Low-Temperature Reduction Synthesis of γ–Fe2O3−x@biochar Catalysts and Their Combining with Peroxymonosulfate for Quinclorac Degradation
Abstract
1. Introduction
2. Experimental Sections
2.1. Reagents and Materials
2.2. Preparation of Catalysts
2.3. Batch Experimental Processes
2.4. Characterization and Analytical Methods
3. Results and Discussion
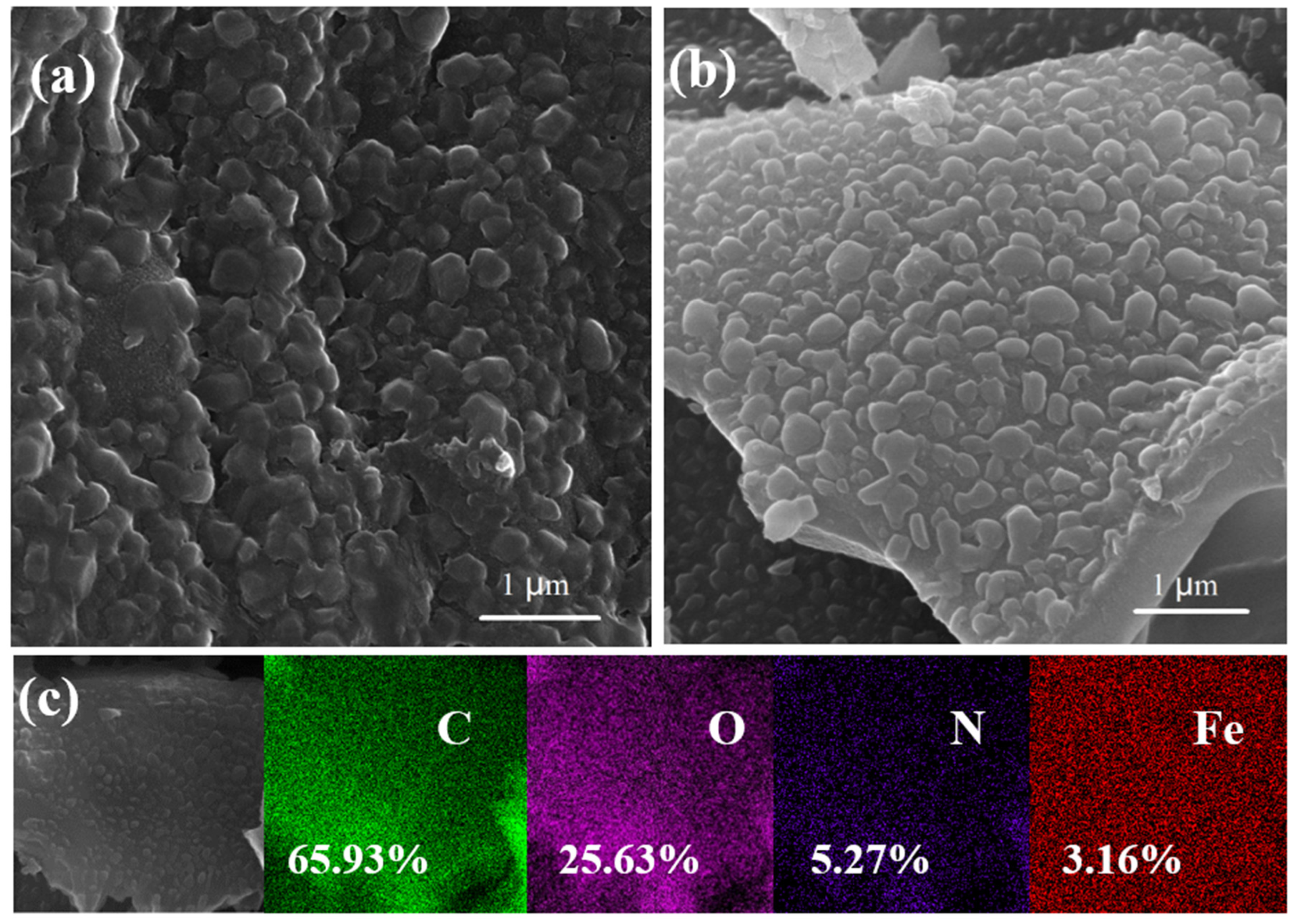
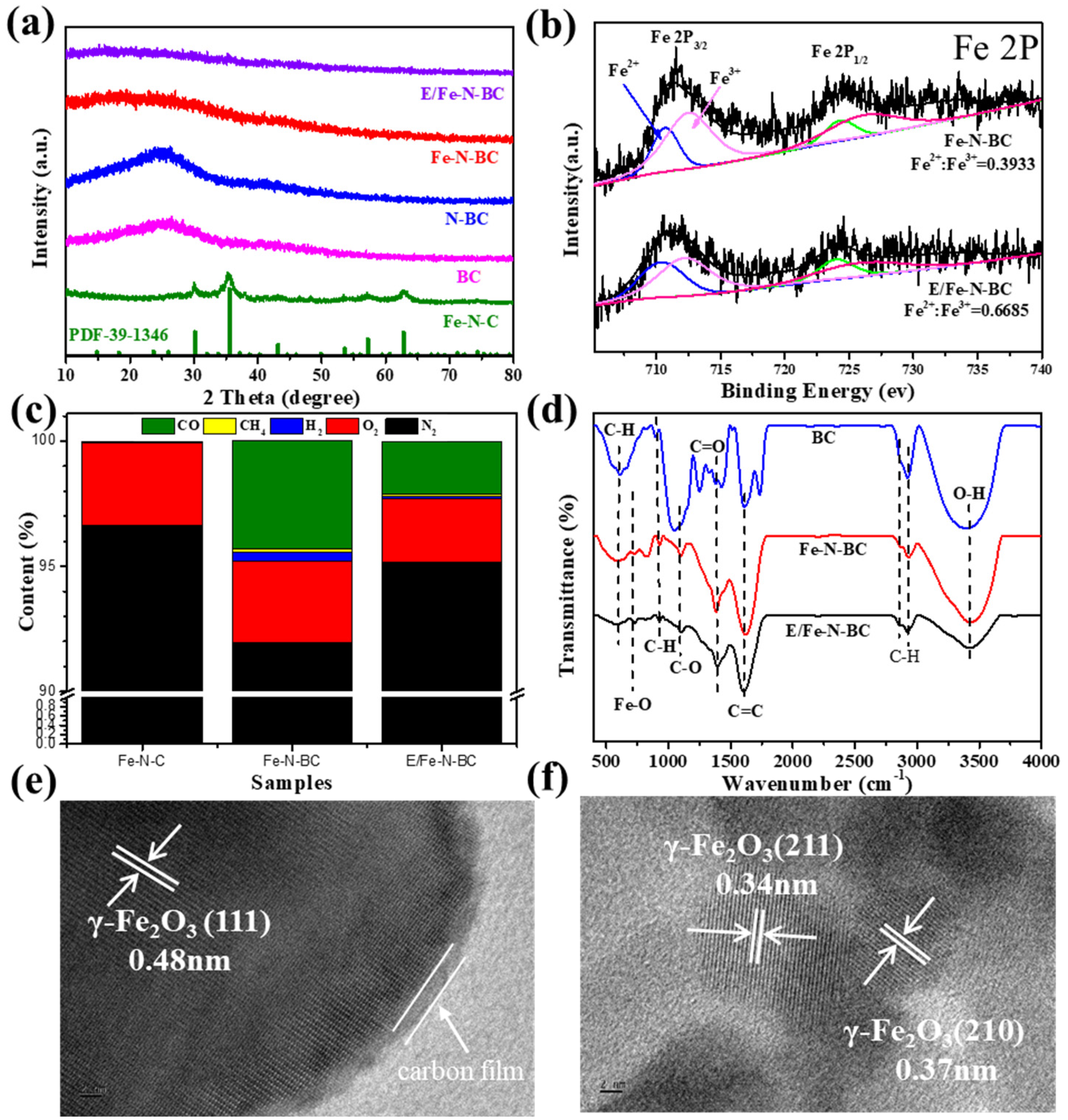
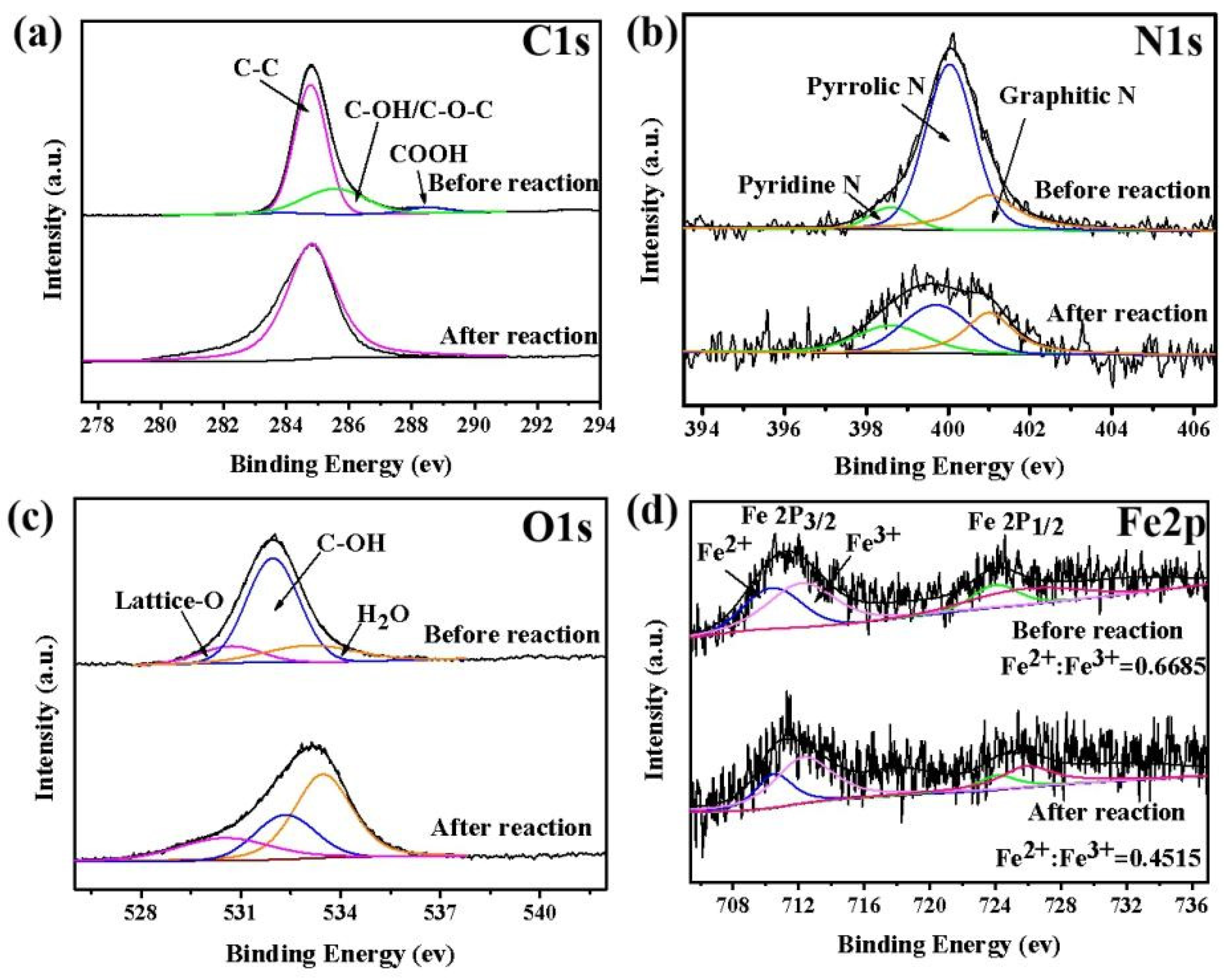
3.1. Characterization of Catalysts
3.2. Catalytic PMS for the Removal of QNC
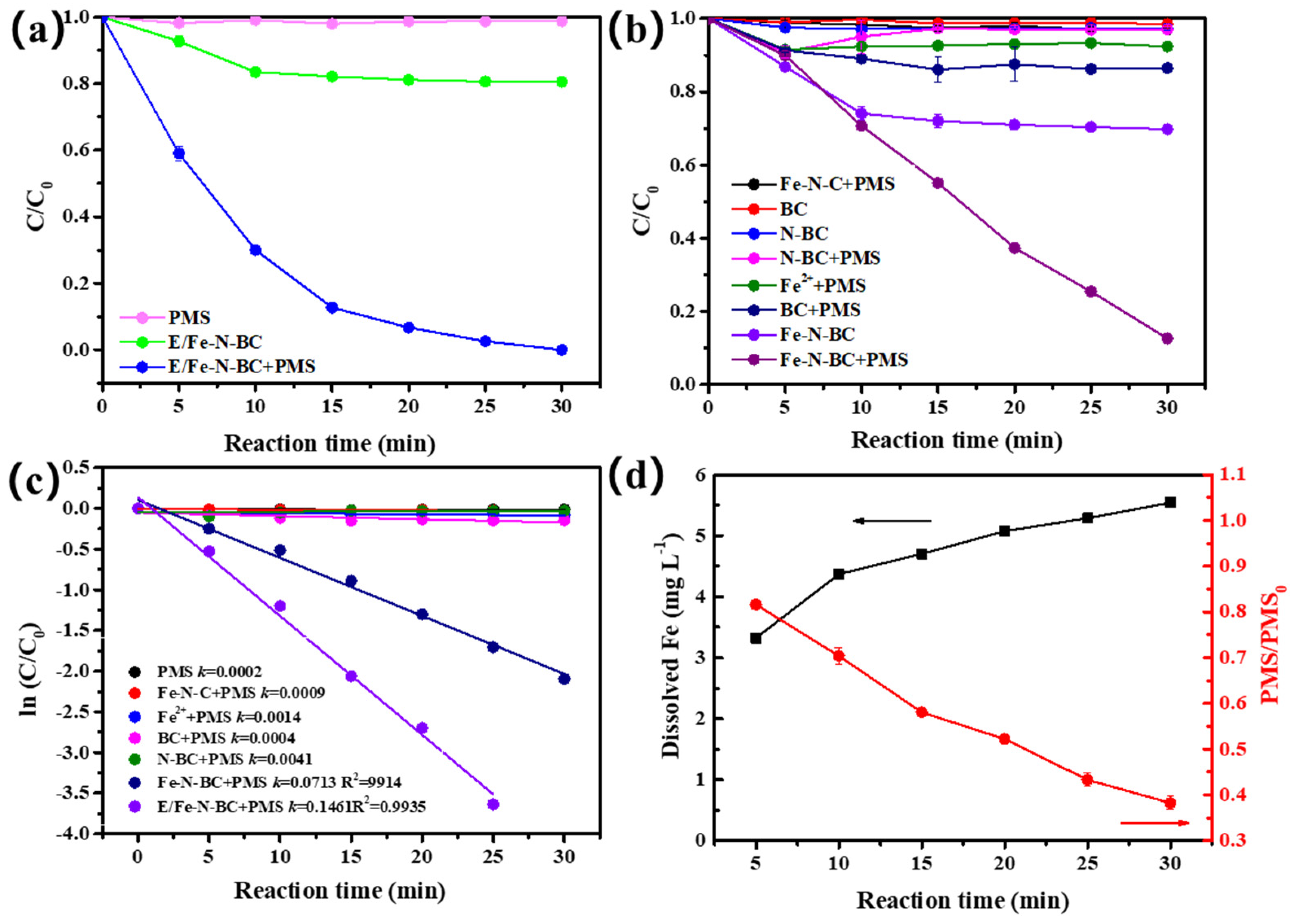
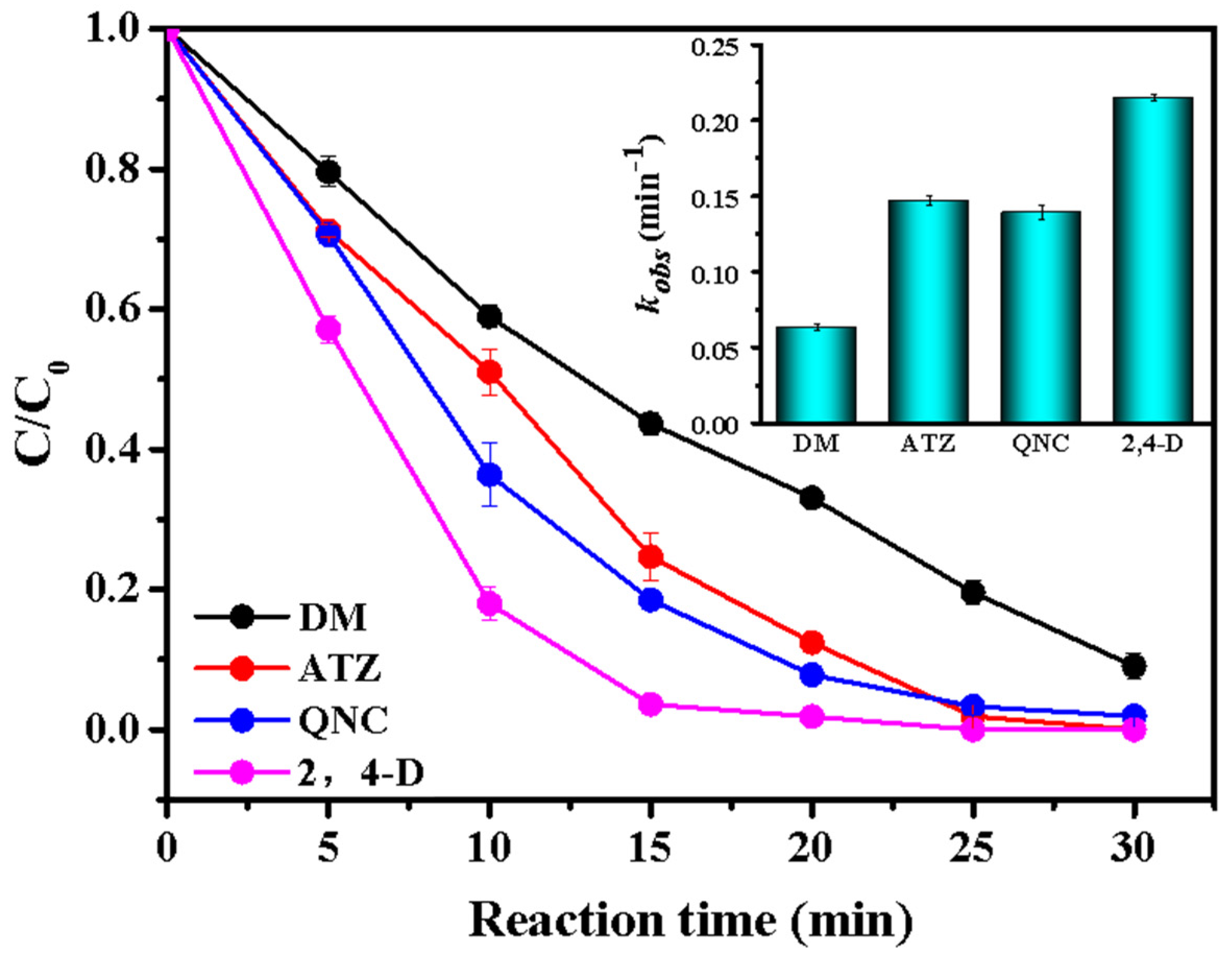
3.2.1. Evaluation for the Catalytic Activity
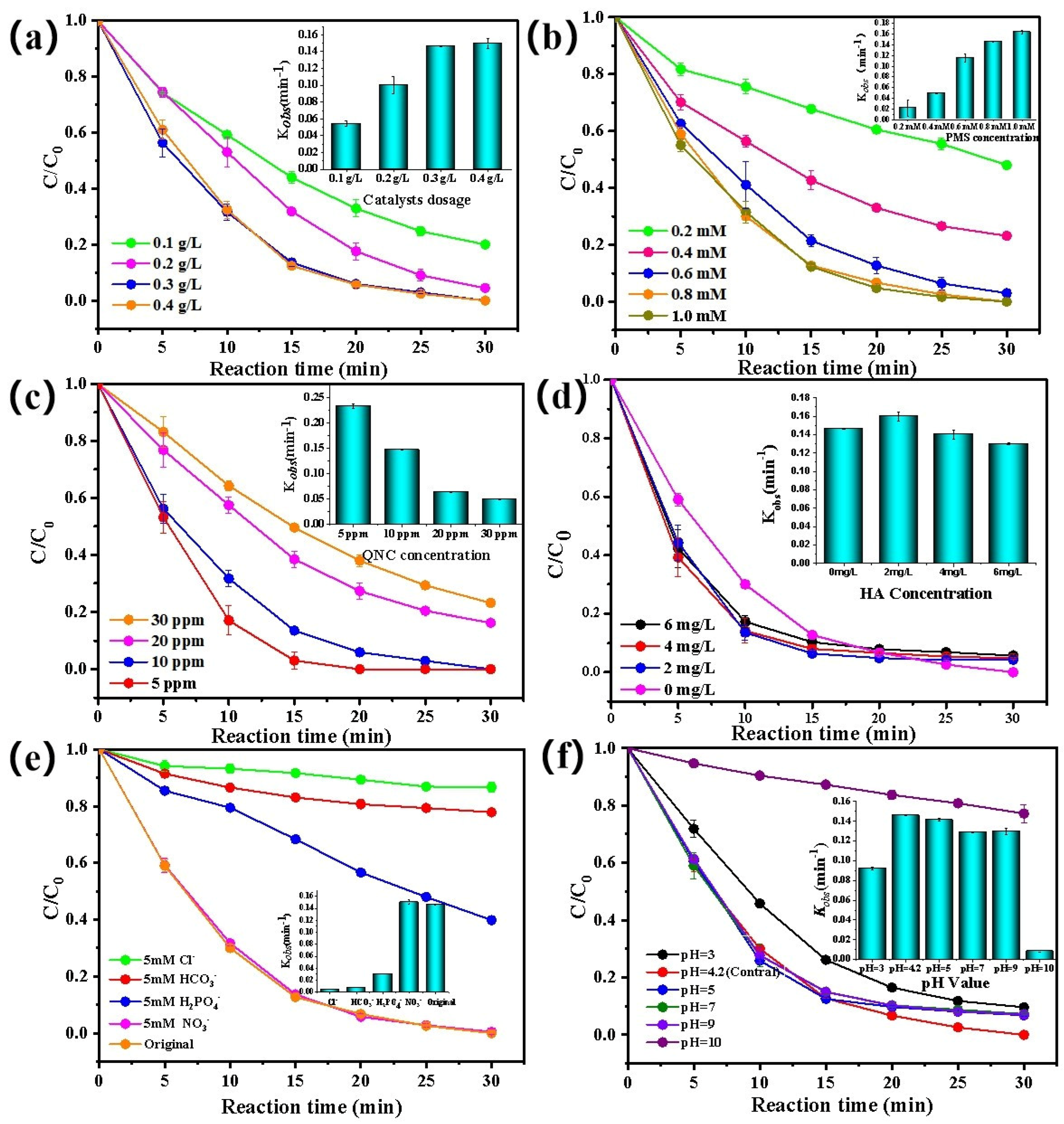
3.2.2. Effect of Catalyst Dosage on the Removal of QNC
3.2.3. Effect of PMS Concentration on the Removal of QNC
3.2.4. Effect of Initial QNC Concentration on the Removal of QNC
3.2.5. Effect of HA and Anions on the Removal of QNC
3.2.6. Effect of pH on the Removal of QNC
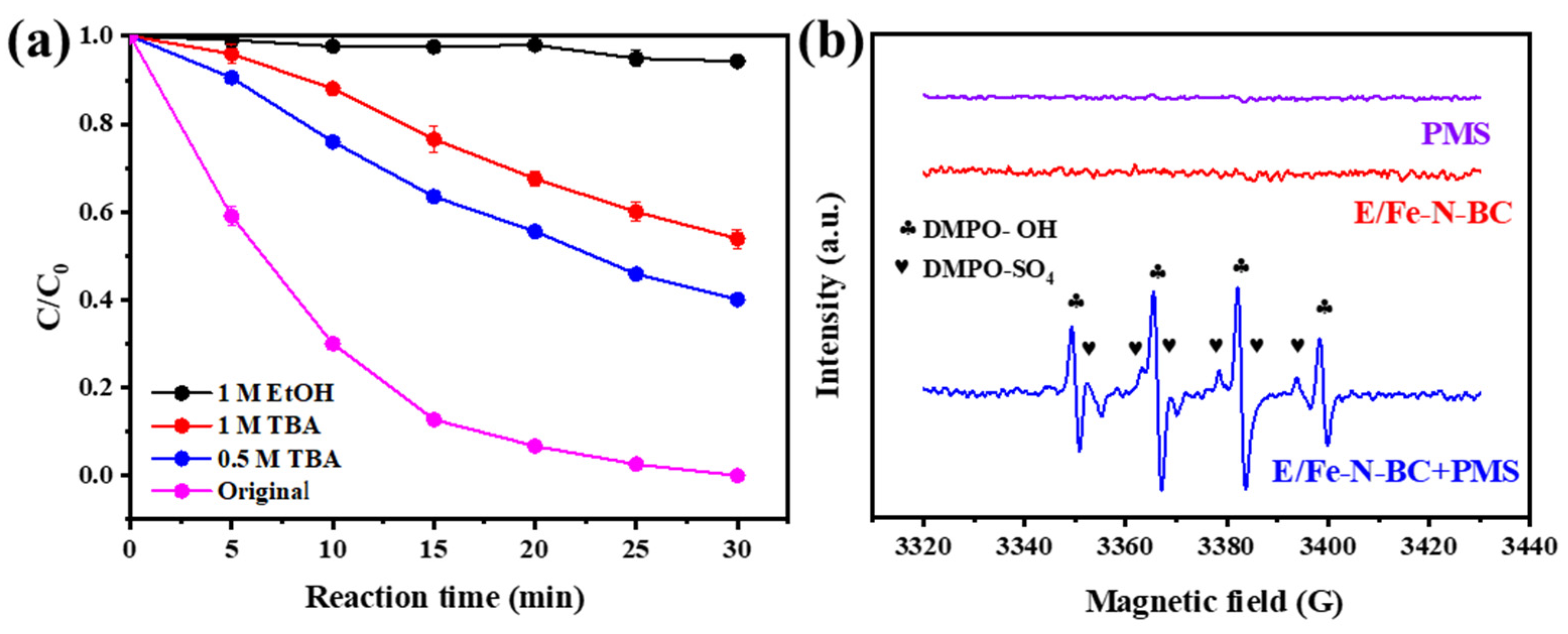
3.3. Radicals Identification
3.4. Possible Degradation Mechanisms
3.5. Stability and Practical Application of E/Fe–N–BC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naylor, R. Herbicide Use in Asian Rice Production. World Dev. 1994, 22, 55–70. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Scott, R.C.; Smith, K.L.; Still, J.; Meier, J. Herbicide Options for Rice Cutgrass (Leersia oryzoides) Control. Weed Technol. 2009, 23, 1–5. [Google Scholar] [CrossRef]
- Grossmann, K. Quinclorac belongs to a New Class of Highly Selective Auxin Herbicides. Weed Sci. 1998, 46, 707–716. [Google Scholar] [CrossRef]
- Hill, B.D.; Moyer, J.R.; Inaba, D.J.; Doram, R. Effect of moisture on quinclorac dissipation in Lethbridge soil. Can. J. Plant Sci. 1998, 78, 697–702. [Google Scholar] [CrossRef]
- Resgalla, C., Jr.; Noldin, J.A.; Tamanaha, M.S.; Deschamps, F.C.; Eberhardt, D.S.; Rörig, L.R. Risk analysis of herbicide quinclorac residues in irrigated rice areas, Santa Catarina, Brazil. Ecotoxicology 2007, 16, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Li, X.; Xu, X.; Gao, S. Phytotoxicity of four herbicides on Ceratophyllum demersum, Vallisneria natans and Elodea nuttallii. J. Environ. Sci. 2009, 21, 307–312. [Google Scholar] [CrossRef]
- Moraes, B.S.; Loro, V.L.; Glusczak, L.; Pretto, A.; Menezes, C.; Marchezan, E.; de Oliveira Machado, S. Effects of four rice herbicides on some metabolic and toxicology parameters of teleost fish (Leporinus obtusidens). Chemosphere 2007, 68, 1597–1601. [Google Scholar] [CrossRef]
- Neta, P.; Madhavan, V.; Zemel, H.; Fessenden, R.W. Fessenden, Rate Constants and Mechanism of Reaction of SO4 with Aromatic Compounds. J. Am. Chem. Soc. 1977, 99, 163–164. [Google Scholar] [CrossRef]
- Betterton, E.A.; Hoffmann, M.R. Kinetics and Mechanism of the Oxidation of Aqueous Hydrogen Sulfide by Peroxymonosulfate. Environ. Sci. Technol. 1990, 24, 1819–1824. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Jawad, A.; Lang, J.; Liao, Z.; Khan, A.; Ifthikar, J.; Lv, Z.; Long, S.; Chen, Z.; Chen, Z. Activation of persulfate by CuOx@Co-LDH: A novel heterogeneous system for contaminant degradation with broad pH window and controlled leaching. Chem. Eng. J. 2018, 335, 548–559. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Lin, C.; Zhang, X.; Ma, J.; Tan, H.; Ye, W. Degradation of sulfamethoxazole by microwave-activated persulfate: Kinetics, mechanism and acute toxicity. Chem. Eng. J. 2014, 249, 6–14. [Google Scholar] [CrossRef]
- Cashman, M.A.; Kirschenbaum, L.; Holowachuk, J.; Boving, T.B. Identification of hydroxyl and sulfate free radicals involved in the reaction of 1,4-dioxane with peroxone activated persulfate oxidant. J. Hazard. Mater. 2019, 380, 120875. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Mishra, I.M.; Dionysiou, D.D.; Kumar, V. Oxidative removal of Bisphenol A by UV-C/peroxymonosulfate (PMS): Kinetics, influence of co-existing chemicals and degradation pathway. Chem. Eng. J. 2015, 276, 193–204. [Google Scholar] [CrossRef]
- Wei, Z.; Villamena, F.A.; Weavers, L.K. Kinetics and Mechanism of Ultrasonic Activation of Persulfate: An in Situ EPR Spin Trapping Study. Environ. Sci. Technol. 2017, 51, 3410–3417. [Google Scholar] [CrossRef]
- Liang, C.; Lei, J.H. Identification of Active Radical Species in Alkaline Persulfate Oxidation. Water Environ. Res. 2015, 87, 656–659. [Google Scholar] [CrossRef]
- Sun, Q.-T.; Xu, B.-D.; Yang, J.; Qian, T.-T.; Jiang, H. Layered oxides supported Co-Fe bimetal catalyst for carbamazepine degradation via the catalytic activation of peroxymonosulfate. Chem. Eng. J. 2020, 400, 125899. [Google Scholar] [CrossRef]
- Li, J.; Yang, L.; Lai, B.; Liu, C.; He, Y.; Yao, G.; Li, N. Recent progress on heterogeneous Fe-based materials induced persulfate activation for organics removal. Chem. Eng. J. 2021, 414, 128674. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Leiknes, T. Oxidation of Refractory Benzothiazoles with PMS/CuFe2O4: Kinetics and Transformation Intermediates. Environ. Sci. Technol. 2016, 50, 5864–5873. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, H.; Croue, J.P. Production of sulfate radical from peroxymonosulfate induced by a magnetically separable CuFe2O4 spinel in water: Efficiency, stability, and mechanism. Environ. Sci. Technol. 2013, 47, 2784–2791. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ren, Y.; Ji, F.; Lai, B. Heterogeneous catalytic oxidation for the degradation of p-nitrophenol in aqueous solution by persulfate activated with CuFe2O4 magnetic nano-particles. Chem. Eng. J. 2017, 324, 63–73. [Google Scholar] [CrossRef]
- Li, J.; Xu, M.; Yao, G.; Lai, B. Enhancement of the degradation of atrazine through CoFe2O4 activated peroxymonosulfate (PMS) process: Kinetic, degradation intermediates, and toxicity evaluation. Chem. Eng. J. 2018, 348, 1012–1024. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, W.; Liu, J.; Lin, P.; Zhang, J.; Huang, T.; Liu, K. Mesoporous sulfur-doped CoFe2O4 as a new Fenton catalyst for the highly efficient pollutants removal. Appl. Catal. B: Environ. 2021, 295, 120273. [Google Scholar] [CrossRef]
- Xian, G.; Niu, L.; Zhang, G.; Zhou, N.; Long, Z.; Zhi, R. An efficient CuO-γFe2O3 composite activates persulfate for organic pollutants removal: Performance, advantages and mechanism. Chemosphere 2020, 242, 125191. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Zhang, G.; Xian, G.; Ren, Z.; Wei, T.; Li, Q.; Zhang, Y.; Zou, Z. Tetracycline degradation by persulfate activated with magnetic γ-Fe2O3/CeO2 catalyst: Performance, activation mechanism and degradation pathway. Sep. Purif. Technol. 2021, 259, 118156. [Google Scholar] [CrossRef]
- Yu, J.; Tang, L.; Pang, Y.; Zeng, G.; Feng, H.; Zou, J.; Wang, J.; Feng, C.; Zhu, X.; Ouyang, X.; et al. Hierarchical porous biochar from shrimp shell for persulfate activation: A two-electron transfer path and key impact factors. Appl. Catal. B Environ. 2020, 260, 118160. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, Z.; Chen, Z.; Bi, X.T. Reduction of hematite (Fe2O3) to metallic iron (Fe) by CO in a micro fluidized bed reaction analyzer: A multistep kinetics study. Powder Technol. 2017, 316, 410–420. [Google Scholar] [CrossRef]
- Hammam, A.; Cao, Y.; El-Geassy, A.-H.A.; El-Sadek, M.H.; Li, Y.; Wei, H.; Omran, M.; Yu, Y. Non-Isothermal Reduction Kinetics of Iron Ore Fines with Carbon-Bearing Materials. Metals 2021, 11, 1137. [Google Scholar] [CrossRef]
- Guo, D.; Cui, B.; Chen, Z.; Yan, W.; Ji, B.; Zhang, Q.; Liu, Y.; Luo, S.; Hu, M.; Ruan, R. Biomass enhances the reduction of oxidized pellets with carbon monoxide. Bioresour. Technol. 2021, 331, 124973. [Google Scholar] [CrossRef]
- Bhosale, T.T.; Shinde, H.M.; Gavade, N.L.; Babar, S.B.; Gawade, V.V.; Sabale, S.R.; Kamble, R.J.; Shirke, B.S.; Garadkar, K.M. Biosynthesis of SnO2 nanoparticles by aqueous leaf extract of Calotropis gigantea for photocatalytic applications. J. Mater. Sci. Mater. Electron. 2018, 29, 6826–6834. [Google Scholar] [CrossRef]
- Long, F.; He, J.; Zhang, M.; Wu, X.; Mo, S.; Zou, Z.; Zhou, Y. Microwave-hydrothermal synthesis of Co-doped FeS2 as a visible-light photocatalyst. J. Mater. Sci. 2015, 50, 1848–1854. [Google Scholar] [CrossRef]
- Pang, J.; Guo, P.; Zhao, P.; Cao, C.; Zhang, D. Influence of Size of Hematite Powder on Its Reduction Kinetics by H2 at Low Temperature. J. Iron Steel Res. Int. 2009, 16, 7–11. [Google Scholar] [CrossRef]
- Pal, S.; De, G. A New Approach for the Synthesis of Au-Ag Alloy Nanoparticle Incorporated SiO2 Films. Chem. Mater 2005, 17, 6161–6166. [Google Scholar] [CrossRef]
- Montoya-Zamora, J.M.; Martinez-De La Cruz, A.; Cuéllar, E.L. Synthesis of BiOI photocatalyst by microwave method using EDTA as retarder of the reaction. Res. Chem. Intermed. 2016, 43, 2545–2563. [Google Scholar] [CrossRef]
- Hu, P.; Su, H.; Chen, Z.; Yu, C.; Li, Q.; Zhou, B.; Alvarez, P.J.J.; Long, M. Selective Degradation of Organic Pollutants Using an Efficient Metal-Free Catalyst Derived from Carbonized Polypyrrole via Peroxymonosulfate Activation. Environ. Sci. Technol. 2017, 51, 11288–11296. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Xie, M.; Kong, L.; Natarajan, V.; Ma, L.; Zhan, J. The magnetic biochar derived from banana peels as a persulfate activator for organic contaminants degradation. Chem. Eng. J. 2019, 372, 294–303. [Google Scholar] [CrossRef]
- Guo, R.; Wang, Y.; Li, J.; Cheng, X.; Dionysiou, D.D. Sulfamethoxazole degradation by visible light assisted peroxymonosulfate process based on nanohybrid manganese dioxide incorporating ferric oxide. Appl. Catal. B Environ. 2020, 278, 119297. [Google Scholar] [CrossRef]
- Wu, G.; Tan, X.; Li, G.; Hu, C. Effect of preparation method on the physical and catalytic property of nanocrystalline Fe2O3. J. Alloy. Compd. 2010, 504, 371–376. [Google Scholar] [CrossRef]
- Ponomar, V.P.; Brik, O.B.; Cherevko, Y.I.; Ovsienko, V.V. Kinetics of hematite to magnetite transformation by gaseous reduction at low concentration of carbon monoxide. Chem. Eng. Res. Des. 2019, 148, 393–402. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, X.; Chen, B.; Ma, J.; Niu, X.; Zhang, D.; Lin, Z.; Fu, M.; Zhou, S. Enhanced adsorption of sulfamethoxazole from aqueous solution by Fe-impregnated graphited biochar. J. Clean. Prod. 2020, 256, 120662. [Google Scholar] [CrossRef]
- Babar, S.; Gavade, N.; Shinde, H.; Mahajan, P.; Lee, K.H.; Mane, N.; Deshmukh, A.; Garadkar, K.; Bhuse, V. Evolution of Waste Iron Rust into Magnetically Separable g-C3N4-Fe2O3 Photocatalyst: An Efficient and Economical Waste Management Approach. ACS Appl. Nano Mater. 2018, 1, 4682–4694. [Google Scholar] [CrossRef]
- Tang, L.; Liu, Y.; Wang, J.; Zeng, G.; Deng, Y.; Dong, H.; Feng, H.; Wang, J.; Peng, B. Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: Electron transfer mechanism. Appl. Catal. B Environ. 2018, 231, 1–10. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, P.; Xu, S.; Cheng, G.; Li, Z.; Li, Y.; Li, K.; Ma, S. Fabrication of Fe3O4 and graphitized porous biochar composites for activating peroxymonosulfate to degrade p-hydroxybenzoic acid: Insights on the mechanism. Chem. Eng. J. 2019, 375, 121980. [Google Scholar] [CrossRef]
- Liang, C.; Wang, Z.S.; Bruell, C.J. Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere 2007, 66, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Xiao, S.; Lin, Y.; Yu, P.; Zhong, M.-E.; Yang, L.; Wang, H.; Su, L.; Liao, C.; Zhou, Y.; et al. Attapulgite-supported nano-Fe0/peroxymonsulfate for quinclorac removal: Performance, mechanism and degradation pathway. Chem. Eng. J. 2019, 360, 104–114. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, Y.; Wu, G.; Wei, F.; Li, X.; Chen, H.; Wang, S. Sulfate radicals induced from peroxymonosulfate by cobalt manganese oxides (Co(x)Mn(3-x)O4) for Fenton-Like reaction in water. J. Hazard. Mater. 2015, 296, 128–137. [Google Scholar] [CrossRef]
- Wang, L.; Lan, X.; Peng, W.; Wang, Z. Uncertainty and misinterpretation over identification, quantification and transformation of reactive species generated in catalytic oxidation processes: A review. J. Hazard. Mater. 2021, 408, 124436. [Google Scholar] [CrossRef]
- Fang, G.; Gao, J.; Dionysiou, D.D.; Liu, C.; Zhou, D. Activation of persulfate by quinones: Free radical reactions and implication for the degradation of PCBs. Environ. Sci. Technol. 2013, 47, 4605–4611. [Google Scholar] [CrossRef]
- Peng, L.; Duan, X.; Shang, Y.; Gao, B.; Xu, X. Engineered carbon supported single iron atom sites and iron clusters from Fe-rich Enteromorpha for Fenton-like reactions via nonradical pathways. Appl. Catal. B Environ. 2021, 287, 119963. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Z.; Zhu, Y.; Li, T.; Hu, C. New Insights into the Generation of Singlet Oxygen in the Metal-Free Peroxymonosulfate Activation Process: Important Role of Electron-Deficient Carbon Atoms. Environ. Sci. Technol. 2020, 54, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Liu, C.; Ji, Y.; Yang, Q.; Chen, L.; Jiang, C.; Cai, T. Mechanism insight of degradation of norfloxacin by magnetite nanoparticles activated persulfate: Identification of radicals and degradation pathway. Chem. Eng. J. 2017, 308, 330–339. [Google Scholar] [CrossRef]
- Zhao, G.; Zou, J.; Chen, X.; Liu, L.; Wang, Y.; Zhou, S.; Long, X.; Yu, J.; Jiao, F. Iron-based catalysts for persulfate-based advanced oxidation process: Microstructure, property and tailoring. Chem. Eng. J. 2021, 421, 127845. [Google Scholar] [CrossRef]
- Zhou, N.; Zu, J.; Yang, L.; Shu, X.; Guan, J.; Deng, Y.; Gong, D.; Ding, C.; Zhong, M.E. Cobalt (0/II) incorporated N-doped porous carbon as effective heterogeneous peroxymonosulfate catalyst for quinclorac degradation. J. Colloid Interface Sci. 2020, 563, 197–206. [Google Scholar] [CrossRef]
- Huang, S.; Su, Y.; Luo, W.; He, Q.; Huang, S.; Zhou, N.; Zhou, Z. Kinetic analysis and in-situ no support catalytic pyrolysis product distribution of Chinese herb residue. J. Anal. Appl. Pyrol. 2021, 156, 105114. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, M.-e.; Tong, G.; Sun, J.; Zhou, N.; Ding, C.; Liu, X.; Merchant, A.; Zhou, X. Low-Temperature Reduction Synthesis of γ–Fe2O3−x@biochar Catalysts and Their Combining with Peroxymonosulfate for Quinclorac Degradation. Int. J. Environ. Res. Public Health 2022, 19, 16790. https://doi.org/10.3390/ijerph192416790
Zhong M-e, Tong G, Sun J, Zhou N, Ding C, Liu X, Merchant A, Zhou X. Low-Temperature Reduction Synthesis of γ–Fe2O3−x@biochar Catalysts and Their Combining with Peroxymonosulfate for Quinclorac Degradation. International Journal of Environmental Research and Public Health. 2022; 19(24):16790. https://doi.org/10.3390/ijerph192416790
Chicago/Turabian StyleZhong, Mei-e, Gongsong Tong, Jingchun Sun, Nan Zhou, Chunxia Ding, Xiangying Liu, Austin Merchant, and Xuguo Zhou. 2022. "Low-Temperature Reduction Synthesis of γ–Fe2O3−x@biochar Catalysts and Their Combining with Peroxymonosulfate for Quinclorac Degradation" International Journal of Environmental Research and Public Health 19, no. 24: 16790. https://doi.org/10.3390/ijerph192416790
APA StyleZhong, M.-e., Tong, G., Sun, J., Zhou, N., Ding, C., Liu, X., Merchant, A., & Zhou, X. (2022). Low-Temperature Reduction Synthesis of γ–Fe2O3−x@biochar Catalysts and Their Combining with Peroxymonosulfate for Quinclorac Degradation. International Journal of Environmental Research and Public Health, 19(24), 16790. https://doi.org/10.3390/ijerph192416790







