Deep Dive into the Long Haul: Analysis of Symptom Clusters and Risk Factors for Post-Acute Sequelae of COVID-19 to Inform Clinical Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Study and Electronic Survey Design
2.2. Depression and Anxiety Screening Questionnaires
2.3. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Incidence and Sequelae of Long COVID Symptomatology
3.3. Sociodemographic and Clinical Predictors of Long COVID
3.4. Sociodemographic and Clinical Correlates of Long COVID Burden of Disease
3.5. Long COVID Symptomatology Reflects Five Underlying Symptom Clusters
3.6. Associations between Symptom Clusters, Anxiety, and Depression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, S.; Rebolledo, P.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Pawelek, P.; Gaughan, C. Technical article: Updated estimates of the prevalence of post-acute symptoms among people with coronavirus (COVID-19) in the UK: 26 April 2020 to 1 August 2021. Office for National Statistics. 2021; pp. 1–19. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/technicalarticleupdatedestimatesoftheprevalenceofpostacutesymp-tomsamongpeoplewithcoronaviruscovid19intheuk/26april2020to1august2021 (accessed on 1 October 2022).
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V.; on behalf of the WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Al Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Hayes, L.D.; Ingram, J.; Sculthorpe, N.F. More than 100 persistent symptoms of SARS-CoV-2 (Long COVID): A scoping review. Front. Med. 2021, 8, 750378. [Google Scholar] [CrossRef]
- Tabacof, L.; Tosto-Mancuso, J.; Wood, J.; Cortes, M.; Kontorovich, A.; McCarthy, D.; Rizk, D.; Rozanski, G.; Breyman, E.; Nasr, L.; et al. Post-acute COVID-19 Syndrome Negatively Impacts Physical Function, Cognitive Function, Health-Related Quality of Life, and Participation. Am. J. Phys. Med. Rehabil. 2020, 101, 48–52. [Google Scholar] [CrossRef]
- Badenoch, J.B.; Rengasamy, E.R.; Watson, C.; Jansen, K.; Chakraborty, S.; Sundaram, R.D.; Hafeez, D.; Burchill, E.; Saini, A.; Thomas, L.; et al. Persistent neuropsychiatric symptoms after COVID-19: A systematic review and meta-analysis. Brain Commun. 2021, 4, fcab297. [Google Scholar] [CrossRef]
- Houben-Wilke, S.; Goërtz, Y.M.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.V.; van Herck, M.; Burtin, C.; Posthuma, R.; Franssen, F.M.; et al. The Impact of Long COVID-19 on Mental Health: Observational 6-Month Follow-Up Study. JMIR Ment. Health 2022, 9, e33704. [Google Scholar] [CrossRef]
- Burton, A.; Aughterson, H.; Fancourt, D.; Philip, K.E.J. Factors shaping the mental health and well-being of people experiencing persistent COVID-19 symptoms or ‘long COVID’: Qualitative study. BJPsych Open 2022, 8, e72. [Google Scholar] [CrossRef]
- Calabria, M.; García-Sánchez, C.; Grunden, N.; Pons, C.; Arroyo, J.A.; Gómez-Anson, B.; Estévez García, M.D.C.; Belvís, R.; Morollón, N.; Vera Igual, J.; et al. Post-COVID-19 fatigue: The contribution of cognitive and neuropsychiatric symptoms. J. Neurol. 2022, 269, 3990–3999. [Google Scholar] [CrossRef]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.-M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef]
- Institute of Health Metrics and Evaluation. Global Health Data Exchange (GHDx). Available online: https://vizhub.healthdata.org/gbd-results (accessed on 1 July 2022).
- World Health Organization. Mental Health and COVID-19: Early evidence of the pandemic’s impact. Sci. Brief 2022, 1–11. Available online: https://www.who.int/campaigns/connecting-the-world-to-combat-coronavirus/healthyathome/healthyathome---mental-health?gclid=EAIaIQobChMImqawgOX6-wIVSHRgCh3TNAmXEAAYASAAEgK82vD_BwE (accessed on 1 July 2022).
- Thompson, E.J.; Williams, D.M.; Walker, A.J.; Mitchell, R.E.; Niedzwiedz, C.L.; Yang, T.C.; Huggins, C.F.; Kwong, A.S.F.; Silverwood, R.J.; Di Gessa, G.; et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat. Commun. 2022, 13, 3528. [Google Scholar] [CrossRef]
- Carfi, A.; Bernabei, R.; Landi, F.; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID1-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Writing Committee for the COMEBAC Study Group; Morin, L.; Savale, L.; Pham, T.; Colle, R.; Figueiredo, S.; Harrois, A.; Gasnier, M.; Lecoq, A.-L.; Meyrignac, O.; et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA 2021, 325, 1525–1534. [Google Scholar] [CrossRef]
- Lüdecke D (2021). sjPlot: Data Visualization for Statistics in Social Science. R Package Version 2.8.10. Available online: https://CRAN.R-project.org/package=sjPlot (accessed on 1 July 2022).
- Goldhaber, N.H.; Ogan, W.S.; Greaves, A.; Tai-Seale, M.; Sitapati, A.; A Longhurst, C.; E Horton, L. Population-based evaluation of post-acute COVID-19 chronic sequelae in patients who tested positive for SARS-CoV-2. Open Forum Infect. Dis. 2022, 9, ofac495. [Google Scholar] [CrossRef]
- UC Health Center. UC Health Center for Data-Driven Insights and Inovation (CDI2); Database; UC Health Center: Christchurch, New Zealand, 2022; Unpublished Results. [Google Scholar]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Bahmer, T.; Borizikowsky, C.; Lieb, W.; Horn, W.; Krist, L.; Fricke, J.; Scheibenbogen, C.; Rabe, K.F.; Maetzler, W.; Maetzler, C.; et al. Severity, predictors and clinical correlates of Post-COVID syndrome (PCS) in Germany: A prospective, multi-centre, population-based cohort study. Lancet 2022, 51, 101549. [Google Scholar] [CrossRef]
- Townsend, L.; Dowds, J.; O’Brien, K.; Sheill, G.; Dyer, A.H.; O’Kelly, B.; Hynes, J.P.; Mooney, A.; Dunne, J.; Cheallaigh, C.N.; et al. Peristent poor health after COVID-19 is not associated with respiratory complications or initial disease severity. Ann. Am. Thorac. Surg. 2021, 18, 997–1003. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 2020, 15, e024078. [Google Scholar] [CrossRef]
- Bai, F.; Tomasoni, D.; Falcinella, C.; Barbanotti, D.; Castoldi, R.; Mulè, G.; Augello, M.; Mondatore, D.; Allegrini, M.; Cona, A.; et al. Female gender is associated with long COVID syndrome: A prospective cohort study. Clin. Microbiol. Infect. 2022, 28, 611.e9–611.e16. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Martín-Guerrero, J.D.; Pellicer-Valero, Ó.J.; Navarro-Pardo, E.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Arias-Navalón, J.A.; Cigarán-Méndez, M.; Hernández-Barrera, V.; Arendt-Nielsen, L. Female Sex Is a Risk Factor Associated with Long-Term Post-COVID Related-Symptoms but Not with COVID-19 Symptoms: The LONG-COVID-EXP-CM Multicenter Study. J. Clin. Med. 2022, 11, 413. [Google Scholar] [CrossRef]
- Thye, A.Y.; Law, J.W.; Tan, L.T.; Pusparajah, P.; Ser, H.L.; Thurairajasingam, S.; Letchumanan, V.; Lee, L.H. Psychological Symptoms in COVID-19 Patients: Insights into Pathophysiology and Risk Factors of Long COVID-19. Biology 2022, 11, 61. [Google Scholar] [CrossRef]
- Han, Q.; Zheng, B.; Daines, L.; Sheikh, A. Long-Term Sequelae of COVID-19: A Systematic Review and Meta-Analysis of One-Year Follow-Up Studies on Post-COVID Symptoms. Pathogens 2022, 11, 269. [Google Scholar] [CrossRef]
- Hastie, C.E.; Lowe, D.J.; McAuley, A.; Winter, A.J.; Mills, N.L.; Black, C.; Scott, J.T.; O’Donnell, C.A.; Blane, D.N.; Browne, S.; et al. Outcomes among confirmed cases and a matched comparison group in the Long-COVID in Scotland study. Nat. Commun. 2022, 13, 5663. [Google Scholar] [CrossRef]
- Albtoosh, A.S.; Toubasi, A.A.; Oweidat, K.A.; Hansuneh, M.M.; Alshurafa, A.H.; Alfaqheri, D.L.; Farah, R.I. New symptoms and prevalence of postacute COVID-19 syndrome among nonhospitalized COVID-19 survivors. Sci. Rep. 2022, 12, 16921. [Google Scholar] [CrossRef]
- Parkin, A.; Davison, J.; Tarrant, R.; Ross, D.; Halpin, S.; Simms, A.; Salman, R.; Sivan, M. A Multidisciplinary NHS COVID-19 Service to Manage Post-COVID-19 Syndrome in the Community. J. Prim. Care Community Health 2022, 12, 21501327211010994. [Google Scholar] [CrossRef]
- Postolache, T.T.; Benros, M.E.; Brenner, L.A. Targetable biological mechanisms implicated in emergent psychiatric conditions associated with SARS-CoV-2 infection. JAMA Psychiatry 2020, 78, 353–354. [Google Scholar] [CrossRef]
- Troyer, E.A.; Kohn, J.N.; Hong, S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020, 87, 34–39. [Google Scholar] [CrossRef]
- Tsoukali, E.; Sifrim, D. Investigation of extraesophageal gastroesophageal reflux disease. Ann. Gastroenterol. 2013, 26, 290–295. [Google Scholar]
- Pauwels, A.; Blondeau, K.; Dupont, L.; Sifrim, D. Cough and gastroesophageal reflux: From the gastroenterologist end. Pulm. Pharmacol. Ther. 2009, 22, 135–138. [Google Scholar] [CrossRef]
- Decalmer, S.; Stovold, R.; Houghton, L.A.; Pearson, J.; Ward, C.; Kelsall, A.; Jones, H.; McGuinness, K.; Woodcock, A.; Smith, J.A. Chronic cough: Relationship between microaspiration, gastroesophageal reflux, and cough frequency. Chest 2012, 142, 958–964. [Google Scholar] [CrossRef]
- Bongiovanni, A.; Parisi, G.F.; Scuderi, M.G.; Licari, A.; Brambilla, I.; Marseglia, G.L.; Leonardi, S. Gastroesophageal reflux and respiratory diseases: Does a real link exist? Minerva Pediatr. 2019, 71, 515–523. [Google Scholar] [CrossRef]
- Vahia, I.V.; Jeste, D.V.; Reynolds, C.F., III. Older adults and the mental health effects of COVID-19. JAMA 2020, 324, 2253–2254. [Google Scholar] [CrossRef]
- Lee, E.E.; Depp, C.; Palmer, B.W.; Glorioso, D.; Daly, R.; Liu, J.; Tu, X.M.; Kim, H.C.; Tarr, P.; Yamada, Y. Jeste DV, High prevalence and adverse health effects of loneliness in community-dwelling adults across the lifespan. Int. Psychogeriatr. 2019, 31, 1447–1462. [Google Scholar] [CrossRef]
- Kohn, J.N.; Jester, D.J.; Dilmore, A.H.; Thomas, M.L.; Daly, R.; Jeste, D.V. Trends, heterogeneity, and correlates of mental health and psychological well-being later in life: Study of 590 community-dwelling adults aged 40-104 years. Aging Ment. Health 2022, 1–10. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, F.; Hou, W.; Silver, Z.; Wong, C.Y.; Chang, O.; Drakos, A.; Zuo, Q.K.; Huang, E. The prevalence of depressive symptoms, anxiety symptoms and sleep disturbances in higher education students during the COVID-19 pandemic: A systematic review and meta-analysis. Psychiatry Res. 2021, 301, 113863. [Google Scholar] [CrossRef]
- Wathelet, M.; Duhem, S.; Vaiva, G.; Baubet, T.; Habran, E.; Veerapa, E.; Debien, C.; Molenda, S.; Horn, M.; Grandgenèvre, P.; et al. Factors associated with mental health disorders among university students in France confined during the COVID-19 pandemic. JAMA Netw. Open 2020, 3, e20255. [Google Scholar] [CrossRef]
- Varma, P.; Junge, M.; Meaklim, H.; Jackson, M.L. Younger people are more vulnerable to stress, anxiety and depression during COVID-19 pandemic: A global cross-sectional survey. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110236. [Google Scholar] [CrossRef] [PubMed]
- Request the Complete HPI Data File, California Healthy Places Index. 2022. Available online: https://www.healthyplacesindex.org/request-hpi-data-file (accessed on 1 July 2022).
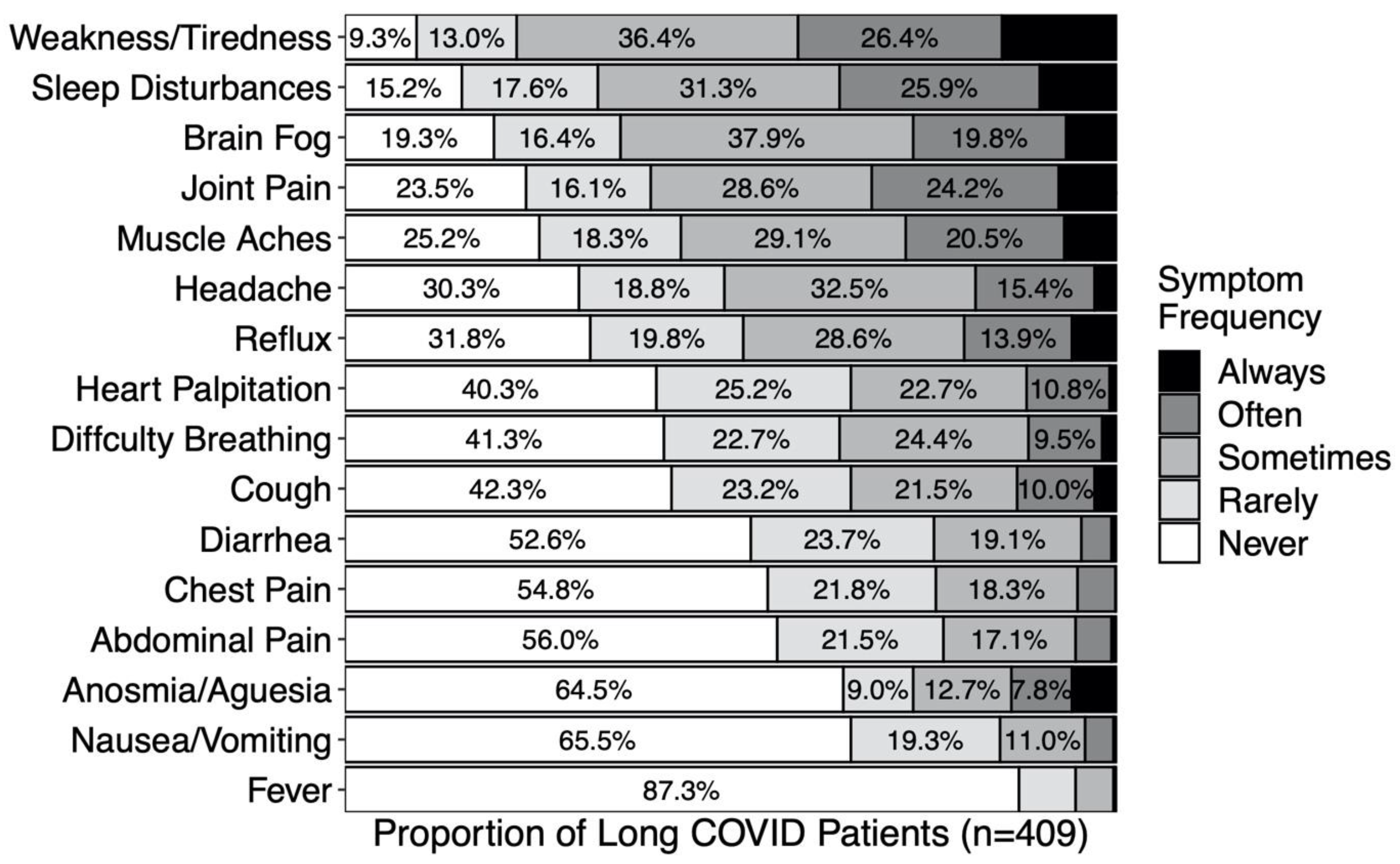
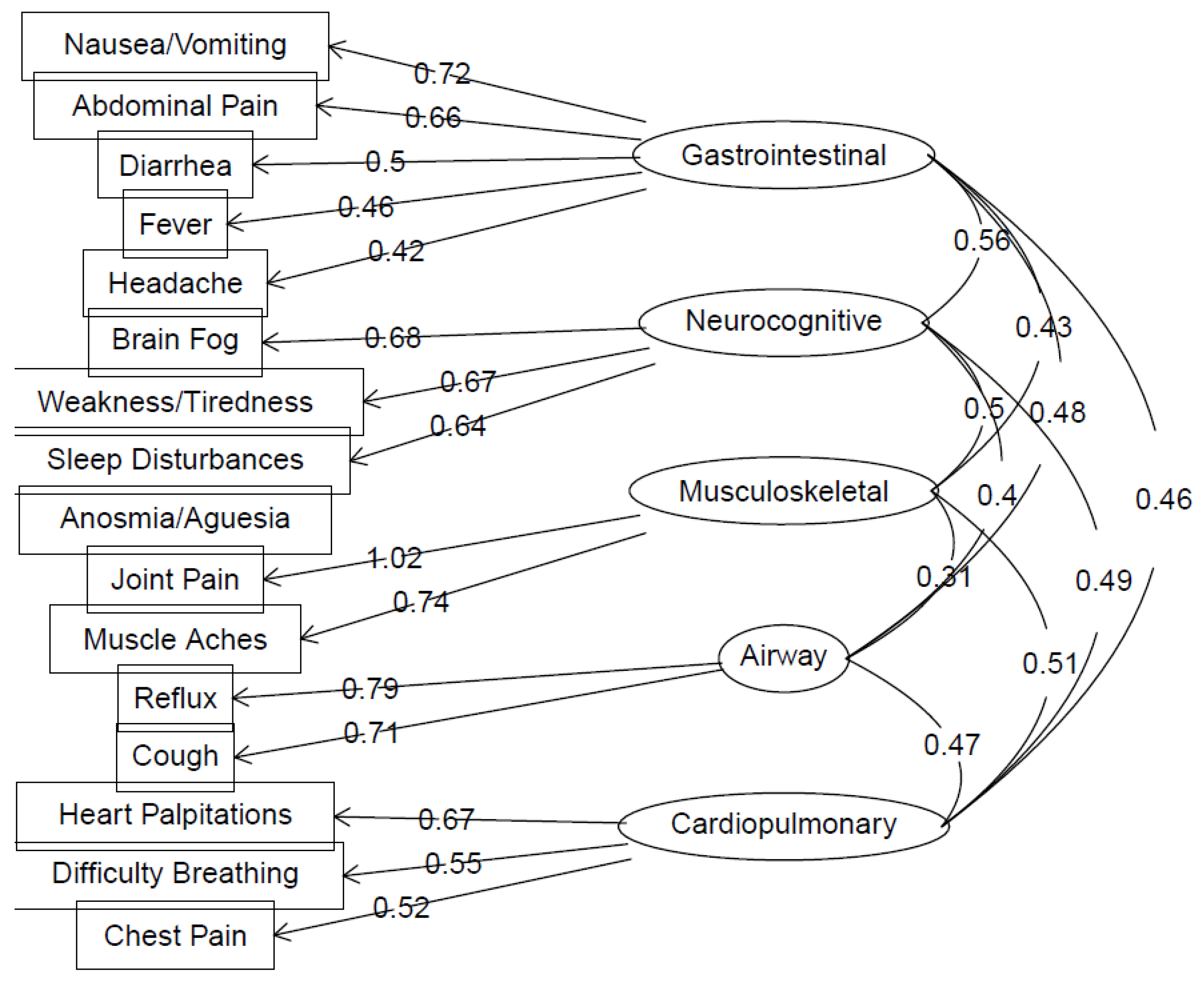
| No Long COVID Symptoms Reported (n = 488) | Long COVID Symptoms Reported (n = 421) | Test Statistics (99% CI) | |
|---|---|---|---|
| Age (years) | 51.4 (16.3) | 52.2 (15.0) | d = 0.05 (−0.12–0.22) |
| Race (%White/Black/AAPI/Other/NR) | 56.8/4.7/9.2/19.7/9.6 | 50.8/4.0/10.7/24.7/9.7 | X24 = 4.80, p > 0.01 |
| Sex (% Female) | 56.4% | 63.4% | OR = 1.34 (1.02–1.77) |
| HPI (Percentile score) | 58.0 (24.8) | 56.6 (27.5) | d = −0.06 (−0.23–0.12) |
| Acute COVID-19 hospitalization | 6.8% | 24.7% | OR = 4.53 (2.59–8.22) |
| Pre-COVID self-rated health | 4.27 (0.74) | 4.04 (0.80) | d = −0.29 (−0.46–−0.12) |
| Post-COVID self-rated health | 4.24 (0.73) | 3.25 (0.91) | d = −1.21 (−1.39–−1.02) |
| Monoclonal antibody treatment | 5.9% | 11.5% | OR = 2.05 (1.06–4.07) |
| Days since COVID+ result | 315 (3.4) | 348 (4.5) | d = 0.41 (0.23, 0.58) |
| GI Cluster | MSK Cluster | NC Cluster | AW Cluster | CP Cluster | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | Std. Beta | 99% CI | Std. Beta | 99% CI | Std. Beta | 99% CI | Std. Beta | 99% CI | Std. Beta | 99% CI |
| Age | −0.09 * | −0.18–0.00 | 0.21 *** | 0.11–0.30 | −0.03 | −0.11–0.04 | 0.03 | −0.06–0.12 | −0.01 | −0.08–0.07 |
| Sex [female] | 0.03 | −0.16–0.21 | −0.01 | −0.21–0.19 | 0.11 | −0.04–0.27 | −0.09 | −0.27–0.10 | −0.02 | −0.18–0.13 |
| Race [Black] | 0.06 | −0.39–0.51 | 0.07 | −0.42–0.57 | −0.10 | −0.48–0.29 | 0.04 | −0.41–0.50 | −0.14 | −0.53–0.26 |
| Race [AAPI] | −0.13 | −0.41–0.15 | 0.20 | −0.12–0.51 | −0.12 | −0.36–0.12 | −0.1 | −0.39–0.19 | 0.11 | −0.14–0.36 |
| Race [Other] | 0.05 | −0.17–0.26 | 0.27 *** | 0.04–0.51 | −0.01 | −0.19–0.17 | −0.15 | −0.37–0.07 | 0.01 | −0.18–0.19 |
| Race [NR] | −0.02 | −0.32–0.27 | 0.20 | −0.12–0.53 | 0.04 | −0.21–0.29 | −0.26 * | −0.56–0.04 | −0.01 | −0.26–0.25 |
| HPI percentile | −0.06 | −0.14–0.03 | −0.04 | −0.14–0.05 | 0.00 | −0.07–0.07 | −0.01 | −0.10–0.08 | 0.00 | −0.08–0.07 |
| COVID−19 hospitalization | 0.04 | −0.17–0.25 | 0.17 | −0.07–0.40 | 0.15 * | −0.02–0.33 | 0.01 | −0.20–0.23 | 0.11 | −0.08–0.29 |
| Pre-COVID self-rated health | −0.07 | −0.18–0.03 | −0.02 | −0.14–0.10 | −0.08 * | −0.18–0.01 | 0.01 | −0.10–0.12 | −0.01 | −0.10–0.09 |
| No. of symptoms | 0.81 *** | 0.72–0.90 | 0.69 *** | 0.59–0.79 | 0.79 *** | 0.71–0.87 | 0.72 *** | 0.63–0.81 | 0.78 *** | 0.70–0.86 |
| Observations | 377 | 377 | 377 | 377 | 377 | |||||
| R2/R2 adjusted | 0.638/0.629 | 0.569/0.559 | 0.701/0.694 | 0.562/0.551 | 0.682/0.674 | |||||
| Depression | Anxiety | |||
|---|---|---|---|---|
| OR | 99% CI | OR | 99% CI | |
| Sociodemographics | ||||
| Age | 0.98 | 0.95–1.01 | 0.96 ** | 0.94–0.99 |
| Sex [female] | 0.51 * | 0.21–1.19 | 0.68 | 0.28–1.66 |
| HPI Percentile | 1.01 | 0.99–1.02 | 1.01 | 0.99–1.03 |
| Race [Black] | 1.76 | 0.24–9.95 | 0.77 | 0.05–5.26 |
| Race [AAPI] | 0.63 | 0.13–2.46 | 0.29 * | 0.05–1.23 |
| Race [Other] | 1.37 | 0.52–3.61 | 1.09 | 0.42–2.76 |
| Race [NR] | 2.04 | 0.54–7.30 | 0.7 | 0.14–2.70 |
| Clinical characteristics | ||||
| COVID-19 hospitalization | 1.97 * | 0.81–4.81 | 1.37 | 0.54–3.47 |
| Pre-COVID self-rated health | 0.76 | 0.47–1.22 | 0.69 * | 0.42–1.11 |
| No. of Symptoms | 0.96 | 0.70–1.31 | 1.29 * | 0.95–1.77 |
| Symptom Clusters | ||||
| GI | 0.93 | 0.50–1.72 | 0.62 * | 0.33–1.15 |
| Neurocognitive | 5.86 *** | 2.71–13.78 | 2.83 *** | 1.36–6.14 |
| MSK | 0.99 | 0.55–1.77 | 0.77 | 0.41–1.40 |
| Airway | 0.91 | 0.46–1.78 | 0.83 | 0.42–1.58 |
| Cardiopulmonary | 0.89 | 0.44–1.80 | 1.19 | 0.60–2.40 |
| Observations | 377 | 375 | ||
| R2 Tjur | 0.281 | 0.256 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldhaber, N.H.; Kohn, J.N.; Ogan, W.S.; Sitapati, A.; Longhurst, C.A.; Wang, A.; Lee, S.; Hong, S.; Horton, L.E. Deep Dive into the Long Haul: Analysis of Symptom Clusters and Risk Factors for Post-Acute Sequelae of COVID-19 to Inform Clinical Care. Int. J. Environ. Res. Public Health 2022, 19, 16841. https://doi.org/10.3390/ijerph192416841
Goldhaber NH, Kohn JN, Ogan WS, Sitapati A, Longhurst CA, Wang A, Lee S, Hong S, Horton LE. Deep Dive into the Long Haul: Analysis of Symptom Clusters and Risk Factors for Post-Acute Sequelae of COVID-19 to Inform Clinical Care. International Journal of Environmental Research and Public Health. 2022; 19(24):16841. https://doi.org/10.3390/ijerph192416841
Chicago/Turabian StyleGoldhaber, Nicole H., Jordan N. Kohn, William Scott Ogan, Amy Sitapati, Christopher A. Longhurst, Angela Wang, Susan Lee, Suzi Hong, and Lucy E. Horton. 2022. "Deep Dive into the Long Haul: Analysis of Symptom Clusters and Risk Factors for Post-Acute Sequelae of COVID-19 to Inform Clinical Care" International Journal of Environmental Research and Public Health 19, no. 24: 16841. https://doi.org/10.3390/ijerph192416841
APA StyleGoldhaber, N. H., Kohn, J. N., Ogan, W. S., Sitapati, A., Longhurst, C. A., Wang, A., Lee, S., Hong, S., & Horton, L. E. (2022). Deep Dive into the Long Haul: Analysis of Symptom Clusters and Risk Factors for Post-Acute Sequelae of COVID-19 to Inform Clinical Care. International Journal of Environmental Research and Public Health, 19(24), 16841. https://doi.org/10.3390/ijerph192416841






