Optimal Assessment of Nutritional Status in Older Subjects with the Chronic Obstructive Pulmonary Disease—A Comparison of Three Screening Tools Used in the GLIM Diagnostic Algorithm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Respiratory Functional Tests
- GOLD 1 (mild obturation) FEV1 ≥ 80%
- GOLD 2 (moderate obturation) FEV1 ≥ 50%
- GOLD 3 (severe obturation) FEV1 ≥ 30%
- GOLD 4 (very severe obturation) FEV1 < 30%
2.2. Assessment of Cognitive Performance
2.3. Diagnostics of Malnutrition
2.3.1. Mini Nutritional Assessment—Short Form Questionnaire
2.3.2. Malnutrition Universal Screening Tool (MUST)
2.3.3. Nutritional Risk Screening 2002 (NRS-2002)
2.3.4. Global Leadership Initiative on Malnutrition (GLIM) Criteria
Phenotypic Criteria
Etiologic Criteria
2.4. Diagnostics of Sarcopenia
2.5. Statistical Analysis
- -
- Analysis of variance (ANOVA)—for samples with normal distribution and homogeneity of variance;
- -
- Kruskal–Wallis test—for samples not fulfilling the homogeneity of variance criterion.
- -
- Student t-test—for samples with normal distribution and homogeneity of variance;
- -
- Mann–Whitney U test—for samples not following a normal distribution.
3. Results
3.1. Study Population Characteristics
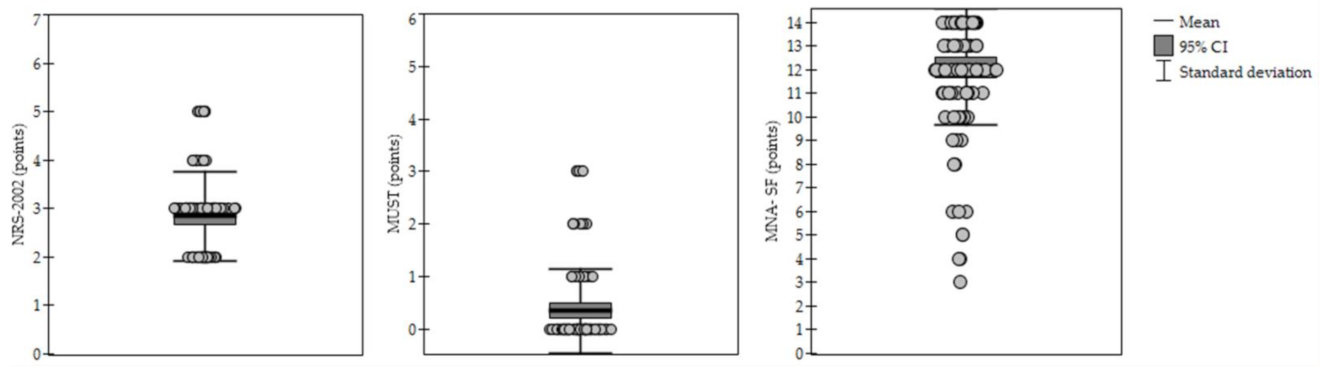
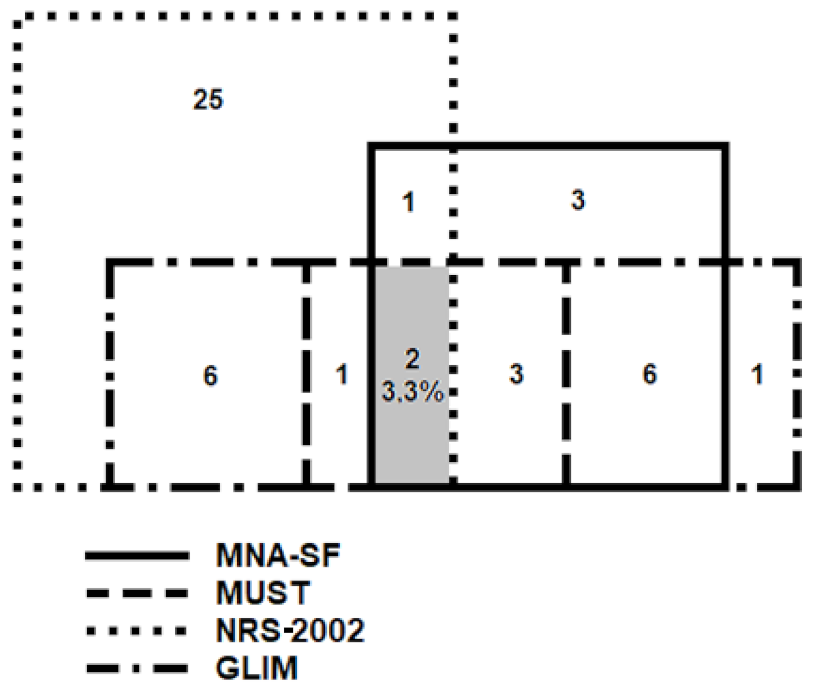
3.2. Malnutrition According to Various Diagnostic Methods
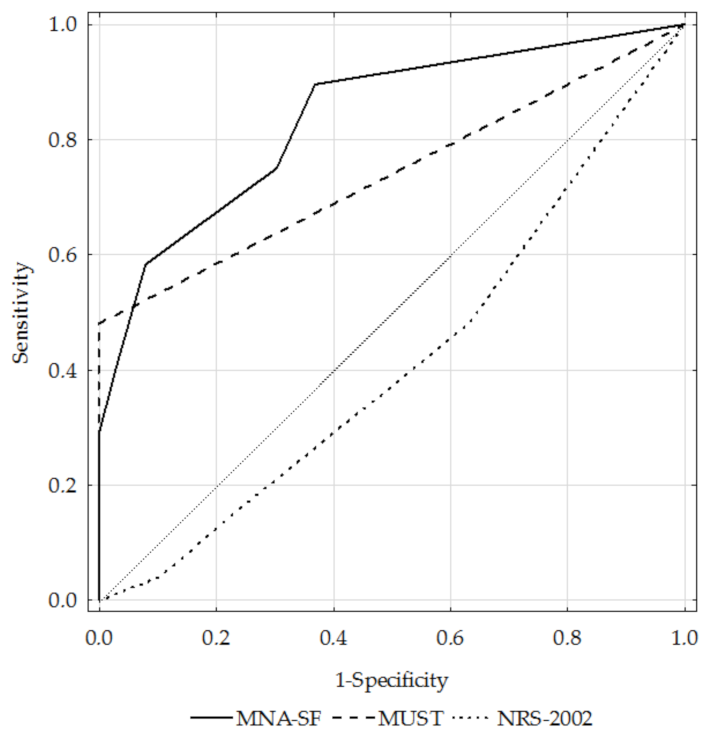
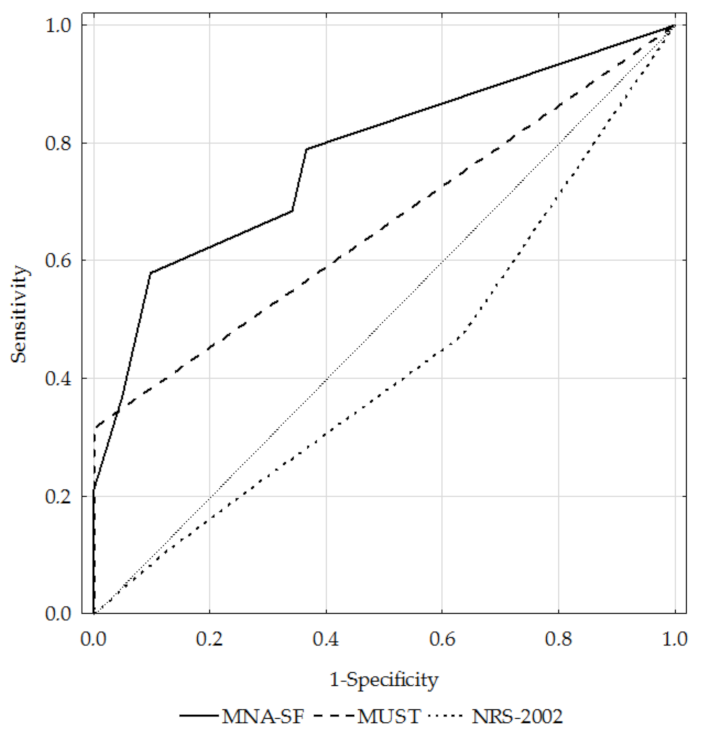
3.3. Diagnostic Performance of the MNA-SF, MUST, and NRS-2002 Questionnaires
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Norman, K.; Pichard, C.; Lochs, H.; Pirlich, M. Prognostic Impact of Disease-Related Malnutrition. Clin. Nutr. 2008, 27, 5–15. [Google Scholar] [CrossRef]
- Volkert, D.; Kiesswetter, E.; Cederholm, T.; Donini, L.M.; Eglseer, D.; Norman, K.; Schneider, S.M.; Ströbele-Benschop, N.; Torbahn, G.; Wirth, R.; et al. Development of a Model on Determinants of Malnutrition in Aged Persons: A MaNuEL Project. Gerontol. Geriatr. Med. 2019, 5, 2333721419858438. [Google Scholar] [CrossRef] [PubMed]
- Schols, A.M.; Ferreira, I.M.; Franssen, F.M.; Gosker, H.R.; Janssens, W.; Muscaritoli, M.; Pison, C.; Rutten-van Mölken, M.; Slinde, F.; Steiner, M.C.; et al. Nutritional Assessment and Therapy in COPD: A European Respiratory Society Statement. Eur. Respir. J. 2014, 44, 1504–1520. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment Short-Form (MNA-SF): A Practical Tool for Identification of Nutritional Status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for Undernutrition in Geriatric Practice: Developing the Short-Form Mini-Nutritional Assessment (MNA-SF). J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M366–M372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corish, C.A.; Bardon, L.A. Malnutrition in Older Adults: Screening and Determinants. Proc. Nutr. Soc. 2019, 78, 372–379. [Google Scholar] [CrossRef]
- Guyonnet, S.; Rolland, Y. Screening for Malnutrition in Older People. Clin. Geriatr. Med. 2015, 31, 429–437. [Google Scholar] [CrossRef]
- Murphy, J.; Mayor, A.; Forde, E. Identifying and Treating Older Patients with Malnutrition in Primary Care: The MUST Screening Tool. Br. J. Gen. Pract. 2018, 68, 344–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Tang, M.; Zhang, Q.; Zhang, K.-P.; Guo, Z.-Q.; Xu, H.-X.; Yuan, K.-T.; Yu, M.; Braga, M.; Cederholm, T.; et al. The GLIM Criteria as an Effective Tool for Nutrition Assessment and Survival Prediction in Older Adult Cancer Patients. Clin. Nutr. 2021, 40, 1224–1232. [Google Scholar] [CrossRef]
- Shimizu, A.; Maeda, K.; Honda, T.; Ishida, Y.; Ueshima, J.; Nagami, S.; Nagano, A.; Inoue, T.; Murotani, K.; Kayashita, J.; et al. Comparison between the Global Leadership Initiative on Malnutrition and the European Society for Clinical Nutrition and Metabolism Definitions for the Prevalence of Malnutrition in Geriatric Rehabilitation Care. Geriatr. Gerontol. Int. 2020, 20, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Kakavas, S.; Karayiannis, D.; Bouloubasi, Z.; Poulia, K.A.; Kompogiorgas, S.; Konstantinou, D.; Vougas, V. Global Leadership Initiative on Malnutrition Criteria Predict Pulmonary Complications and 90-Day Mortality after Major Abdominal Surgery in Cancer Patients. Nutrients 2020, 12, 3726. [Google Scholar] [CrossRef]
- Allard, J.P.; Keller, H.; Gramlich, L.; Jeejeebhoy, K.N.; Laporte, M.; Duerksen, D.R. GLIM Criteria Has Fair Sensitivity and Specificity for Diagnosing Malnutrition When Using SGA as Comparator. Clin. Nutr. 2020, 39, 2771–2777. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Iwai, K.; Namikawa, N.; Matsuda, S.; Wakano, C.; Heya, H.; Yamanaka, M. The Relationship between Existing Nutritional Indicators and Global Leadership Initiative on Malnutrition (GLIM) Criteria: A One-Institution Cross-Sectional Analysis. Clin. Nutr. 2020, 39, 3099–3104. [Google Scholar] [CrossRef] [PubMed]
- Sobrini, P.; Sánchez-Castellano, C.; Cruz-Jentoft, A.J. MNA-SF as a Screening Tool for Malnutrition Diagnosed with the Glim Criteria in Older Persons with Cancer. Eur. Geriatr. Med. 2021, 12, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.B.; Reijnierse, E.M.; Lim, W.K.; Maier, A.B. Prevalence of Malnutrition Comparing the GLIM Criteria, ESPEN Definition and MST Malnutrition Risk in Geriatric Rehabilitation Patients: RESORT. Clin. Nutr. 2020, 39, 3504–3511. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Lo Buglio, A.; Quiete, S.; Pellegrino, G.; Dobrakowski, M.; Kasperczyk, A.; Kasperczyk, S.; Vendemiale, G. Comparison of Three Nutritional Screening Tools with the New Glim Criteria for Malnutrition and Association with Sarcopenia in Hospitalized Older Patients. J. Clin. Med. 2020, 9, 1898. [Google Scholar] [CrossRef]
- Lengelé, L.; Bruyère, O.; Beaudart, C.; Reginster, J.-Y.; Locquet, M. Malnutrition, Assessed by the Global Leadership Initiative on Malnutrition (GLIM) Criteria but Not by the Mini Nutritional Assessment (MNA), Predicts the Incidence of Sarcopenia over a 5-Year in the SarcoPhAge Cohort. Aging Clin. Exp. Res. 2021, 33, 1507–1517. [Google Scholar] [CrossRef]
- Kaluźniak-Szymanowska, A.; Krzymińska-Siemaszko, R.; Lewandowicz, M.; Deskur-Śmielecka, E.; Stachnik, K.; Wieczorowska-Tobis, K. Diagnostic Performance and Accuracy of the MNA-SF against GLIM Criteria in Community-Dwelling Older Adults from Poland. Nutrients 2021, 13, 2183. [Google Scholar] [CrossRef]
- Dubé, B.-P.; Laveneziana, P. Effects of Aging and Comorbidities on Nutritional Status and Muscle Dysfunction in Patients with COPD. J. Thorac. Dis. 2018, 10, S1355–S1366. [Google Scholar] [CrossRef] [PubMed]
- Gea, J.; Sancho-Muñoz, A.; Chalela, R. Nutritional Status and Muscle Dysfunction in Chronic Respiratory Diseases: Stable Phase versus Acute Exacerbations. J. Thorac. Dis. 2018, 10, S1332–S1354. [Google Scholar] [CrossRef] [PubMed]
- Nordén, J.; Grönberg, A.M.; Bosaeus, I.; Forslund, H.B.; Hulthén, L.; Rothenberg, E.; Karlsson, J.; Wallengren, O.; Slinde, F. Nutrition Impact Symptoms and Body Composition in Patients with COPD. Eur. J. Clin. Nutr. 2015, 69, 256–261. [Google Scholar] [CrossRef]
- 2021 GOLD Reports. Available online: https://goldcopd.org/2021-gold-reports/ (accessed on 3 August 2021).
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanik, W.; Lazarewicz, M. The Polish Version of the Abbreviated Mental Test Score (AMTS)—Methodology Issues. Psychiatr. Psychol. Klin. 2017, 17, 203–207. [Google Scholar] [CrossRef]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z.; Ad Hoc ESPEN Working Group. Nutritional Risk Screening (NRS 2002): A New Method Based on an Analysis of Controlled Clinical Trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Krzymińska-Siemaszko, R.; Fryzowicz, A.; Czepulis, N.; Kaluźniak-Szymanowska, A.; Dworak, L.B.; Wieczorowska-Tobis, K. The Impact of the Age Range of Young Healthy Reference Population on the Cut-off Points for Low Muscle Mass Necessary for the Diagnosis of Sarcopenia. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4321–4332. [Google Scholar] [CrossRef] [PubMed]
- Power, L.; Mullally, D.; Gibney, E.R.; Clarke, M.; Visser, M.; Volkert, D.; Bardon, L.; de van der Schueren, M.A.E.; Corish, C.A. A Review of the Validity of Malnutrition Screening Tools Used in Older Adults in Community and Healthcare Settings—A MaNuEL Study. Clin. Nutr. ESPEN 2018, 24, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marco, E.; Sánchez-Rodríguez, D.; Dávalos-Yerovi, V.N.; Duran, X.; Pascual, E.M.; Muniesa, J.M.; Rodríguez, D.A.; Aguilera-Zubizarreta, A.; Escalada, F.; Duarte, E. Malnutrition According to ESPEN Consensus Predicts Hospitalizations and Long-Term Mortality in Rehabilitation Patients with Stable Chronic Obstructive Pulmonary Disease. Clin. Nutr. 2019, 38, 2180–2186. [Google Scholar] [CrossRef]
- Keogh, E.; Mark Williams, E. Managing Malnutrition in COPD: A Review. Respir. Med. 2021, 176, 106248. [Google Scholar] [CrossRef] [PubMed]
- Mete, B.; Pehlivan, E.; Gülbaş, G.; Günen, H. Prevalence of Malnutrition in COPD and Its Relationship with the Parameters Related to Disease Severity. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 3307–3312. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Rodriguez, D.; Locquet, M.; Reginster, J.-Y.; Cavalier, E.; Bruyère, O.; Beaudart, C. Mortality in Malnourished Older Adults Diagnosed by ESPEN and GLIM Criteria in the SarcoPhAge Study. J. Cachexia Sarcopenia Muscle 2020, 11, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.E.; Burgel, C.F.; Lima, J.; Chites, V.S.; Saragiotto, C.B.; Rabito, E.I.; Silva, F.M. GLIM Criteria for Malnutrition Diagnosis of Hospitalized Patients Presents Satisfactory Criterion Validity: A Prospective Cohort Study. Clin. Nutr. 2021, 40, 4366–4372. [Google Scholar] [CrossRef]
- Dávalos-Yerovi, V.; Marco, E.; Sánchez-Rodríguez, D.; Duran, X.; Meza-Valderrama, D.; Rodríguez, D.A.; Muñoz, E.; Tejero-Sánchez, M.; Muns, M.D.; Guillén-Solà, A.; et al. Malnutrition According to GLIM Criteria Is Associated with Mortality and Hospitalizations in Rehabilitation Patients with Stable Chronic Obstructive Pulmonary Disease. Nutrients 2021, 13, 369. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, L.; Wang, H.; Hao, Q.; Dong, B.; Yang, M. Malnutrition-Sarcopenia Syndrome Predicts Mortality in Hospitalized Older Patients. Sci. Rep. 2017, 7, 3171. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| GOLD 1+2 n = 60 | GOLD 3+4 n = 64 | p-Value | ||
|---|---|---|---|---|
| Age (years) | 69.8 ± 6.5 | 69.0 ± 5.7 | 0.4832 | |
| MNA-SF | Risk of malnutrition | 15 (25.0) | 19 (29.7) | 0.5587 |
| No risk of malnutrition | 45 (75.0) | 45 (70.3) | ||
| MUST | Risk of malnutrition | 6 (10.0) | 17 (26.6) | 0.0177 |
| No risk of malnutrition | 54 (90.0) | 47 (73.4) | ||
| NRS-2002 | Risk of malnutrition | 35 (58.3) | 36 (56.3) | 0.8147 |
| No risk of malnutrition | 25 (41.7) | 28 (43.8) | ||
| Height (cm) | 166.6 ± 9.4 | 166.3 ± 9.2 | 0.8641 | |
| Weight (kg) | 82.8 ± 19.1 | 73.5 ± 21.1 | 0.0048 | |
| BMI (kg/m2) | 29.8 ± 6.2 | 26.4 ± 6.6 | 0.0046 | |
| BFM (kg) | 28.3 ± 12.4 | 24.2 ± 13.0 | 0.0591 | |
| SMM (kg) | 30.2 ± 6.7 | 26.9 ± 6.5 | 0.0071 | |
| PBF (%) | 33.1 ± 9.6 | 31.2 ± 10.2 | 0.2901 | |
| FFM (kg) | 47.5 ± 11.0 | 42.4 ± 10.9 | 0.0117 | |
| Low ALM index | 7 (11.7) | 22 (34.4) | 0.0028 | |
| FEV1/FVC EX | 59.4 ± 7.6 | 43.2 ± 11.4 | <0.0001 | |
| FEV1 | 66.7 ± 13.2 | 35.6 ± 7.1 | <0.0001 | |
| GLIM, n = 48 | MNA-SF + GLIM, n = 28 | MUST + GLIM, n = 23 | NRS-2002 + GLIM, n = 23 | p-Value | |
|---|---|---|---|---|---|
| Age | 69.1 ± 6.3 | 69.4 ± 6.0 | 68.8 ± 5.1 | 69.8 ± 6.7 | 0.9509 |
| AMTS | 9.5 ± 0.7 | 9.6 ± 0.6 | 9.7 ± 0.7 | 9.7 ± 0.6 | 0.7974 |
| Sex | 0.3503 | ||||
| Women | 20 (41.7) | 16 (57.1) | 12 (52.2) | 8 (34.8) | |
| Men | 28 (58.3) | 12 (42.9) | 11 (47.8 | 15 (65.2) | |
| GOLD | |||||
| 1+2 | 19 (39.6) | 11 (39.3) | 6 (26.1) | 9 (39.1) | 0.7006 |
| 3+4 | 29 (60.4) | 17 (60.7) | 17 (73.9) | 14 (60.9) | |
| FEV1/FVC EX | 48.1 ± 10.9 | 49.3 ± 10.9 | 47.1 ± 11.1 | 46.3 ± 10.9 | 0.7799 |
| FEV1 | 48.9 ± 20.2 | 49.3 ± 19.5 | 43.8 ± 18.6 | 48.5 ± 21.3 | 0.7811 |
| 6MWT (m) | 317.1 ± 134.2 | 281.0 ± 143.4 | 286.8 ± 152.7 | 332.8 ± 145.4 | 0.5403 |
| Height (cm) | 165.4 ± 9.4 | 162.8 ± 10.2 | 163.7 ± 8.6 | 165.1 ± 10.5 | 0.5409 |
| Weight (kg) | 66.2 ± 18.6 | 61.0 ± 19.2 | 55.0 ± 12.9 | 67.5 ± 18.5 | 0.0318 |
| BMI (kg/m2) | 24.0 ± 5.8 | 22.8 ± 6.0 | 20.4 ± 3.6 | 24.6 ± 5.6 | 0.0249 |
| BFM (kg) | 19.3 ± 11.1 | 17.6 ± 11.1 | 13.5 ± 6.8 | 20.4 ± 10.9 | 0.0758 |
| SMM (kg) | 25.5 ± 6.3 | 23.4 ± 6.3 | 22.3 ± 4.6 | 25.6 ± 6.2 | 0.0745 |
| PBF (%) | 27.6 ± 9.8 | 26.9 ± 10.0 | 23.6 ± 7.4 | 29.0 ± 9.0 | 0.2336 |
| FFM (kg) | 40.0 ± 10.8 | 36.3 ± 10.9 | 34.4 ± 8.0 | 40.5 ± 10.8 | 0.0706 |
| Low ALM index | 29 (60.4) | 19 (67.9) | 18 (78.3) | 15 (65.2) | 0.5200 |
| Sarcopenia | 16 (33.3) | 12 (42.9) | 12 (52.2) | 9 (39.1) | 0.4923 |
| No sarcopenia | 32 (66.7) | 16 (57.1) | 11 (47.8) | 14 (60.9) |
| MNA-SF | MUST | NRS-2002 | ||
|---|---|---|---|---|
| Sensitivity (%) | Total | 58.3 | 47.9 | 47.9 |
| GOLD 1+2 | 57.9 | 31.6 | 47.4 | |
| GOLD 3+4 | 58.6 | 58.6 | 48.3 | |
| Specificity (%) | Total | 92.1 | 100.0 | 36.8 |
| GOLD 1+2 | 90.2 | 100.0 | 36.6 | |
| GOLD 3+4 | 94.3 | 100.0 | 37.1 | |
| Positive predictive value (%) | Total | 82.4 | 100.0 | 32.4 |
| GOLD 1+2 | 73.3 | 100.0 | 25.7 | |
| GOLD 3+4 | 89.5 | 100.0 | 38.9 | |
| Negative predictive value (%) | Total | 77.8 | 75.3 | 52.8 |
| GOLD 1+2 | 82.2 | 75.9 | 60.0 | |
| GOLD 3+4 | 73.3 | 74.5 | 46.4 | |
| Accuracy (%) | Total | 79.0 | 79.8 | 41.1 |
| GOLD 1+2 | 80.0 | 78.3 | 40.0 | |
| GOLD 3+4 | 78.1 | 81.3 | 42.2 | |
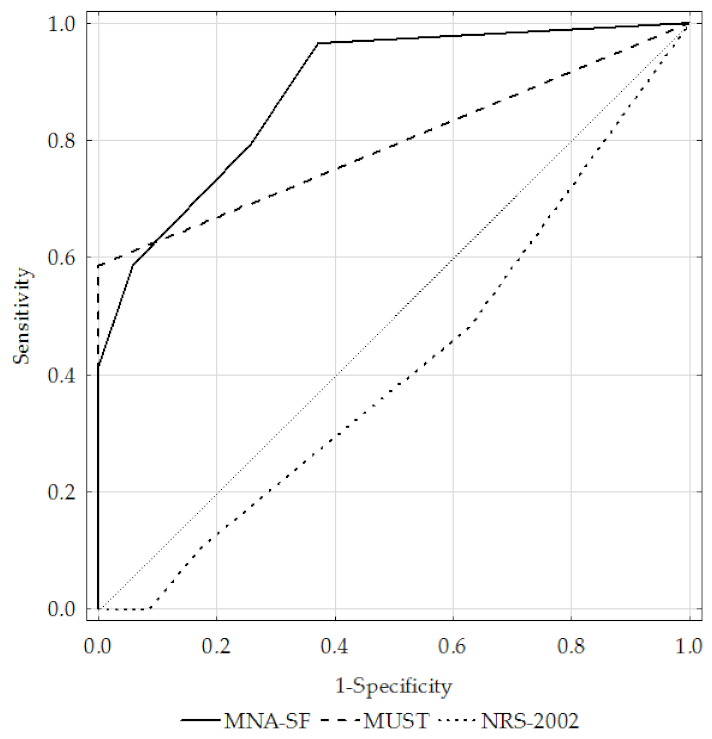
| AUC | Total | 0.84 | 0.74 | 0.41 |
| GOLD 1+2 | 0.78 | 0.66 | 0.42 | |
| GOLD 3+4 | 0.89 | 0.79 | 0.41 | |
| Kappa coefficient | Total | 0.533 | 0.530 | 0.140 |
| GOLD 1+2 | 0.510 | 0.387 | 0.131 | |
| GOLD 3+4 | 0.545 | 0.608 | 0.143 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaluźniak-Szymanowska, A.; Krzymińska-Siemaszko, R.; Wieczorowska-Tobis, K.; Deskur-Śmielecka, E. Optimal Assessment of Nutritional Status in Older Subjects with the Chronic Obstructive Pulmonary Disease—A Comparison of Three Screening Tools Used in the GLIM Diagnostic Algorithm. Int. J. Environ. Res. Public Health 2022, 19, 1025. https://doi.org/10.3390/ijerph19031025
Kaluźniak-Szymanowska A, Krzymińska-Siemaszko R, Wieczorowska-Tobis K, Deskur-Śmielecka E. Optimal Assessment of Nutritional Status in Older Subjects with the Chronic Obstructive Pulmonary Disease—A Comparison of Three Screening Tools Used in the GLIM Diagnostic Algorithm. International Journal of Environmental Research and Public Health. 2022; 19(3):1025. https://doi.org/10.3390/ijerph19031025
Chicago/Turabian StyleKaluźniak-Szymanowska, Aleksandra, Roma Krzymińska-Siemaszko, Katarzyna Wieczorowska-Tobis, and Ewa Deskur-Śmielecka. 2022. "Optimal Assessment of Nutritional Status in Older Subjects with the Chronic Obstructive Pulmonary Disease—A Comparison of Three Screening Tools Used in the GLIM Diagnostic Algorithm" International Journal of Environmental Research and Public Health 19, no. 3: 1025. https://doi.org/10.3390/ijerph19031025
APA StyleKaluźniak-Szymanowska, A., Krzymińska-Siemaszko, R., Wieczorowska-Tobis, K., & Deskur-Śmielecka, E. (2022). Optimal Assessment of Nutritional Status in Older Subjects with the Chronic Obstructive Pulmonary Disease—A Comparison of Three Screening Tools Used in the GLIM Diagnostic Algorithm. International Journal of Environmental Research and Public Health, 19(3), 1025. https://doi.org/10.3390/ijerph19031025






