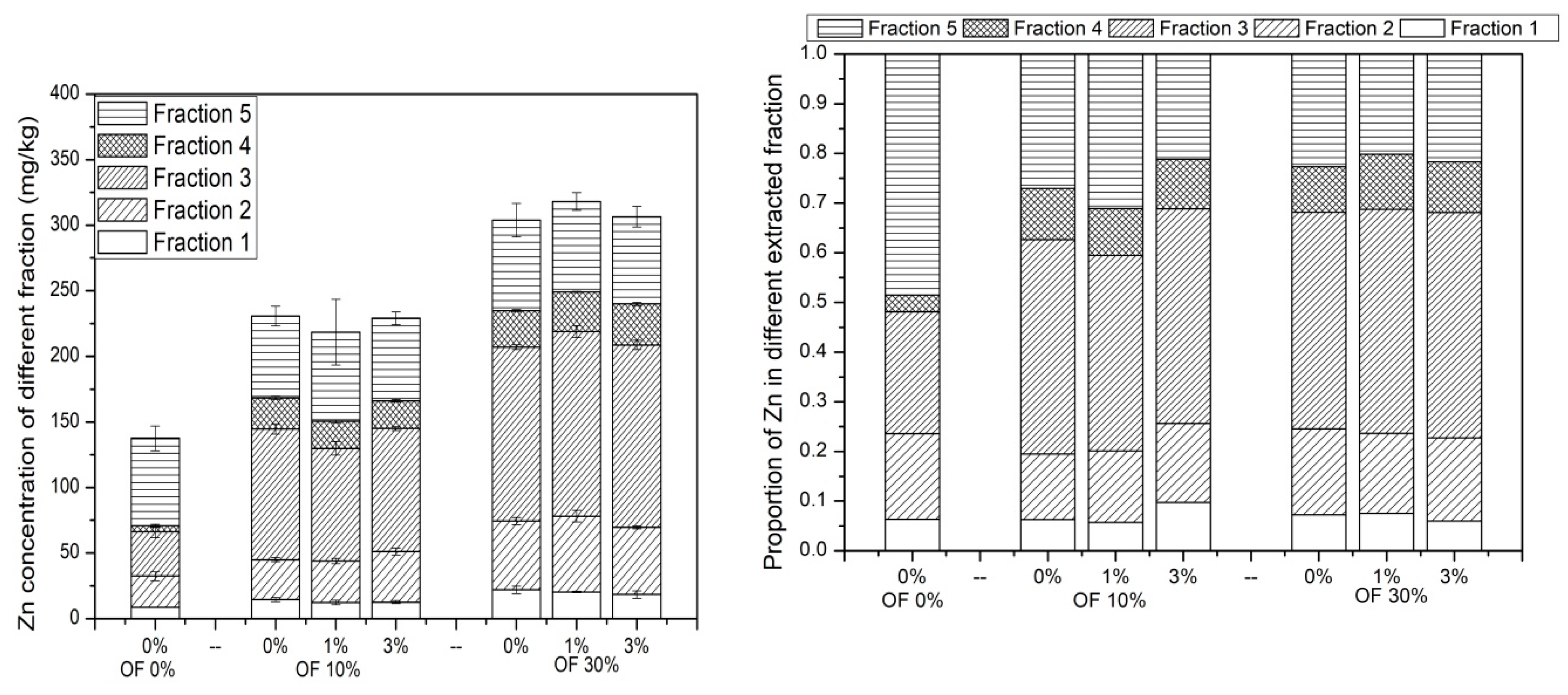
3.5. Chemical Speciation of Zn in Soil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yearbook, C.A.H. Chinese Animal Husbandry Yearbook; China Agriculture Press: Beijing, China, 2006. [Google Scholar]
- Liu, S.; Cai, Y.; Zhu, H.; Tan, Z. Potential and constraints in the development of animal industries in China. J. Sci. Food Agric. 2012, 92, 1025–1030. [Google Scholar] [CrossRef]
- Weber, J.; Karczewska, A.; Drozd, J.; Licznar, M.; Licznar, S.; Jamroz, E.; Kocowicz, A. Agricultural and ecological aspects of a sandy soil as affected by the application of municipal solid waste composts. Soil Biol. Biochem. 2007, 39, 1294–1302. [Google Scholar] [CrossRef]
- Baker, L.R.; White, P.M.; Pierzynski, G.M. Changes in microbial properties after manure, lime, and bentonite application to a heavy metal-contaminated mine waste. Appl. Soil Ecol. 2011, 48, 1–10. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, W.; Ma, Q.; Zhou, H. Accumulation of copper and zinc in soil and plant within ten-year application of different pig manure rates. Plant Soil Environ. 2013, 59, 492–499. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Yu, X.; Zhang, M.; Lu, S.; Wu, W.; Wu, J.; Xu, J. Potential risks of copper, zinc, and cadmium pollution due to pig manure application in a soil-rice system under intensive farming: A case study of Nanhu, China. J. Environ. Qual. 2011, 40, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Patrycja, B.; Zofia, S.; Leonardo, F. Interactions of Zn(II) Ions with Humic Acids Isolated from Various Type of Soils. Effect of pH, Zn Concentrations and Humic Acids Chemical Properties. PLoS ONE 2016, 11, e0153626. [Google Scholar]
- Flemming, C.A.; Trevors, J.T. Copper toxicity and chemistry in the environment: A review. Water Air Soil Pollut. 1989, 44, 143–158. [Google Scholar] [CrossRef]
- Mohamed, M.M. Heat capacities, phase transitions and structural properties of cation-exchanged H-mordenite zeolites. Thermochim. Acta 2001, 372, 75–83. [Google Scholar] [CrossRef]
- Shahbaz, A.K.; Adnan Ramzani, P.M.; Saeed, R.; Turan, V.; Iqbal, M.; Lewińska, K.; Abbas, F.; Saqib, M.; Tauqeer, H.M.; Iqbal, M.; et al. Effects of biochar and zeolite soil amendments with foliar proline spray on nickel immobilization, nutritional quality and nickel concentrations in wheat. Ecotoxicol. Environ. Saf. 2019, 173, 182–191. [Google Scholar] [CrossRef]
- Wen, J.; Yi, Y.; Zeng, G. Effects of modified zeolite on the removal and stabilization of heavy metals in contaminated lake sediment using BCR sequential extraction. J. Environ. Manag. 2016, 178, 63–69. [Google Scholar] [CrossRef]
- Zeng, W.A.; Hu, Q.L.; Li, F.; Gu, S.S.; Huang, Y.N.; Cai, H.L.; Zeng, M.; Li, Q.; Tan, L. Effects of Application of Silicon and Zeolite on Chemical Speciation of Cadmium in Soil and its Uptake by Tobacco. Int. J. Agric. Biol. 2018, 20, 452–456. [Google Scholar] [CrossRef]
- Quevauviller, P. Operationally defined extraction procedures for soil and sediment analysis I. Standardization. TrAC Trends Anal. Chem. 1998, 17, 289–298. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Dalorima, T.; Sakimin, S.Z.; Shah, R.M. Utilization of organic fertilisers a potential approaches for agronomic crops: A review. Plant Sci. Today 2021, 8, 190–196. [Google Scholar] [CrossRef]
- Cai, A.D.; Zhang, W.J.; Xu, M.G.; Wang, B.; Wen, S.; Shah, S.A. Soil fertility and crop yield after manure addition to acidic soils in South China. Nutr. Cycl. Agroecosyst. 2018, 111, 61–72. [Google Scholar] [CrossRef]
- Oustani, M.; Tahar Halilat, M.; Haroun, C. Effect of poultry manure on the yield and nutriments uptake of potato under saline conditions of arid regions. Emir. J. Food Agric. 2015, 27, 106–120. [Google Scholar] [CrossRef]
- Boateng, S.A.; Zickermann, J.; Kornahrens, M. Poultry manure effect on growth and yield of maize. West Afr. J. Appl. Ecol. 2006, 9, 1–11. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Yu, T.H.; Yang, X.; Li, H.S. Speciation, Transformation, and Accumulation of Manure-derived Cu and Zn in the Soil-Rice System. Soil Sediment Contam. 2020, 29, 43–52. [Google Scholar] [CrossRef]
- Agbede:, T.M.; Adekiya, A.O. Evaluation of sweet potato (Ipomoea batatas L.) performance and soil properties under tillage methods and poultry manure levels. Emir. J. Food Agric. 2011, 23, 164–177. [Google Scholar]
- Saxena, M.C.; Yadav, D.S. Parallel cropping of soybean with pigeonpea under humid sub-tropical conditions of Pantnagar. Indian J. Agron. 1976, 49, 95–99. [Google Scholar]
- Hue, N.V. Correcting soil acidity of a highly weathered Ultisol with chicken manure and sewage sludge. Commun. Soil Sci. Plant Anal. 1992, 23, 241–264. [Google Scholar] [CrossRef]
- Warren, S.L.; Fonteno, W.C. Changes in physical and chemical properties of a loamy sand soil when amended with composted poultry litter. J. Environ. Hortic. 1993, 11, 186–190. [Google Scholar] [CrossRef]
- Whalen, J.K.; Chang, C.; Clayton, G.W.; Carefoot, J.P. Cattle Manure Amendments Can Increase the pH of Acid Soils. Soil Sci. Soc. Am. J. 2000, 64, 962–966. [Google Scholar] [CrossRef] [Green Version]
- Rillig, M.C.; Wagner, M.; Salem, M.; Antunes, P.M.; George, C.; Ramke, H.G.; Titirici, M.M.; Antonietti, M. Material derived from hydrothermal carbonization: Effects on plant growth and arbuscular mycorrhiza. Appl. Soil Ecol. 2010, 45, 238–242. [Google Scholar] [CrossRef]
- Guo, Z.; Han, J.; Li, J.; Xu, Y.; Wang, X. Effects of long-term fertilization on soil organic carbon mineralization and microbial community structure. PLoS ONE 2019, 14, e0211163. [Google Scholar]
- Querol, X.; Alastuey, A.; Moreno, N.; Alvarez-Ayuso, E.; García-Sánchez, A.; Cama, J.; Ayora, C.; Simón, M. Immobilization of heavy metals in polluted soils by the addition of zeolitic material synthesized from coal fly ash. Chemosphere 2006, 62, 171–180. [Google Scholar] [CrossRef]
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, H.M.; Massadeh, A.M.; Younes, H.A. Natural Jordanian zeolite: Removal of heavy metal ions from water samples using column and batch methods. Environ. Monit. Assess. 2009, 157, 319–330. [Google Scholar] [CrossRef]
- Bao, W.; Zou, H.; Gan, S.; Xu, X.; Ji, G.; Zheng, K. Adsorption of heavy metal ions from aqueous solutions by zeolite based on oil shale ash: Kinetic and equilibrium studies. Chem. Res. Chin. Univ. 2013, 29, 126–131. [Google Scholar] [CrossRef]
- He, K.; Chen, Y.; Tang, Z.; Hu, Y. Removal of heavy metal ions from aqueous solution by zeolite synthesized from fly ash. Environ. Sci. Pollut. Res. 2016, 23, 2778–2788. [Google Scholar] [CrossRef] [PubMed]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Li, S.; Gong, P.; Song, K.; Zhang, H.; Sun, Y.; Qin, Q.; Zhou, B.; Xue, Y. Stabilization of Zinc in Agricultural Soil Originated from Commercial Organic Fertilizer by Natural Zeolite. Int. J. Environ. Res. Public Health 2022, 19, 1210. https://doi.org/10.3390/ijerph19031210
Sun L, Li S, Gong P, Song K, Zhang H, Sun Y, Qin Q, Zhou B, Xue Y. Stabilization of Zinc in Agricultural Soil Originated from Commercial Organic Fertilizer by Natural Zeolite. International Journal of Environmental Research and Public Health. 2022; 19(3):1210. https://doi.org/10.3390/ijerph19031210
Chicago/Turabian StyleSun, Lijuan, Shuangxi Li, Peiyun Gong, Ke Song, Hong Zhang, Yafei Sun, Qin Qin, Bin Zhou, and Yong Xue. 2022. "Stabilization of Zinc in Agricultural Soil Originated from Commercial Organic Fertilizer by Natural Zeolite" International Journal of Environmental Research and Public Health 19, no. 3: 1210. https://doi.org/10.3390/ijerph19031210
APA StyleSun, L., Li, S., Gong, P., Song, K., Zhang, H., Sun, Y., Qin, Q., Zhou, B., & Xue, Y. (2022). Stabilization of Zinc in Agricultural Soil Originated from Commercial Organic Fertilizer by Natural Zeolite. International Journal of Environmental Research and Public Health, 19(3), 1210. https://doi.org/10.3390/ijerph19031210





