The Role of the Ecotoxicology Applied to Seafood as a Tool for Human Health Risk Assessments Concerning Polycyclic Aromatic Hydrocarbons
Abstract
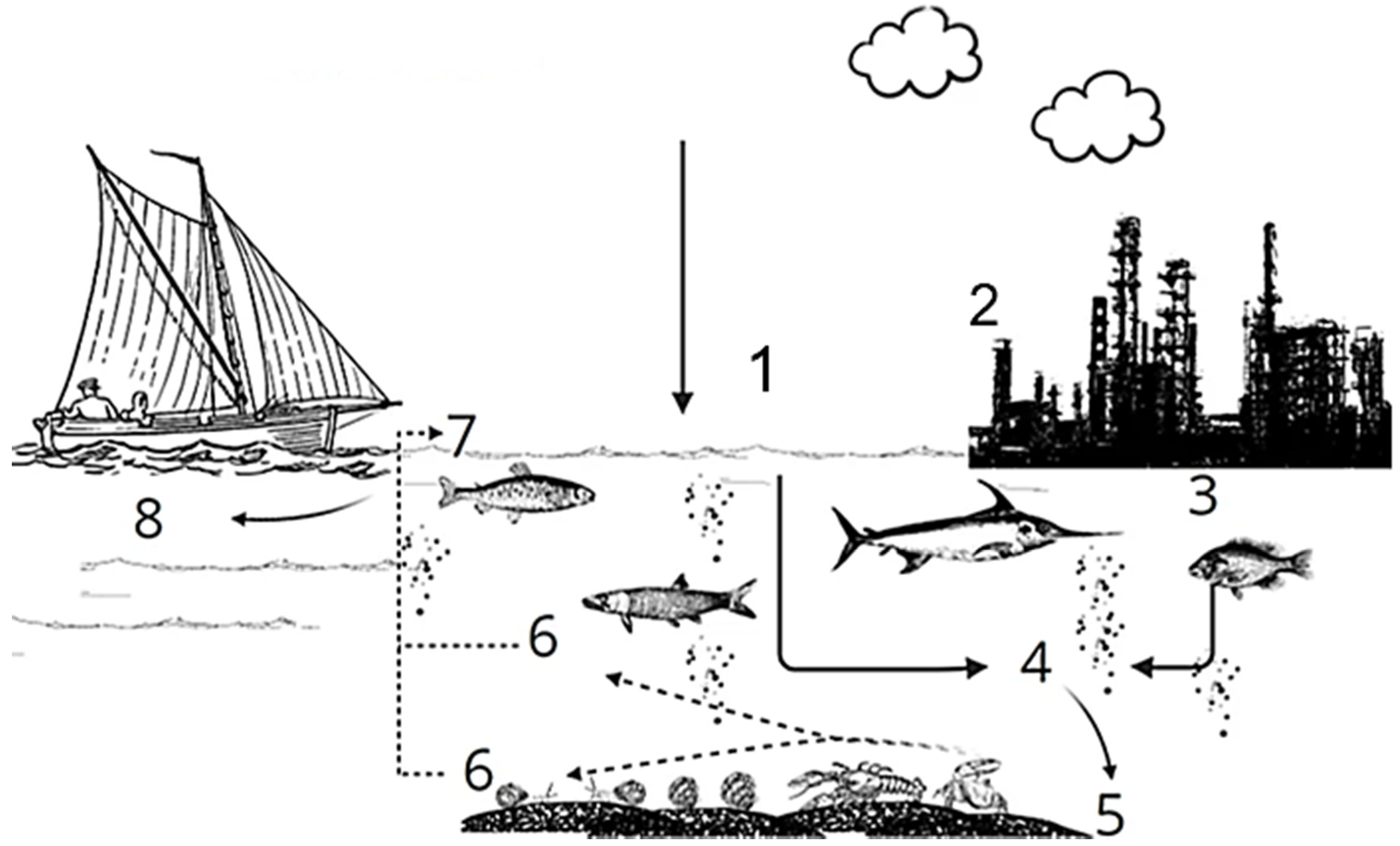
1. Introduction
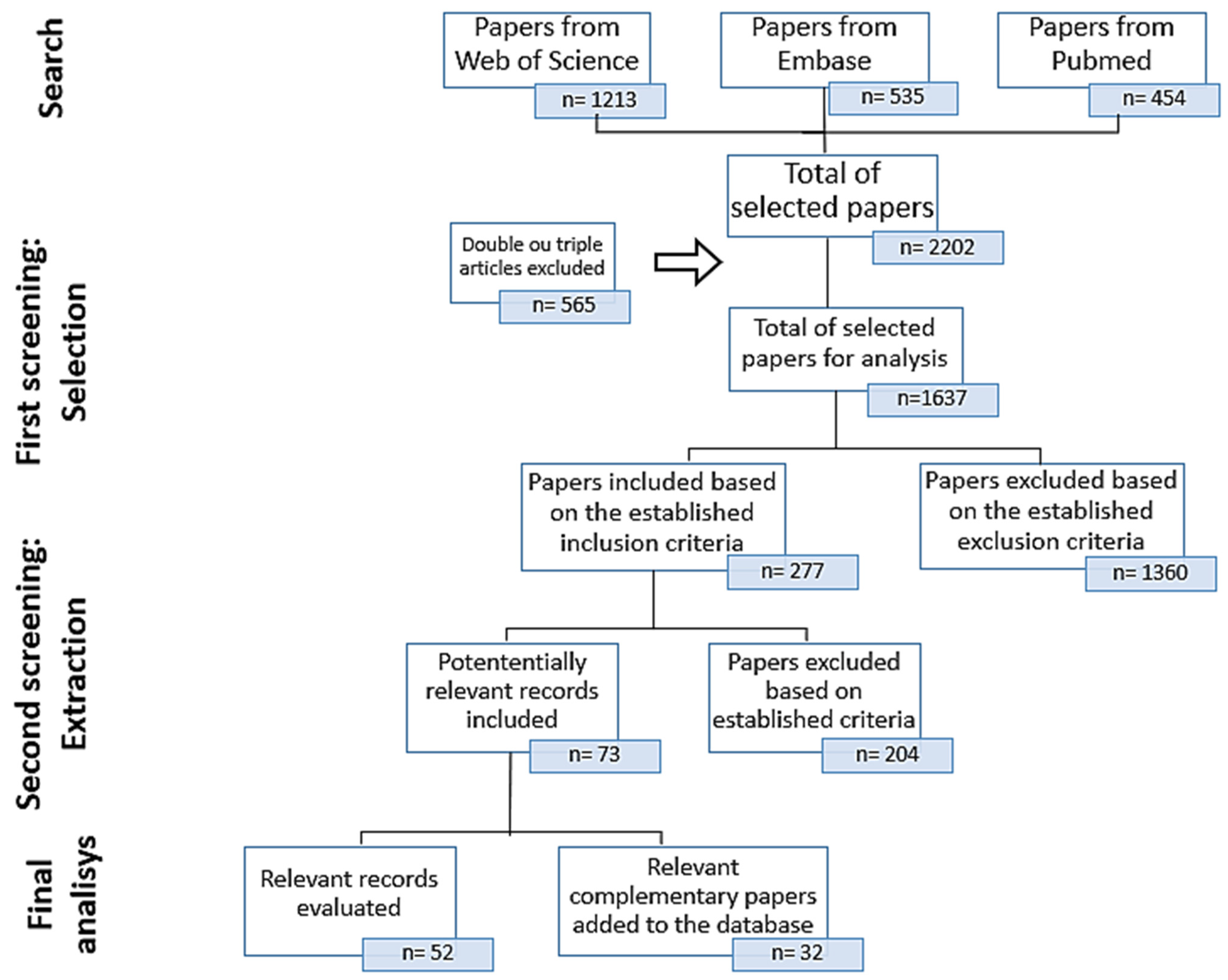
2. Materials and Methods
2.1. Focus Question
2.2. Information Sources
- Search Component 1 (SC1): Population: “Arthropoda” OR “Arthropods” OR “Crustacean” OR “fish” OR “Fish products” OR “Fish culture” OR “Clams” OR “Mussel” OR “Bivalvia.”
- Search Component 2 (SC2): “Ecotoxicology” OR “Environmental toxicology” OR “Effects” OR “Toxicity” OR “Toxicology” OR “Bioassay” OR “Lethal concentration” OR “Effect concentration” OR “Environmental health” OR “Marine toxicity” OR “Aquatic toxicity” OR “Human health” OR “Human risk”.
- Search Component 3 (SC3): “pah” OR PAH OR “Polycyclic aromatic hydrocarbons” OR “Polynuclear aromatic hydrocarbon.”
- Search Component 4 (SC4): AND NOT (“detection” OR “collected”)
- Ecological risk assessments based on PAH distributions in the aquatic or marine environment;
- Oil spills or oil products and their effects on biota;
- Epidemiological studies to determine the incidence/prevalence of diseases associated with the consumption of contaminated fish;
- Development or applications risk assessment methodologies disregarding the effects presented by investigated organisms (animals, plants, and humans) subjected to specific PAH exposure concentrations.
- Assessments concerning PAH effects on test organisms under controlled conditions;
- Assessments indicating the tested organism, the PAH, test/effect concentration, and the evaluated endpoint;
- Evaluations concerning animals sampled from the environment or kept in the laboratory for laboratory exposures;
- Indications of assay time and affected biomarkers or physiological or morphological alterations.
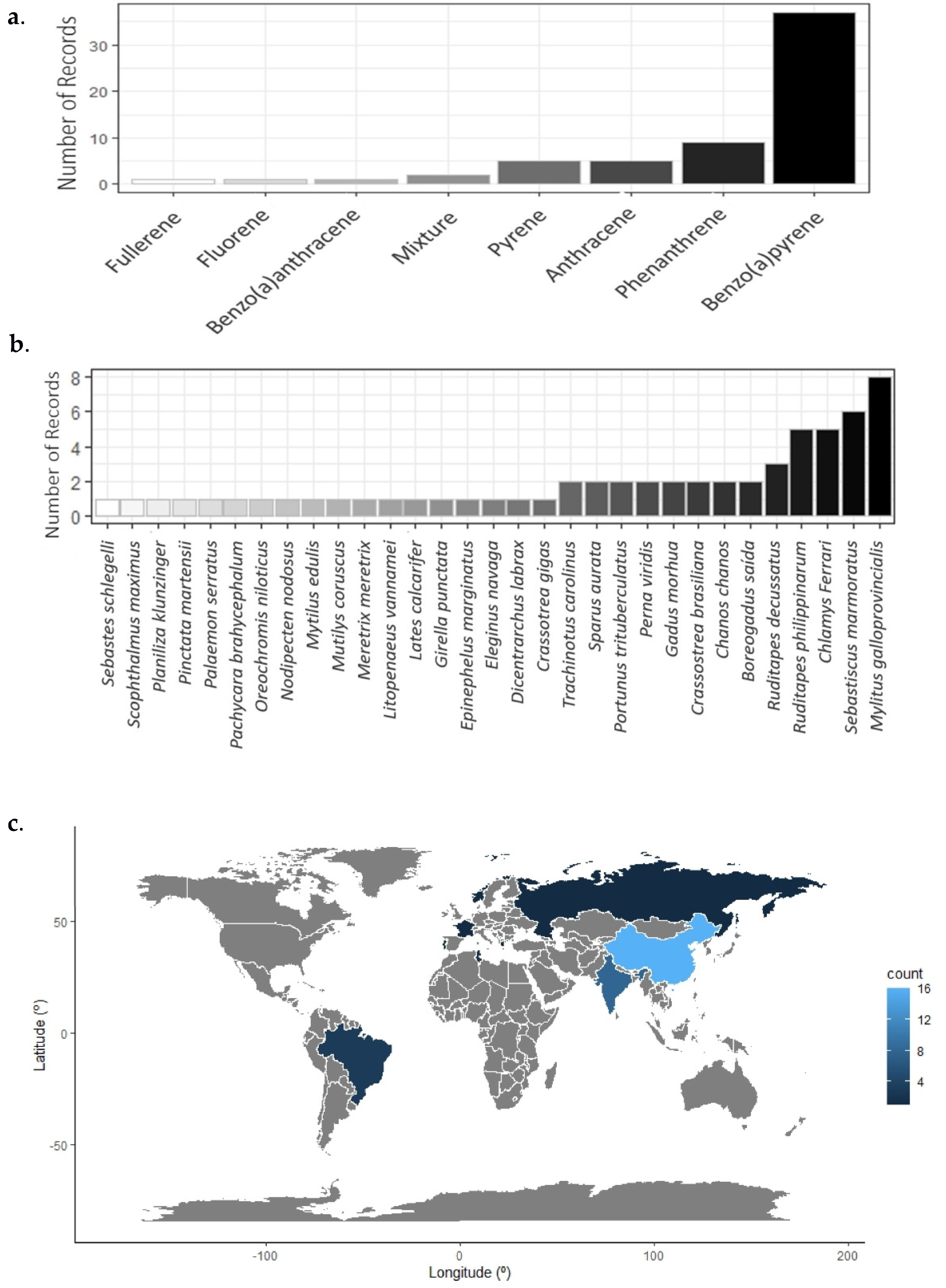
3. Results
4. Discussion
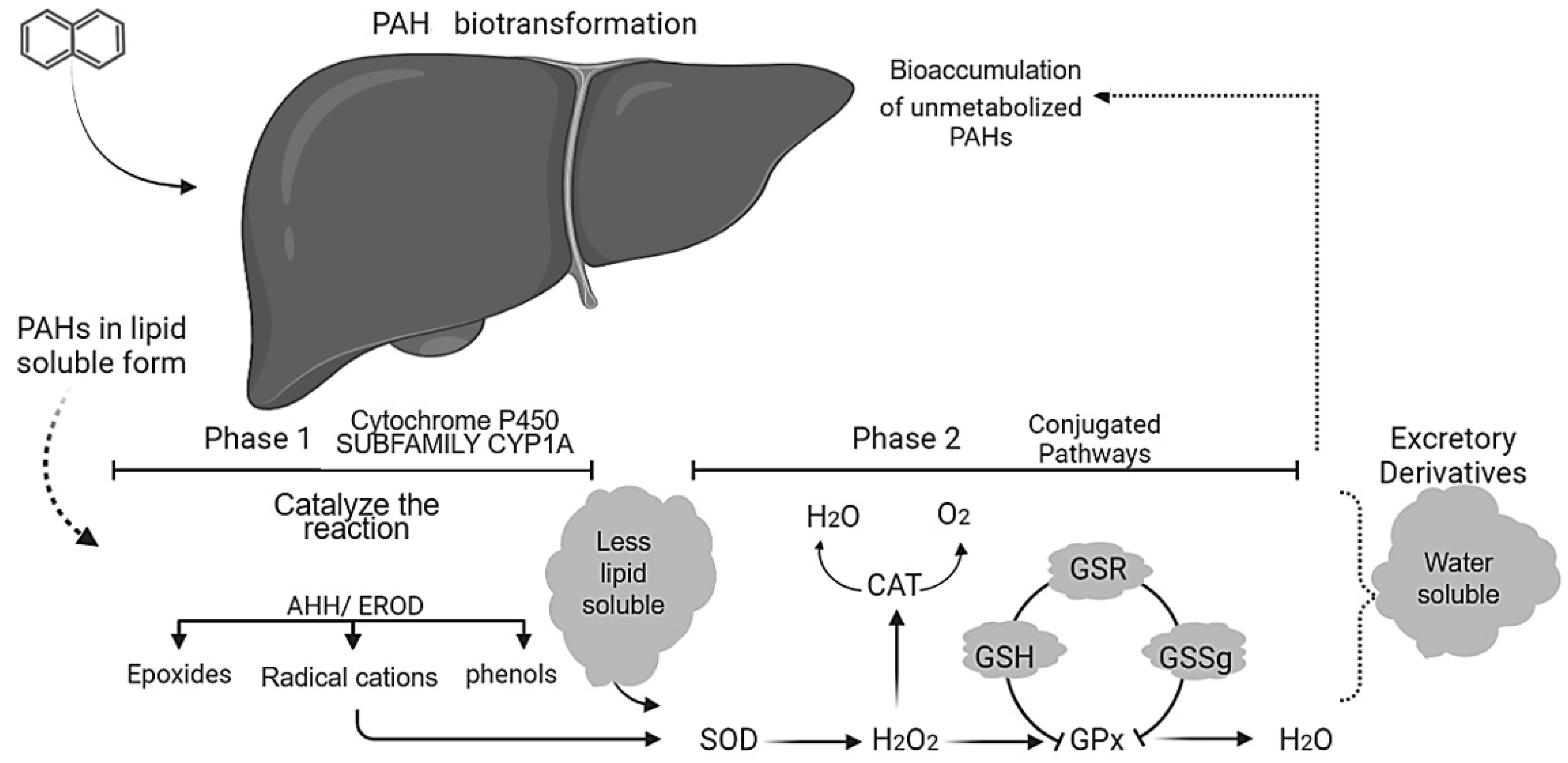
4.1. PAH Assessments
4.2. Volatility
4.3. Molecular Weight Influence
4.4. Lipophilicity
4.5. Environmental Chemistry and PAH Dispersion
4.6. PAH Toxicity in Mixtures
4.7. Factors That Can Affect the Toxicological Response of Animals to PAH
4.8. Aquatic Biota PAH Exposure Studies
4.9. Ecotoxicological Responses
4.10. Biomarker Evaluations
4.11. The Relationship between Toxic Limits and Different Risk Concepts
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bordier, M.; Uea-Anuwong, T.; Binot, A.; Hendrikx, P.; Goutard, F.L. Characteristics of one health surveillance systems: A systematic literature review. Prev. Vet. Med. 2020, 181, 104560. [Google Scholar] [CrossRef]
- Destoumieux-Garzón, D.; Mavingui, P.; Boetsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Fritsch, C.; Giraudoux, P.; Le Roux, F.; Morand, S.; et al. The one health concept: 10 years old and a long road ahead. Front. Vet. Sci. 2018, 5, 14. [Google Scholar] [CrossRef]
- Asagbra, M.C.; Adebayo, A.S.; Anumudu, C.I.; Ugwumba, O.A.; Ugwumba, A.A.A. Polycyclic aromatic hydrocarbons in water, sediment and fish from the Warri River at Ubeji, Niger Delta, Nigeria. Afr. J. Aquat. Sci. 2015, 40, 193–199. [Google Scholar] [CrossRef]
- Achten, C.; Hofmann, T. Native Polycyclic Aromatic Hydrocarbons (PAH) in coals—A hardly recognized source of environmental contamination. Sci. Total Environ. 2009, 407, 2461–2473. [Google Scholar] [CrossRef]
- Naudin, G.; Bastien, P.; Mezzache, S.; Trehu, E.; Bourokba, N.; Appenzeller, B.M.R.; Soeur, J.; Bornschlögl, T. Human pollution exposure correlates with accelerated ultrastructural degradation of hair fibers. Proc. Natl. Acad. Sci. USA 2019, 116, 18410–18415. [Google Scholar] [CrossRef]
- Ranjbar Jafarabadi, A.; Mashjoor, S.; Riyahi Bakhtiari, A.; Jadot, C. Dietary intake of polycyclic aromatic hydrocarbons (PAHs) from coral reef fish in the Persian Gulf—Human health risk assessment. Food Chem. 2020, 329, 127035. [Google Scholar] [CrossRef]
- Li, R.; Hua, P.; Zhang, J.; Krebs, P. Effect of anthropogenic activities on the occurrence of polycyclic aromatic hydrocarbons in aquatic suspended particulate matter: Evidence from Rhine and Elbe Rivers. Water Res. 2020, 179, 115901. [Google Scholar] [CrossRef]
- Ma, W.L.; Zhu, F.J.; Liu, L.Y.; Jia, H.L.; Yang, M.; Li, Y.F. PAHs in Chinese atmosphere: Gas/particle partitioning. Sci. Total Environ. 2019, 693, 133623. [Google Scholar] [CrossRef]
- Moon, H.B.; Kim, H.S.; Choi, M.; Choi, H.G. Intake and Potential Health Risk of Polycyclic Aromatic Hydrocarbons Associated with Seafood Consumption in Korea from 2005 to 2007. Arch. Environ. Contam. Toxicol. 2010, 58, 214–221. [Google Scholar] [CrossRef]
- Sun, S.J.; Zhao, Z.B.; Li, B.; Ma, L.X.; Fu, D.L.; Sun, X.Z.; Thapa, S.; Shen, J.M.; Qi, H.; Wu, Y.N. Occurrence, composition profiles and risk assessment of polycyclic aromatic hydrocarbons in municipal sewage sludge in China. Environ. Pollut. 2019, 245, 764–770. [Google Scholar] [CrossRef]
- Sun, L.; Zuo, Z.; Luo, H.; Chen, M.; Zhong, Y.; Chen, Y.; Wang, C. Chronic exposure to phenanthrene influences the Spermatogenesis of Male Sebastiscus marmoratus: U-Shaped effects and the reason for them. Environ. Sci. Technol. 2011, 45, 10212–10218. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.G.; Ke, C.L.; Liu, Q.; Lin, Q. Polycyclic aromatic hydrocarbons (PAHs) in sediments of Zhelin Bay, the largest mariculture base on the eastern Guangdong coast, South China: Characterization and risk implications. Mar. Pollut. Bull. 2016, 110, 603–608. [Google Scholar] [CrossRef]
- D’Adamo, P.; Fassone, L.; Gedeon, A.; Janssen, E.A.M.; Bione, S.; Bolhuis, P.A.; Barth, P.G.; Wilson, M.; Haan, E.; Örstavik, K.H.; et al. The X-Linked Gene G4.5 Is Responsible for Different Infantile Dilated Cardiomyopathies. Am. J. Hum. Genet. 1997, 61, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, H.; Liu, B.; Wang, X. Toxic effects of benzo[a]pyrene (Bap) and Aroclor1254 on embryogenesis, larval growth, survival and metamorphosis of the bivalve Meretrix meretrix. Ecotoxicology 2012, 21, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Y.; Colella, N.S.; Liang, Y.; Brédas, J.L.; Houk, K.N.; Briseno, A.L. Unconventional, Chemically Stable, and Soluble Two-Dimensional Angular Polycyclic Aromatic Hydrocarbons: From Molecular Design to Device Applications. Acc. Chem. Res. 2015, 48, 500–509. [Google Scholar] [CrossRef]
- Oleagoitia, M.B.Z.; Manterola, A.L.; Maurolagoitia, J.I.; de Dicastillo, M.D.M.L.; Álvarez, J.; Barandiaran, M.A.; Loibide, A.I.; Santa-Marina, L. Polycyclic aromatic hydrocarbons (PAHs) in air associated with particles PM 2.5 in the Basque Country (Spain). Air Qual. Atmos. Health 2019, 12, 107–114. [Google Scholar] [CrossRef]
- Syed, J.H.; Iqbal, M.; Zhong, G.; Katsoyiannis, A.; Yadav, I.C.; Li, J.; Zhang, G. Polycyclic aromatic hydrocarbons (PAHs) in Chinese forest soils: Profile composition, spatial variations and source apportionment. Sci. Rep. 2017, 7, 2692. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Kim, K.H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015, 84, 26–38. [Google Scholar] [CrossRef]
- Karlsson, K.; Viklander, M. Polycyclic Aromatic Hydrocarbons (PAH) in Water and Sediment from Gully Pots. Water Air Soil Pollut. 2008, 188, 271–282. [Google Scholar] [CrossRef]
- Lourenço, R.A.; Taniguchi, S.; da Silva, J.; Gallotta, F.D.C.; Bícego, M.C. Polycyclic aromatic hydrocarbons in marine mammals: A review and synthesis. Mar. Pollut. Bull. 2021, 171, 112699. [Google Scholar] [CrossRef]
- Pérez-Cadahía, B.; Laffon, B.; Pásaro, E.; Méndez, J. Evaluation of PAH Bioaccumulation and DNA Damage in Mussels (Mytilus galloprovincialis) Exposed to Spilled Prestige Crude Oil. Comp. Biochem. Physiol.-C Toxicol. Pharmacol. 2004, 138, 453–460. [Google Scholar] [CrossRef]
- Bandowe, B.A.M.; Bigalke, M.; Boamah, L.; Nyarko, E.; Saalia, F.K.; Wilcke, W. Polycyclic Aromatic Compounds (PAHs and Oxygenated PAHs) and Trace Metals in Fish Species from Ghana (West Africa): Bioaccumulation and Health Risk Assessment. Environ. Int. 2014, 65, 135–146. [Google Scholar] [CrossRef]
- Quiroz, R.; Grimalt, J.O.; Fernández, P. Toxicity Assessment of Polycyclic Aromatic Hydrocarbons in Sediments from European High Mountain Lakes. Ecotoxicol. Environ. Saf. 2010, 73, 559–564. [Google Scholar] [CrossRef]
- Dural, M.; Göksu, M.Z.L.; Özak, A.A. Investigation of Heavy Metal Levels in Economically Important Fish Species Captured from the Tuzla Lagoon. Food Chem. 2007, 102, 415–421. [Google Scholar] [CrossRef]
- Dickey, R.W. FDA Risk Assessment of Seafood Contamination after the BP Oil Spill. Environ. Health Perspect. 2012, 120, a54–a55. [Google Scholar] [CrossRef]
- Magalhães, D.D.P.; Ferrão Filho, A.D.S. A ecotoxicologia como ferramenta no biomonitoramento de ecossistemas aquáticos. Oecol. Bras. 2008, 12, 355–381. [Google Scholar] [CrossRef]
- Castaño, A.; Sanchez, P.; Llorente, M.T.; Carballo, M.; De La Torre, A.; Muñoz, M.J. The use of alternative systems for the ecotoxicological screening of complex mixtures on fish populations. Sci. Total Environ. 2000, 247, 337–348. [Google Scholar] [CrossRef]
- Barone, G.; Storelli, A.; Garofalo, R.; Busco, V.P.; Quaglia, N.C.; Centrone, G.; Storelli, M.M. Assessment of mercury and cadmium via seafood consumption in Italy: Estimated dietary intake (EWI) and target hazard quotient (THQ). Food Addit. Contam. Part A 2015, 32, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 9 December 2021).
- Luís de Sá Salomão, A.; Hauser-Davis, R.A.; Marques, M. Critical Knowledge Gaps and Relevant Variables Requiring Consideration When Performing Aquatic Ecotoxicity Assays. Ecotoxicol. Environ. Saf. 2020, 203, 110941. [Google Scholar] [CrossRef] [PubMed]
- Hodson, P.V. The Toxicity to Fish Embryos of PAH in Crude and Refined Oils. Arch. Environ. Contam. Toxicol. 2017, 73, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Govers, H.; Ruepert, C.; Aiking, H. Quantitative Structure-Activity Relationships for Polycyclic Aromatic Hydrocarbons: Correlation between Molecular Connectivity, Physico-Chemical Properties, Bioconcentration and Toxicity in Daphnia Pulex. Chemosphere 1984, 13, 227–236. [Google Scholar] [CrossRef]
- Carls, M.G.; Meador, J.P. A Perspective on the Toxicity of Petrogenic PAHs to Developing Fish Embryos Related to Environmental Chemistry. Hum. Ecol. Risk Assess. 2009, 15, 1084–1098. [Google Scholar] [CrossRef]
- Harvey, R.G. Environmental Chemistry of PAHs. In PAHs and Related Compounds; Springer: Berlin/Heidelberg, Germany, 1998; pp. 1–54. [Google Scholar] [CrossRef]
- Logan, D.T. Perspective on Ecotoxicology of PAHs to Fish. Hum. Ecol. Risk Assess. 2007, 13, 302–316. [Google Scholar] [CrossRef]
- Wilke, B.M.; Riepert, F.; Koch, C.; Kühne, T. Ecotoxicological Characterization of Hazardous Wastes. Ecotoxicol. Environ. Saf. 2008, 70, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cruz, M.S.; Barceló, D. Chemical Analysis and Ecotoxicological Effects of Organic UV-Absorbing Compounds in Aquatic Ecosystems. TrAC Trends Anal. Chem. 2009, 28, 708–717. [Google Scholar] [CrossRef]
- Escher, B.I.; Hermens, J.L.M. Modes of Action in Ecotoxicology: Their Role in Body Burdens, Species Sensitivity, QSARs, and Mixture Effects. Environ. Sci. Technol. 2002, 36, 4201–4217. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 9 December 2021).
- Nałȩcz-Jawecki, G.; Sawicki, J. Spirotox—A New Tool for Testing the Toxicity of Volatile Compounds. Chemosphere 1999, 38, 3211–3218. [Google Scholar] [CrossRef]
- Ololade, I.A.; Arogunrerin, I.A.; Oladoja, N.A.; Ololade, O.O.; Alabi, A.B. Concentrations and Toxic Equivalency of Polycyclic Aromatic Hydrocarbons (PAHs) and Polychlorinated Biphenyl (PCB) Congeners in Groundwater around Waste Dumpsites in South-West Nigeria. Arch. Environ. Contam. Toxicol. 2021, 80, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Zacchi, F.L.; de Lima, D.; Flores-Nunes, F.; Mattos, J.J.; Lüchmann, K.H.; de Miranda Gomes, C.H.A.; Bícego, M.C.; Taniguchi, S.; Sasaki, S.T.; Dias Bainy, A.C. Transcriptional Changes in Oysters Crassostrea Brasiliana Exposed to Phenanthrene at Different Salinities. Aquat. Toxicol. 2017, 183, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Le Dû-Lacoste, M.; Akcha, F.; Dévier, M.H.; Morin, B.; Burgeot, T.; Budzinski, H. Comparative Study of Different Exposure Routes on the Biotransformation and Genotoxicity of PAHs in the Flatfish Species, Scophthalmus Maximus. Environ. Sci. Pollut. Res. 2013, 20, 690–707. [Google Scholar] [CrossRef] [PubMed]
- Grintzalis, K.; Georgiou, C.D.; Dailianis, S. Total Thiol Redox Status as a Potent Biomarker of PAH-Mediated Effects on Mussels. Mar. Environ. Res. 2012, 81, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Magara, G.; Elia, A.C.; Syberg, K.; Khan, F.R. Single Contaminant and Combined Exposures of Polyethylene Microplastics and Fluoranthene: Accumulation and Oxidative Stress Response in the Blue Mussel, Mytilus Edulis. J. Toxicol. Environ. Health-Part A Curr. Issues 2018, 81, 761–773. [Google Scholar] [CrossRef]
- Speciale, A.; Zena, R.; Calabrò, C.; Bertuccio, C.; Aragona, M.; Saija, A.; Trombetta, D.; Cimino, F.; lo Cascio, P. Experimental Exposure of Blue Mussels (Mytilus galloprovincialis) to High Levels of Benzo[a]Pyrene and Possible Implications for Human Health. Ecotoxicol. Environ. Saf. 2018, 150, 96–103. [Google Scholar] [CrossRef]
- Barranger, A.; Langan, L.M.; Sharma, V.; Rance, G.A.; Aminot, Y.; Weston, N.J.; Akcha, F.; Moore, M.N.; Arlt, V.M.; Khlobystov, A.N.; et al. Antagonistic Interactions between Benzo[a]Pyrene and Fullerene (C60) in Toxicological Response of Marine Mussels. Nanomaterials 2019, 9, 987. [Google Scholar] [CrossRef]
- Moore, F.; Akhbarizadeh, R.; Keshavarzi, B.; Khabazi, S.; Lahijanzadeh, A.; Kermani, M. Ecotoxicological Risk of Polycyclic Aromatic Hydrocarbons (PAHs) in Urban Soil of Isfahan Metropolis, Iran. Environ. Monit. Assess. 2015, 187, 207. [Google Scholar] [CrossRef] [PubMed]
- Witter, A.E.; Nguyen, M.H.; Baidar, S.; Sak, P.B. Coal-Tar-Based Sealcoated Pavement: A Major PAH Source to Urban Stream Sediments. Environ. Pollut. 2014, 185, 59–68. [Google Scholar] [CrossRef]
- Fisher, T.T.; Law, R.J.; Rumney, H.S.; Kirby, M.F.; Kelly, C. Towards a Scheme of Toxic Equivalency Factors (TEFs) for the Acute Toxicity of PAHs in Sediment. Ecotoxicol. Environ. Saf. 2011, 74, 2245–2251. [Google Scholar] [CrossRef]
- Baird, W.M.; Hooven, L.A.; Mahadevan, B. Carcinogenic Polycyclic Aromatic Hydrocarbon-DNA Adducts and Mechanism of Action. Environ. Mol. Mutagen. 2005, 45, 106–114. [Google Scholar] [CrossRef]
- Song, Y.; Nahrgang, J.; Tollefsen, K.E. Transcriptomic Analysis Reveals Dose-Dependent Modes of Action of Benzo(a)Pyrene in Polar Cod (Boreogadus saida). Sci. Total Environ. 2019, 653, 176–189. [Google Scholar] [CrossRef]
- Hylland, K. Polycyclic Aromatic Hydrocarbon (PAH) Ecotoxicology in Marine Ecosystems. J. Toxicol. Environ. Health 2007, 69, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Hasue, F.M.; Passos, M.J.D.A.C.R.; dos Santos, T.D.C.A.; Rocha, A.J.D.S.; Vignardi, C.P.; Sartorio, P.V.; Gomes, V.; van Ngan, P. Assessment of Genotoxicity and Depuration of Anthracene in the Juvenile Coastal Fish Trachinotus Carolinus Using the Comet Assay. Braz. J. Oceanogr. 2013, 61, 215–222. [Google Scholar] [CrossRef]
- Palanikumar, L.; Kumaraguru, A.K.; Ramakritinan, C.M.; Anand, M. Biochemical Response of Anthracene and Benzo [a] Pyrene in Milkfish Chanos Chanos. Ecotoxicol. Environ. Saf. 2012, 75, 187–197. [Google Scholar] [CrossRef]
- Abramochkin, D.V.; Kompella, S.N.; Shiels, H.A. Phenanthrene Alters the Electrical Activity of Atrial and Ventricular Myocytes of a Polar Fish, the Navaga Cod. Aquat. Toxicol. 2021, 235, 105823. [Google Scholar] [CrossRef]
- Li, R.; Zuo, Z.; Chen, D.; He, C.; Chen, R.; Chen, Y.; Wang, C. Inhibition by Polycyclic Aromatic Hydrocarbons of ATPase Activities in Sebastiscus marmoratus Larvae in Relationship with the Development of Early Life Stages. Mar. Environ. Res. 2011, 71, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Zena, R.; Speciale, A.; Calabrò, C.; Calò, M.; Palombieri, D.; Saija, A.; Cimino, F.; Trombetta, D.; lo Cascio, P. Exposure of Sea Bream (Sparus aurata) to Toxic Concentrations of Benzo[a]Pyrene: Possible Human Health Effect. Ecotoxicol. Environ. Saf. 2015, 122, 116–125. [Google Scholar] [CrossRef]
- Honda, M.; Suzuki, N. Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef]
- Wilhelm Filho, D.; Tribess, T.; Gáspari, C.; Claudio, F.D.; Torres, M.A.; Magalhães, A.R.M. Seasonal Changes in Antioxidant Defenses of the Digestive Gland of the Brown Mussel (Perna perna). Aquaculture 2001, 203, 149–158. [Google Scholar] [CrossRef]
- Maria, V.L.; Gomes, T.; Barreira, L.; Bebianno, M.J. Impact of Benzo(a)Pyrene, Cu and Their Mixture on the Proteomic Response of Mytilus galloprovincialis. Aquat. Toxicol. 2013, 144–145, 284–295. [Google Scholar] [CrossRef]
- Saeed, T.; Ali, L.N.; Al-Bloushi, A.; Al-Hashash, H.; Al-Bahloul, M.; Al-Khabbaz, A.; Al-Khayat, A. Effect of Environmental Factors on Photodegradation of Polycyclic Aromatic Hydrocarbons (PAHs) in the Water-Soluble Fraction of Kuwait Crude Oil in Seawater. Mar. Environ. Res. 2011, 72, 143–150. [Google Scholar] [CrossRef]
- Vieira, L.R.; Guilhermino, L. Multiple Stress Effects on Marine Planktonic Organisms: Influence of Temperature on the Toxicity of Polycyclic Aromatic Hydrocarbons to Tetraselmis Chuii. J. Sea Res. 2012, 72, 94–98. [Google Scholar] [CrossRef]
- McCloskey, J.T.; Oris, J.T. Effect of Anthracene and Solar Ultraviolet Radiation Exposure on Gill ATPase and Selected Hematologic Measurements m the Bluegill Sunfish (Lepomis macrochirus). Aquat. Toxicol. 1993, 24, 207–217. [Google Scholar] [CrossRef]
- Serafin, J.; Guffey, S.C.; Bosker, T.; Griffitt, R.J.; de Guise, S.; Perkins, C.; Szuter, M.; Sepúlveda, M.S. Combined Effects of Salinity, Temperature, Hypoxia, and Deepwater Horizon Oil on Fundulus Grandis Larvae. Ecotoxicol. Environ. Saf. 2019, 181, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, M.; Blenkle, H.; Salowsky, H.; Bluhm, K.; Schiwy, S.; Tiehm, A.; Hollert, H. Genotoxicity of Heterocyclic PAHs in the Micronucleus Assay with the Fish Liver Cell Line RTL-W1. PLoS ONE 2014, 9, e85692. [Google Scholar] [CrossRef] [PubMed]
- Olson, G.M.; Meyer, B.M.; Portier, R.J. Assessment of the Toxic Potential of Polycyclic Aromatic Hydrocarbons (PAHs) Affecting Gulf Menhaden (Brevoortia patronus) Harvested from Waters Impacted by the BP Deepwater Horizon Spill. Chemosphere 2016, 145, 322–328. [Google Scholar] [CrossRef]
- Mansour, C.; Guardiola, F.A.; Esteban, M.Á.; Mosbahi, D.S. Combination of Polycyclic Aromatic Hydrocarbons and Temperature Exposure: In Vitro Effects on Immune Response of European Clam (Ruditapes decussatus). Fish Shellfish Immunol. 2017, 67, 110–118. [Google Scholar] [CrossRef]
- Ramachandran, S.D.; Sweezey, M.J.; Hodson, P.V.; Boudreau, M.; Courtenay, S.C.; Lee, K.; King, T.; Dixon, J.A. Influence of Salinity and Fish Species on PAH Uptake from Dispersed Crude Oil. Mar. Pollut. Bull. 2006, 52, 1182–1189. [Google Scholar] [CrossRef]
- De Campos, M.F.; lo Nostro, F.L.; da Cuña, R.H.; Moreira, R.G. Endocrine Disruption of Phenanthrene in the Protogynous Dusky Grouper Epinephelus Marginatus (Serranidae: Perciformes). Gen. Comp. Endocrinol. 2018, 257, 255–263. [Google Scholar] [CrossRef]
- Almeida, J.R.; Gravato, C.; Guilhermino, L. Biological Parameters towards Polycyclic Aromatic Hydrocarbons Pollution: A Study with Dicentrarchus Labrax L. Exposed to the Model Compound Benzo(a)Pyrene. Water Air Soil Pollut. 2012, 223, 4709–4722. [Google Scholar] [CrossRef]
- Guven, O.; Bach, L.; Munk, P.; Dinh, K.V.; Mariani, P.; Nielsen, T.G. Microplastic Does Not Magnify the Acute Effect of PAH Pyrene on Predatory Performance of a Tropical Fish (Lates calcarifer). Aquat. Toxicol. 2018, 198, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Dailianis, S.; Tsarpali, V.; Melas, K.; Karapanagioti, H.K.; Manariotis, I.D. Aqueous Phenanthrene Toxicity after High-Frequency Ultrasound Degradation. Aquat. Toxicol. 2014, 147, 32–40. [Google Scholar] [CrossRef]
- Page, D.S.; Chapman, P.M.; Landrum, P.F.; Neff, J.; Elston, R. A Perspective on the Toxicity of Low Concentrations of Petroleum-Derived Polycyclic Aromatic Hydrocarbons to Early Life Stages of Herring and Salmon. Hum. Ecol. Risk Assess. Int. J. 2012, 18, 229–260. [Google Scholar] [CrossRef]
- Thorpe, K.L.; Gross-Sorokin, M.; Johnson, I.; Brighty, G.; Tyler, C.R. An Assessment of the Model of Concentration Addition for Predicting the Estrogenic Activity of Chemical Mixtures in Wastewater Treatment Works Effluents. Environ. Health Perspect. 2006, 114, 90–97. [Google Scholar] [CrossRef][Green Version]
- Glomstad, B.; Altin, D.; Sørensen, L.; Liu, J.; Jenssen, B.M.; Booth, A.M. Carbon Nanotube Properties Influence Adsorption of Phenanthrene and Subsequent Bioavailability and Toxicity to Pseudokirchneriella Subcapitata. Environ. Sci. Technol. 2016, 50, 2660–2668. [Google Scholar] [CrossRef]
- Dale, K.; Yadetie, F.; Müller, M.B.; Pampanin, D.M.; Gilabert, A.; Zhang, X.; Tairova, Z.; Haarr, A.; Lille-Langøy, R.; Lyche, J.L.; et al. Proteomics and Lipidomics Analyses Reveal Modulation of Lipid Metabolism by Perfluoroalkyl Substances in Liver of Atlantic Cod (Gadus morhua). Aquat. Toxicol. 2020, 227, 105590. [Google Scholar] [CrossRef]
- Farkas, J.; Bergum, S.; Nilsen, E.W.; Olsen, A.J.; Salaberria, I.; Ciesielski, T.M.; Baczek, T.; Konieczna, L.; Salvenmoser, W.; Jenssen, B.M. The Impact of TiO2 Nanoparticles on Uptake and Toxicity of Benzo(a)Pyrene in the Blue Mussel (Mytilus edulis). Sci. Total Environ. 2015, 511, 469–476. [Google Scholar] [CrossRef]
- Song, Q.; Chen, H.; Li, Y.; Zhou, H.; Han, Q.; Diao, X. Toxicological Effects of Benzo(a)Pyrene, DDT and Their Mixture on the Green Mussel Perna Viridis Revealed by Proteomic and Metabolomic Approaches. Chemosphere 2016, 144, 214–224. [Google Scholar] [CrossRef]
- Wang, H.; Cui, L.; Cheng, H.; Zhang, Y.; Diao, X.; Wang, J. Comparative Studies on the Toxicokinetics of Benzo[a]Pyrene in Pinctada Martensii and Perna Viridis. Bull. Environ. Contam. Toxicol. 2017, 98, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Badreddine, S.; Abdelhafidh, K.; Dellali, M.; Mahmoudi, E.; Sheehan, D.; Hamouda, B. The Effects of Anthracene on Biochemical Responses of Mediterranean Mussels Mytilus galloprovincialis. Chem. Ecol. 2017, 33, 309–324. [Google Scholar] [CrossRef]
- FAO. Fishery status for capture production. In FAO Yearbook for 1996; United Nations: Rome, Italy, 1998; Volume 82, 678p. [Google Scholar]
- Zanaty, M.I.; Sawada, N.; Kitani, Y.; Nassar, H.F.; Mahmoud, H.M.; Hayakawa, K.; Sekiguchi, T.; Ogiso, S.; Tabuchi, Y.; Urata, M.; et al. Influence of Benz[a]Anthracene on Bone Metabolism and on Liver Metabolism in Nibbler Fish, Girella Punctata. Int. J. Environ. Res. Public Health 2020, 17, 1391. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Pan, L.; Zhang, H. Identification of a CYP3A-like Gene and CYPs MRNA Expression Modulation Following Exposure to Benzo[a]Pyrene in the Bivalve Mollusk Chlamys farreri. Mar. Environ. Res. 2014, 94, 7–15. [Google Scholar] [CrossRef]
- Tian, S.; Pan, L.; Sun, X. An Investigation of Endocrine Disrupting Effects and Toxic Mechanisms Modulated by Benzo[a]Pyrene in Female Scallop Chlamys farreri. Aquat. Toxicol. 2013, 144–145, 162–171. [Google Scholar] [CrossRef]
- Tian, S.; Pan, L.; Tao, Y.; Sun, X. Environmentally Relevant Concentrations of Benzo[a]Pyrene Affect Steroid Levels and Affect Gonad of Male Scallop Chlamys farreri. Ecotoxicol. Environ. Saf. 2015, 114, 150–156. [Google Scholar] [CrossRef]
- Li, L.; Ping, X.; Li, Z.; Xv, G.; Wang, C.; Jiang, M. Effects of Oxidation Defense System Exposure to Benzo(a)Pyrene on CYP450 Gene Expression and EROD Activity in Crassostrea Gigas and Mytilus Coruscus. Environ. Pollut. Bioavailab. 2021, 33, 206–213. [Google Scholar] [CrossRef]
- Yadetie, F.; Brun, N.R.; Vieweg, I.; Nahrgang, J.; Karlsen, O.A.; Goksøyr, A. Transcriptome Responses in Polar Cod (Boreogadus saida) Liver Slice Culture Exposed to Benzo[a]Pyrene and Ethynylestradiol: Insights into Anti-Estrogenic Effects. Toxicol. Vitr. 2021, 75, 105193. [Google Scholar] [CrossRef]
- Ren, X.; Pan, L.; Wang, L. Metabolic Enzyme Activities, Metabolism-Related Genes Expression and Bioaccumulation in Juvenile White Shrimp Litopenaeus Vannamei Exposed to Benzo[a]Pyrene. Ecotoxicol. Environ. Saf. 2014, 104, 79–86. [Google Scholar] [CrossRef] [PubMed]
- García-Tavera, J.L.; Valdés-Lozano, D.; Poblete-Naredo, I.; Albores-Medina, A.; Zapata-Pérez, O. Bile Benzo[a]Pyrene Concentration and Hepatic CYP1A Induction in Hypoxic Adult Tilapia (Oreochromis niloticus). Chemosphere 2013, 92, 16–23. [Google Scholar] [CrossRef]
- Strobel, A.; Mark, F.C.; Segner, H.; Burkhardt-Holm, P. Expression of Aryl Hydrocarbon Receptor–Regulated Genes and Superoxide Dismutase in the Antarctic Eelpout Pachycara Brachycephalum Exposed to Benzo[a]Pyrene. Environ. Toxicol. Chem. 2018, 37, 1487–1495. [Google Scholar] [CrossRef]
- Soltani, T.; Safahieh, A.; Zolgharnain, H.; Matroodi, S. Interactions of Oxidative DNA Damage and CYP1A Gene Expression with the Liver Enzymes in Klunzinger’s Mullet Exposed to Benzo[a]Pyrene. Toxicol. Rep. 2019, 6, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Pan, L. Short-Term Exposure to Benzo[a]Pyrene Causes Oxidative Damage and Affects Haemolymph Steroid Levels in Female Crab Portunus Trituberculatus. Environ. Pollut. 2016, 208, 486–494. [Google Scholar] [CrossRef]
- Wen, J.; Pan, L. Short-Term Exposure to Benzo[a]Pyrene Disrupts Reproductive Endocrine Status in the Swimming Crab Portunus Trituberculatus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 174–175, 13–20. [Google Scholar] [CrossRef]
- Liu, D.; Pan, L.; Li, Z.; Cai, Y.; Miao, J. Metabolites Analysis, Metabolic Enzyme Activities and Bioaccumulation in the Clam Ruditapes Philippinarum Exposed to Benzo[a]Pyrene. Ecotoxicol. Environ. Saf. 2014, 107, 251–259. [Google Scholar] [CrossRef]
- Wang, H.; Pan, L.; Si, L.; Ji, R.; Cao, Y. Effects of Nrf2-Keap1 Signaling Pathway on Antioxidant Defense System and Oxidative Damage in the Clams Ruditapes Philippinarum Exposure to PAHs. Environ. Sci. Pollut. Res. 2021, 28, 33060–33071. [Google Scholar] [CrossRef]
- Wang, H.; Pan, L.; Xu, R.; Miao, J.; Si, L.; Pan, L. Comparative Transcriptome Analysis between the Short-Term Stress and Long-Term Adaptation of the Ruditapes Philippinarum in Response to Benzo[a]Pyrene. Aquat. Toxicol. 2018, 204, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, L. In Fl Uence of Sublethal Doses of Acetamiprid and Halosulfuron-Methyl on Metabolites of Zebra Fi Sh (Brachydanio rerio). Aquat. Toxicol. 2017, 191, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.J.; Chung, J.K. Cytochrome P450 1 Enzymes in Black Rockfish, Sebastes Schlegelii: Molecular Characterization and Expression Patterns after Exposure to Benzo[a]Pyrene. Aquat. Toxicol. 2020, 226, 105566. [Google Scholar] [CrossRef]
- Zacchino, V.; Centoducati, G.; Narracci, M.; Selvaggi, M.; Santacroce, M.P. Effects of Benzo[a]Pyrene on Gilthead Sea Breams (Sparusaurata L.) Hepatocytes Exposeidn Vitroto Short and Long Term Trials. Ital. J. Anim. Sci. 2013, 12, 101–106. [Google Scholar] [CrossRef][Green Version]
- Da Silva Rocha, A.J.; Santos, T.C.A.; Gomes, V.; Bícego, M.C.; Barbosa, A.C.R.d.A.; Passos, M.J.d.A.C.R.; Hasue, F.M.; van Ngan, P. Assessment of Trophic Transfer of Benzo(a)Pyrene Genotoxicity from the Post-Larval Pink Shrimp F. Brasiliensis to the Juvenile Florida Pompano T. Carolinus. Environ. Toxicol. Pharmacol. 2012, 34, 969–976. [Google Scholar] [CrossRef]
- Deng, X.; Pan, L.; Cai, Y.; Jin, Q. Transcriptomic Changes in the Ovaries of Scallop Chlamys farreri Exposed to Benzo[a]Pyrene. Genes Genom. 2016, 38, 509–518. [Google Scholar] [CrossRef]
- Xu, R.; Pan, L.; Yang, Y.; Zhou, Y.; Li, D. Temporal Transcriptome Analysis in Female Scallop Chlamys farreri: First Molecular Insights into the Disturbing Mechanism on Lipid Metabolism of Reproductive-Stage Dependence under Benzo[a]Pyrene Exposure. Sci. Total Environ. 2020, 746, 142032. [Google Scholar] [CrossRef] [PubMed]
- Lüchmann, K.H.; Dafre, A.L.; Trevisan, R.; Craft, J.A.; Meng, X.; Mattos, J.J.; Zacchi, F.L.; Dorrington, T.S.; Schroeder, D.C.; Bainy, A.C.D. A Light in the Darkness: New Biotransformation Genes, Antioxidant Parameters and Tissue-Specific Responses in Oysters Exposed to Phenanthrene. Aquat. Toxicol. 2014, 152, 324–334. [Google Scholar] [CrossRef]
- Piazza, R.S.; Trevisan, R.; Flores-Nunes, F.; Toledo-Silva, G.; Wendt, N.; Mattos, J.J.; Lima, D.; Taniguchi, S.; Sasaki, S.T.; Mello, Á.C.P.; et al. Exposure to Phenanthrene and Depuration: Changes on Gene Transcription, Enzymatic Activity and Lipid Peroxidation in Gill of Scallops Nodipecten Nodosus. Aquat. Toxicol. 2016, 177, 146–155. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zuo, Z.; Shi, X.; Sun, L.; Wang, C. Pyrene Exposure Influences the Thyroid Development of Sebastiscus marmoratus Embryos. Aquat. Toxicol. 2012, 124–125, 28–33. [Google Scholar] [CrossRef]
- Ali, N. Polycyclic Aromatic Hydrocarbons (PAHs) in Indoor Air and Dust Samples of Different Saudi Microenvironments; Health and Carcinogenic Risk Assessment for the General Population. Sci. Total Environ. 2019, 696, 133995. [Google Scholar] [CrossRef] [PubMed]
- Gravato, C.; Almeida, J.R.; Silva, C.; Oliveira, C.; Soares, A.M.V.M. Using a Multibiomarker Approach and Behavioural Responses to Assess the Effects of Anthracene in Palaemon Serratus. Aquat. Toxicol. 2014, 149, 94–102. [Google Scholar] [CrossRef]
- Olatunji, O.S.; Fatoki, O.S.; Opeolu, B.O.; Ximba, B.J. Benzo[a]Pyrene and Benzo[k]Fluoranthene in Some Processed Fish and Fish Products. Int. J. Environ. Res. Public Health 2015, 12, 940–951. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Report on the Ecological Risk Assessment Guidelines Strategic Planning Workshop; EPA 630/R-92-002; Risk Assessment Forum: Washington, DC, USA, 1992.
- Suter, G.W. A Critique of Ecosystem Health Concepts and Indexes. Environ. Toxicol. Chem. 1993, 12, 1533–1539. [Google Scholar] [CrossRef]
- Van Leeuwen, C.J.; Verhaar, H.J.M.; Hermens, J.L.M. Quality Criteria and Risk Assessment for Mixtures of Chemicals in the Aquatic Environment. Hum. Ecol. Risk Assess. Int. J. 2008, 2, 419–425. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, J.; Luan, Y.; Li, Y.; Ma, M.; Xu, J.; Han, S. Distribution and Ecosystem Risk Assessment of Polycyclic Aromatic Hydrocarbons in the Luan River, China. Ecotoxicology 2010, 19, 827–837. [Google Scholar] [CrossRef]
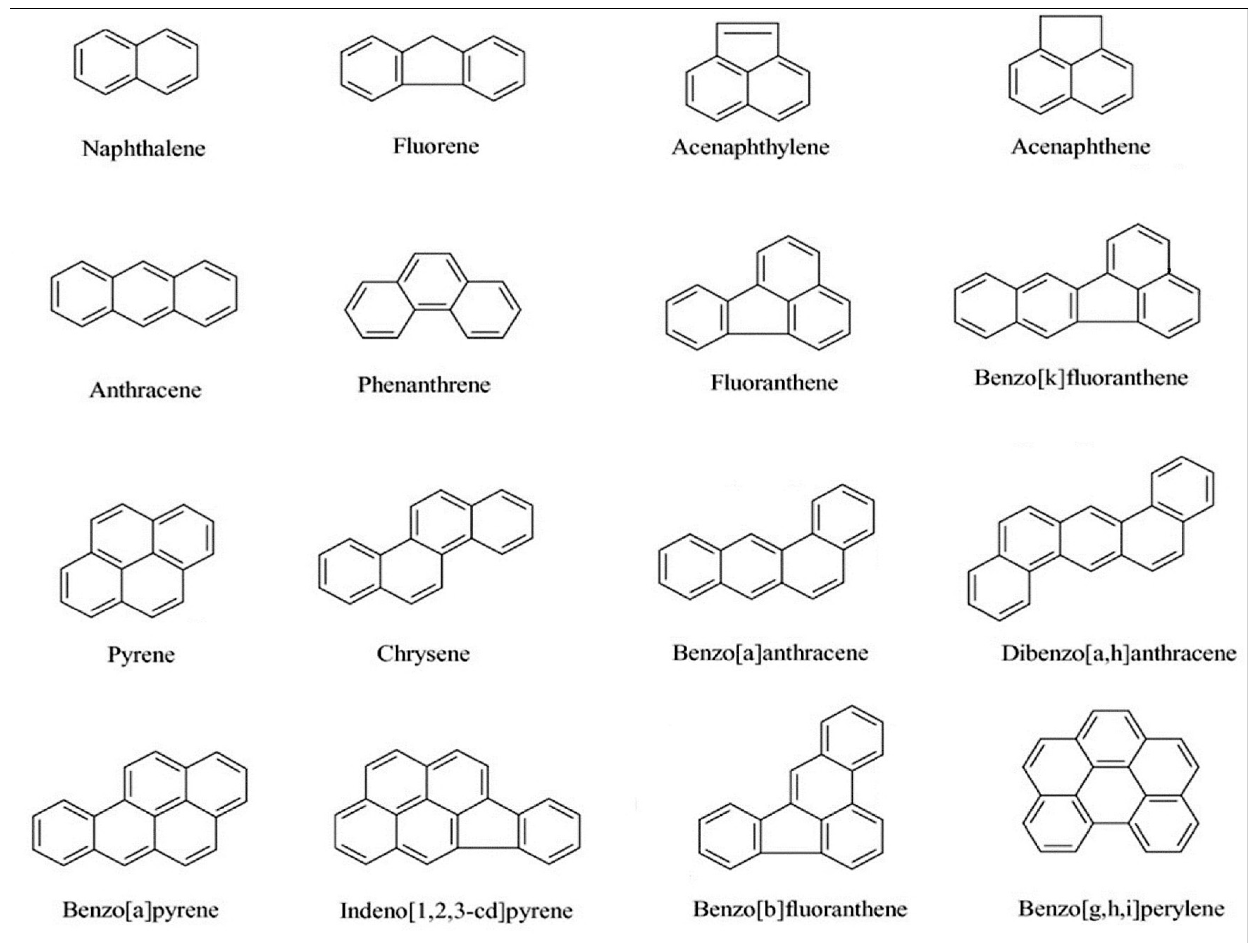
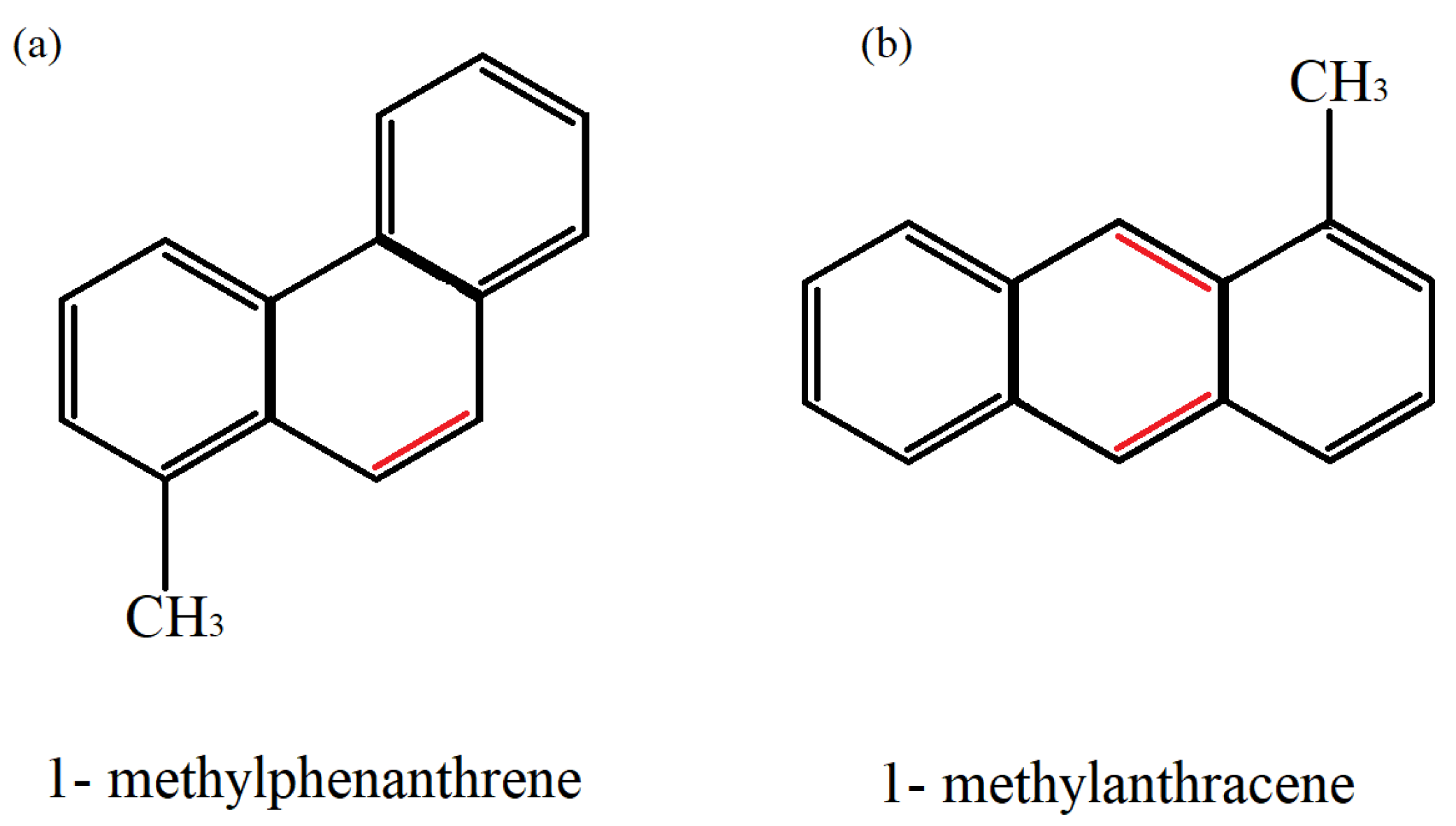

| Name | Structural Formula | Molecular Weight | Partition Coefficient (Log KOW) | Vapour Pressure (25 °C) |
|---|---|---|---|---|
| Anthracene | C13H8 | 180.20 | 3.58 | 5.7 × 10−5 |
| Fluorene | C13H10 | 166.22 | 4.18 | 6.00 × 10−4 |
| Phenanthrene | C14H10 | 178.23 | 4.46 | 1.21 × 10−4 |
| Methylphenanthrene | C15H12 | 192.25 | 4.97 | 1.50 × 10−5 |
| Methylanthracene | C15H12 | 192.25 | - | 5.34 × 10−6 |
| Pyrene | C16H10 | 202.25 | 4.88 | 4.50 × 10−6 |
| Fluoranthene | C16H10 | 202.25 | 5.16 | 9.22 × 10−6 |
| Benzo(a)antracene | C18H12 | 228.3 | 5.76 | 2.1 × 10−7 |
| Crysene | C18H12 | 228.3 | 5.73 | 6.23 × 10−9 |
| Benzo(b)fluoranthene | C20H12 | 252.3 | 5.78 (0.0015 mg L−1) | 5.00 × 10−7 |
| Benzo(k)fluoranthene | C20H12 | 252.3 | 6.11 | 9.65 × 10−10 |
| Benzo(e)pyrene | C20H12 | 252.3 | 6.44 | 5.70 × 10−9 |
| Benzo(a)pyrene | C20H12 | 252.3 | 6.13 | 5.49 × 10−9 |
| Indeno[1,2,3-cd]pyrene | C22H12 | 276.3 | 6.70 | 1.3 × 10−10 |
| Dibenz(a,h)anthracene | C22H14 | 278.3 | 6.50 | 9.55 × 10−10 |
| Benzo(g,h,i)perilene | C22H12 | 276.3 | 6.63 (9.41 mg L−1) * | 1.0 × 10−10 |
| Coronene | C24H12 | 300.4 | - | 2.17 × 10−12 |
| Species | Common Name | Compound | PAH Concentrations in Single Treatments | Compound Concentrations in Single Treatments | Total Mixture Concentrations | Reference |
|---|---|---|---|---|---|---|
| Mytilus galloprovincialis | Mediterranean Mussel | PHE ANT | 100 µg L−1 100 µg L−1 | - | 100 µg L−1 (50 µg L−1 each) | [45] |
| Gadus morhua | Atlantic cod | NAP, PHE, dibenzothiophene (DBT), PYR BaP, FLU | - | - | 12.64 µg kg−1 8.38 µg kg−1 0.58 µg kg−1 1.45 µg kg−1 1.93 µg kg−1 15.03 µg kg−1 | [78] |
| Scophthalmus Maximus | Turbot | NAP, ANT, PHE, FLU, PYR, CHR, BaP | - | - | 10,600 mg L−1 10,200 mg L−1 7500 mg L−1 13,300 mg L−1 3300 mg L−1 15,500 mg L−1 5200 mg L−1 | [44] |
| Mytilus galloprovincialis | Mediterranean Mussel | BaP C60 | 5, 50, and 100 µg L−1 of BaP | 10, 100, and 1000 µg L−1 of C60 | 1000 µg L−1 of C60 + 5 µg L−1 of BaP 1000 µg L−1 of C60 + 50 µg L−1 of BaP 1000 µg L−1 of C60 +100 µg L−1 of BaP | [48] |
| Mytilus galloprovincialis | Mediterranean Mussel | BaP (Cu) | 10 µg L−1 of BaP | 10 µg L−1 of Cu | 10 µg L−1 of BaP + 10 µg L−1 of Cu | [62] |
| Lates calcarifer | Barramundi | PYR MPs | 100 nM of PYR | 100 MP L−1 | 100 nM of PYR + 100 particles L−1 | [73] |
| Mytilus edulis | Blue mussel | FLU MPs | 50, 10 µg L−1 of FLU | 100, 1000 MP mL−1 | 100 µg L−1 of FLU + 1000 MP mL−1 50 µg L−1 of FLU + 100 MP mL−1 | [46] |
| Mytilus edulis | Blue mussel | BaP TiO2NP | 20 µg L−1 of BaP | 0.2, 2 mg L of TiO2 | 20 µg L−1 BaP + 0.2 mg L−1 TiO2NP 20 µg L−1 BaP + 2 mg L−1 TiO2NP | [79] |
| Perna viridis | Green mussel | BaP DDT | 10 µg L−1 of BaP | 10 µg L−1 of DDT | 20 µg L−1 (10 µg L−1 of each one) | [80] |
| Species | Common Name | Reference | Compound | Concentrations | Exposure Time |
|---|---|---|---|---|---|
| Trachinotus carolinus | Florida pompano | [55] | Anthracene | 8–32 µg L−1 | 24 h * |
| Girella punctata | Largescale blackfish | [84] | Benzo(a)anthracene | 1 and 10 ng/d dose | 10 days |
| Chanos chanos | Milkfish | [56] | Benzo(a)pyrene | 0.002–0.031 mg L−1 | 96 h |
| Chlamys farreri | Farrer’s scallop | [85,86,87] | Benzo(a)pyrene | 0.025–10 µg L−1 | 10 days |
| Crassostrea gigas | Pacifi cupped oyster | [88] | Benzo(a)pyrene | 0.2–5 µg L−1 | 15 days |
| Dicentrarchus labrax | Sea bass | [72] | Benzo(a)pyrene | 2–256 µg L−1 | 96 h |
| Gadus morhua | Common cod | [89] | Benzo(a)pyrene | 2.52–252.3 µg L−1 | 48 h |
| Litopenaeus vannamei and Mytilus coruscus | White shrimp and Korean mussel | [90] | Benzo(a)pyrene | 0.03–3 µg L−1 | 21 days |
| Mytilus galloprovincialis | Mediterranean mussel | [48] | Benzo(a)pyrene | 5–100 µg L−1 | 3 days |
| Mytilus galloprovincialis | Mediterranean mussel | [47] | Benzo(a)pyrene | 0.5 and 1 mg L−1 | 72 h |
| Oreochromis niloticus | Tilapia | [91] | Benzo(a)pyrene | 20 mg kg−1 | 120 h |
| Pachycara brachycephalum | - | [92] | Benzo(a)pyrene | 10 and 100 mg L−1 | 10 days |
| Perna viridis and Pinctada martensii | Brown mussel and Japanese Pearl-oyster | [81] | Benzo(a)pyrene | 2–16 µg L−1 | 14 days * |
| Planiliza klunzinger | Klunzinger’s mullet | [93] | Benzo(a)pyrene | 5–50 mg kg−1 | 14 days |
| Portunus trituberculatus | gazami crab | [94,95] | Benzo(a)pyrene | 0.1–2.5 µg L−1 | 10 days |
| Ruditapes philippinarum | Manila clam | [96] | Benzo(a)pyrene | 0.03–3 µg L−1 | 21 days |
| Ruditapes philippinarum | Manila clam | [97,98] | Benzo(a)pyrene | 4 µg L−1 | 5 and 15 days |
| Ruditapes philippinarum | Manila clam | [99] | Benzo(a)pyrene | 0.02 and 0.2 µmol L−1 | 96 h |
| Sebastes schlegelii | Korean rockfish | [100] | Benzo(a)pyrene | 2–200 µg g bw−1 | 48 h |
| Sebastiscus marmoratus | Sea ruffle | [58] | Benzo(a)pyrene | 0.01–1 µg L−1 | 6 days |
| Sparus aurata | Gilt-head | [59] | Benzo(a)pyrene | 2 mg L−1 | 72 h |
| Sparus aurata | Gilt-head | [101] | Benzo(a)pyrene | 10−4 to 106 µg L−1 | 72 h |
| Trachinotus carolinus | Florida pompano | [102] | Benzo(a)pyrene | 1–8 mg L−1 | 10 days |
| Crassostrea brasiliana | Mangrove oyster | [43] | Phenanthrene | 100 µg L−1 | 96 h |
| Chlamys farreri | Farrer’s scallop | [103] | Benzo(a)pyrene | 1–8 mg L−1 | 10 days |
| Chlamys farreri | Farrer’s scallop | [104] | Benzo(a)pyrene | 1–8 mg L−1 | 29 days |
| Meretrix meretrix | Asiatic hard clam | [14] | Benzo(a)pyrene | 1–8 mg L−1 | 24 h |
| Mytilus edulis | Blue mussel | [46] | Fluoranthene | 50 and 100 µg L−1 | 96 h |
| Ruditapes decussatus | Carpet shell | [69] | Fluorene | 0.1–1 mg L−1 | 24 h |
| Boreogadus saida | Polar cod | [80] | Benzo(a)pyrene | 0.1 and 480 µg L−1 | 14 days |
| Crassostrea brasiliana | Mangrove oyster | [105] | Phenanthrene | 100 and 1000 µg L−1 | 24 h |
| Eleginus navaga | Atlantic navaga | [57] | Phenanthrene | 1–30 µmol L−1 | - |
| Epinephelus marginatus | Dusky grouper | [71] | Phenanthrene | 0.47–3.76 mg L−1 | 96 h |
| Nodipecten nodosus | Lions-paw scallop | [106] | Phenanthrene | 50 and 200 µg L−1 | 96 h |
| Sebastiscus marmoratus | Sea ruffle | [10] | Phenanthrene | 0.06–6 μg L−1 | 50 days |
| Lates calcarifer | Barramundi | [73] | Pyrene | 1–275 nM | 24 h |
| Sebastiscus marmoratus | Sea ruffle | [107] | Pyrene | 10.2–102 mg L−1 | 5 days |
| Mytilus galloprovincialis | Mediterranean mussel | [82] | Anthracene | 0.05, 0.15, 0.4 µg L−1 | 8 days |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Pinho, J.V.; Rodrigues, P.d.A.; Guimarães, I.D.L.; Monteiro, F.C.; Ferrari, R.G.; Hauser-Davis, R.A.; Conte-Junior, C.A. The Role of the Ecotoxicology Applied to Seafood as a Tool for Human Health Risk Assessments Concerning Polycyclic Aromatic Hydrocarbons. Int. J. Environ. Res. Public Health 2022, 19, 1211. https://doi.org/10.3390/ijerph19031211
de Pinho JV, Rodrigues PdA, Guimarães IDL, Monteiro FC, Ferrari RG, Hauser-Davis RA, Conte-Junior CA. The Role of the Ecotoxicology Applied to Seafood as a Tool for Human Health Risk Assessments Concerning Polycyclic Aromatic Hydrocarbons. International Journal of Environmental Research and Public Health. 2022; 19(3):1211. https://doi.org/10.3390/ijerph19031211
Chicago/Turabian Stylede Pinho, Julia Vianna, Paloma de Almeida Rodrigues, Ivelise Dimbarre Lao Guimarães, Francielli Casanova Monteiro, Rafaela Gomes Ferrari, Rachel Ann Hauser-Davis, and Carlos Adam Conte-Junior. 2022. "The Role of the Ecotoxicology Applied to Seafood as a Tool for Human Health Risk Assessments Concerning Polycyclic Aromatic Hydrocarbons" International Journal of Environmental Research and Public Health 19, no. 3: 1211. https://doi.org/10.3390/ijerph19031211
APA Stylede Pinho, J. V., Rodrigues, P. d. A., Guimarães, I. D. L., Monteiro, F. C., Ferrari, R. G., Hauser-Davis, R. A., & Conte-Junior, C. A. (2022). The Role of the Ecotoxicology Applied to Seafood as a Tool for Human Health Risk Assessments Concerning Polycyclic Aromatic Hydrocarbons. International Journal of Environmental Research and Public Health, 19(3), 1211. https://doi.org/10.3390/ijerph19031211







