Carbonyl Profiles of Electronic Nicotine Delivery System (ENDS) Aerosols Reflect Both the Chemical Composition and the Numbers of E-Liquid Ingredients–Focus on the In Vitro Toxicity of Strawberry and Vanilla Flavors
Abstract
1. Introduction
2. Methods
2.1. ENDS Aerosol Generation and Chemical Characterization
2.2. In Vitro Assay
2.3. Cell Culture
2.4. Air-Liquid Interface (ALI) Exposures
2.5. Cell Viability
2.6. Extracellular Reactive Oxygen Species (ROS) Measurements
2.7. Extracellular Nitric Oxide (NO) Measurements
2.8. RNA Extraction and qRT-PCR
2.9. Statistical Analyses
3. Results
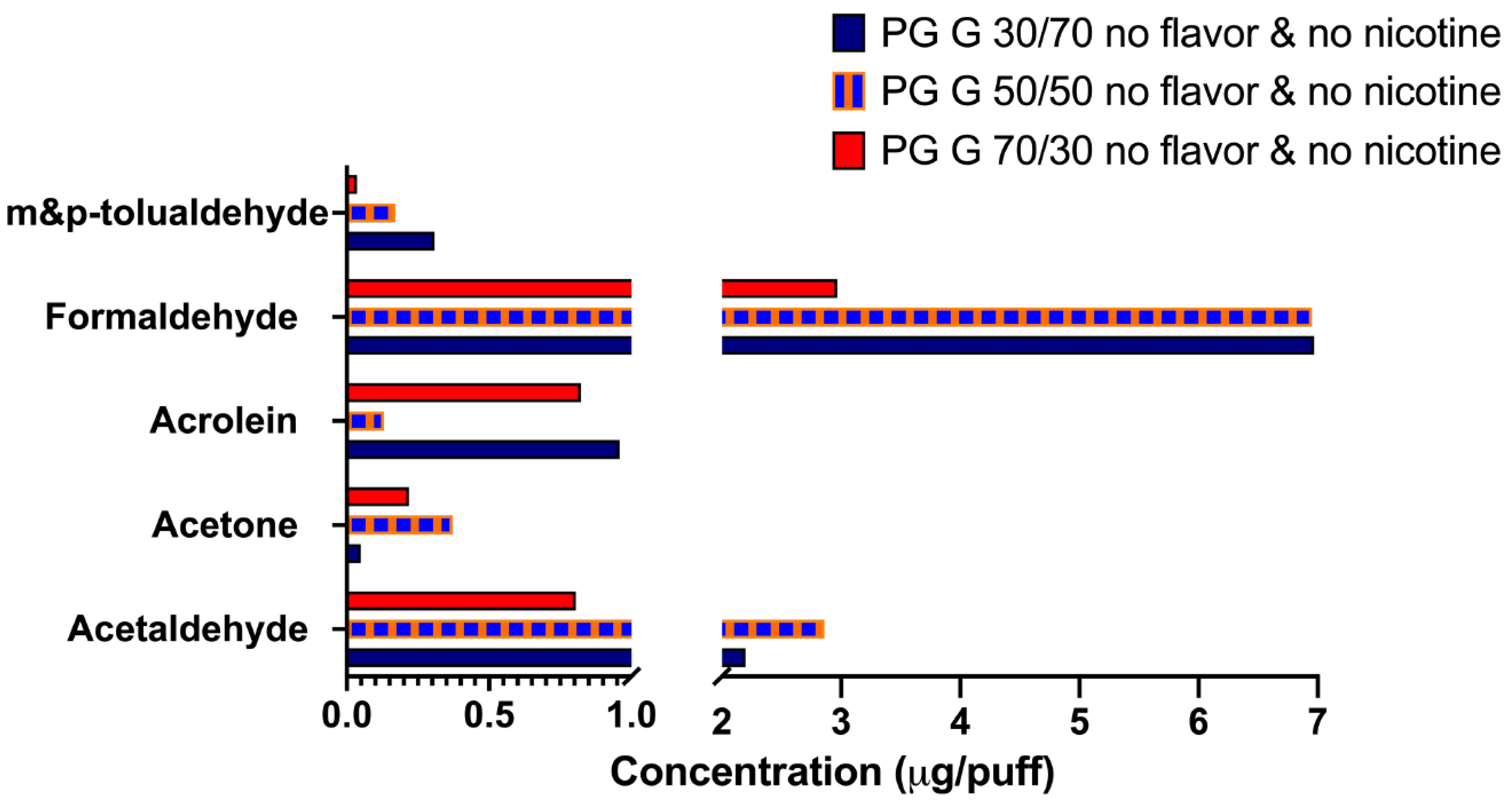
3.1. ENDS Aerosols Composed Solely of PG and G Generated by a Tank-Style Device Contain Carbonyls
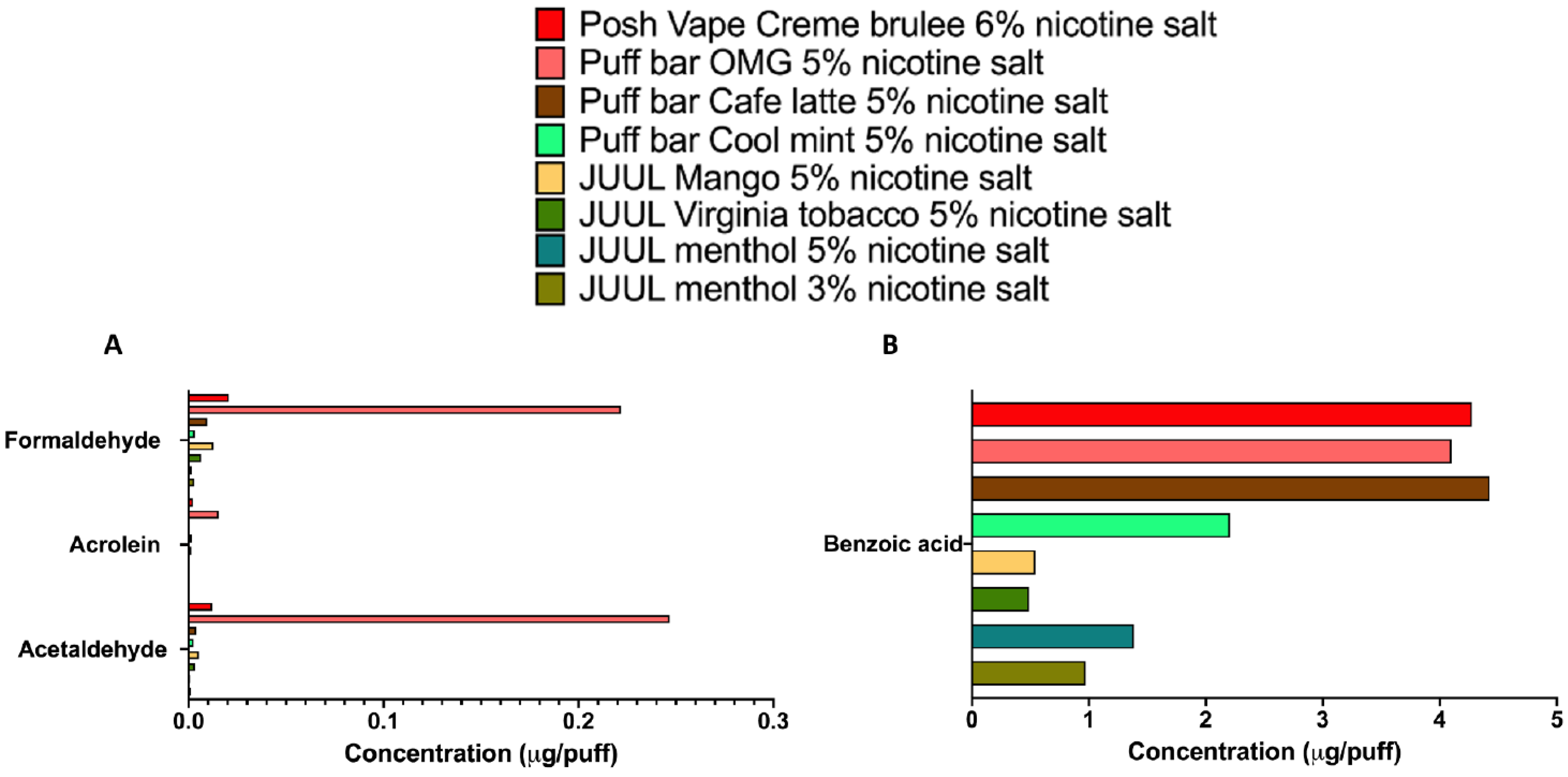
3.2. Carbonyls Are Present in Strawberry-, Vanilla- and Catalan Cream-Flavored E-Cig Aerosols
3.3. ENDS Aerosols Produced by Fourth Generation Devices Contain Trace Levels of Carbonyls but Generate High Levels of Benzoic Acid
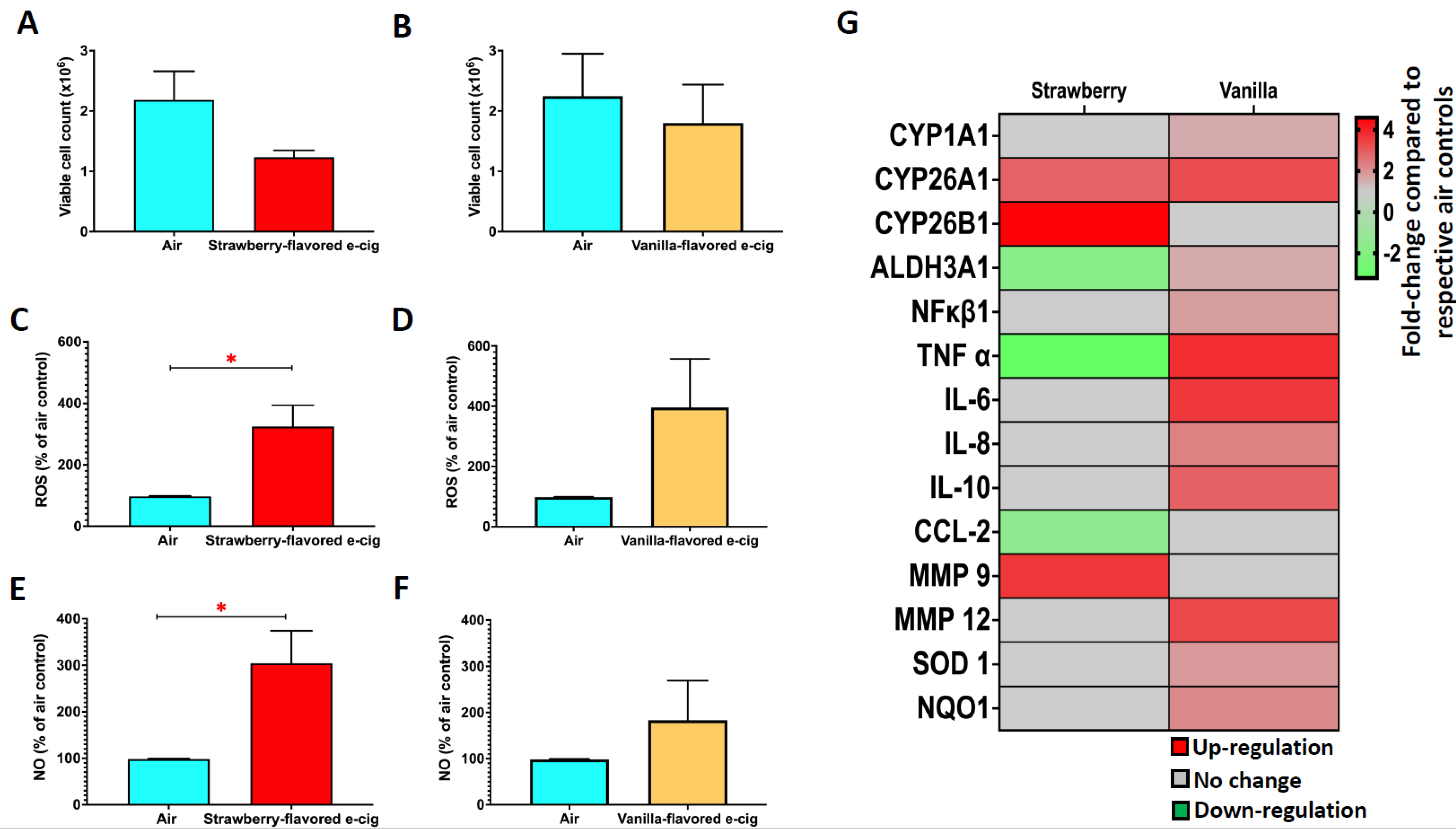
3.4. 1-h of Exposure at the ALI to Strawberry-Flavored E-Cig Aerosols Cause Oxidative Stress, While Vanilla-Flavored e-Cig Aerosols Induce Transcriptional Changes in BEAS-2B Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirbolouk, M.; Boakye, E.; Obisesan, O.; Osei, A.D.; Dzaye, O.; Osuji, N.; Erhabor, J.; Stokes, A.C.; El-Shahawy, O.; Rodriguez, C.J.; et al. E-cigarette use among high school students in the United States prior to the COVID-19 pandemic: Trends, correlates, and sources of acquisition. Prev. Med. Rep. 2022, 29, 101925. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.; Reyes-Guzman, C.; Grana, R.; Choi, K.; Freedman, N.D. Demographic Characteristics, Cigarette Smoking, and e-Cigarette Use Among US Adults. JAMA Netw. Open 2020, 3, e2020694. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Duan, Z.; Kwok, J.; Binns, S.; Vera, L.E.; Kim, Y.; Szczypka, G.; Emery, S.L. Vaping versus JUULing: How the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob. Control 2019, 28, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Hickman, E.; Payton, A.; Duffney, P.; Wells, H.; Ceppe, A.S.; Brocke, S.; Bailey, A.; Rebuli, M.E.; Robinette, C.; Ring, B.; et al. Biomarkers of Airway Immune Homeostasis Differ Significantly with Generation of E-Cigarettes. Am. J. Respir. Crit. Care Med. 2022, 206, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhan, Y.; Wang, L.; Leischow, S.J.; Zeng, D.D. Analysis of symptoms and their potential associations with e-liquids’ components: A social media study. BMC Public Health 2016, 16, 674. [Google Scholar] [CrossRef]
- Morean, M.E.; Kong, G.; Cavallo, D.A.; Camenga, D.R.; Krishnan-Sarin, S. Nicotine concentration of e-cigarettes used by adolescents. Drug Alcohol Depend. 2016, 167, 224–227. [Google Scholar] [CrossRef]
- Zare, S.; Nemati, M.; Zheng, Y. A systematic review of consumer preference for e-cigarette attributes: Flavor, nicotine strength, and type. PLoS ONE 2018, 13, e0194145. [Google Scholar] [CrossRef]
- Romberg, A.R.; Lo, E.J.M.; Cuccia, A.F.; Willett, J.G.; Xiao, H.; Hair, E.C.; Vallone, D.M.; Marynak, K.; King, B.A. Patterns of nicotine concentrations in electronic cigarettes sold in the United States, 2013–2018. Drug Alcohol Depend. 2019, 203, 1–7. [Google Scholar] [CrossRef]
- Stefaniak, A.B.; Ranpara, A.C.; Virji, M.A.; LeBouf, R.F. Influence of E-Liquid Humectants, Nicotine, and Flavorings on Aerosol Particle Size Distribution and Implications for Modeling Respiratory Deposition. Front. Public Health 2022, 10, 782068. [Google Scholar] [CrossRef]
- Sleiman, M.; Logue, J.M.; Montesinos, V.N.; Russell, M.L.; Litter, M.I.; Gundel, L.A.; Destaillats, H. Emissions from Electronic Cigarettes: Key Parameters Affecting the Release of Harmful Chemicals. Environ. Sci. Technol. 2016, 50, 9644–9651. [Google Scholar] [CrossRef]
- Geiss, O.; Bianchi, I.; Barrero-Moreno, J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int. J. Hyg. Environ. Health 2016, 219, 268–277. [Google Scholar] [CrossRef]
- Kosmider, L.; Sobczak, A.; Fik, M.; Knysak, J.; Zaciera, M.; Kurek, J.; Goniewicz, M. Carbonyl Compounds in Electronic Cigarette Vapors: Effects of Nicotine Solvent and Battery Output Voltage. Nicotine Tob. Res. 2014, 16, 1319–1326. [Google Scholar] [CrossRef]
- Leigh, N.J.; I Lawton, R.; A Hershberger, P.; Goniewicz, M.L. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS). Tob. Control. 2016, 25, ii81–ii87. [Google Scholar] [CrossRef]
- Stefaniak, A.B.; LeBouf, R.F.; Ranpara, A.C.; Leonard, S.S. Toxicology of flavoring- and cannabis-containing e-liquids used in electronic delivery systems. Pharmacol. Ther. 2021, 224, 107838. [Google Scholar] [CrossRef]
- Chen, Z.; Zeng, D.D. Mining online e-liquid reviews for opinion polarities about e-liquid features. BMC Public Health 2017, 17, 1–7. [Google Scholar] [CrossRef]
- Russell, C.; McKeganey, N.; Dickson, T.; Nides, M. Changing patterns of first e-cigarette flavor used and current flavors used by 20,836 adult frequent e-cigarette users in the USA. Harm Reduct. J. 2018, 15, 33. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The airway epithelium in asthma. Nat. Med. 2012, 18, 684–692. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, M.; Ou, Y.; Adcock, I.M.; Yao, X. Mechanisms and biomarkers of airway epithelial cell damage in asthma: A review. Clin. Respir. J. 2021, 15, 1027–1045. [Google Scholar] [CrossRef]
- Vareille, M.; Kieninger, E.; Edwards, M.R.; Regamey, N. The Airway Epithelium: Soldier in the Fight against Respiratory Viruses. Clin. Microbiol. Rev. 2011, 24, 210–229. [Google Scholar] [CrossRef]
- Noël, A.; Hossain, E.; Perveen, Z.; Zaman, H.; Penn, A.L. Sub-ohm vaping increases the levels of carbonyls, is cytotoxic, and alters gene expression in human bronchial epithelial cells exposed at the air–liquid interface. Respir. Res. 2020, 21, 305. [Google Scholar] [CrossRef]
- Pinkston, R.; Zaman, H.; Hossain, E.; Penn, A.L.; Noël, A. Cell-specific toxicity of short-term JUUL aerosol exposure to human bronchial epithelial cells and murine macrophages exposed at the air–liquid interface. Respir. Res. 2020, 21, 269. [Google Scholar] [CrossRef] [PubMed]
- Noël, A.; Verret, C.M.; Hasan, F.; Lomnicki, S.; Morse, J.; Robichaud, A.; Penn, A.L. Generation of Electronic Cigarette Aerosol by a Third-Generation Machine-Vaping Device: Application to Toxicological Studies. J. Vis. Exp. 2018, 138, e58095. [Google Scholar] [CrossRef] [PubMed]
- CORESTA. Recommended method Nº 81. Routine Analytical Machine for e-Cigarette Aerosol Generation and Collection–Definitions and Standard Conditions. June 2015. Available online: https://www.coresta.org/sites/default/files/technical_documents/main/CRM_81.pdf (accessed on 22 October 2022.).
- Dautzenberg, B.; Bricard, D. Real-time characterization of e-cigarettes use: The 1 million puffs study. J. Addict. Res. Ther. 2015, 6, 4172. [Google Scholar] [CrossRef]
- Klager, S.; Vallarino, J.; MacNaughton, P.; Christiani, D.C.; Lu, Q.; Allen, J.G. Flavoring Chemicals and Aldehydes in E-Cigarette Emissions. Environ. Sci. Technol. 2017, 51, 10806–10813. [Google Scholar] [CrossRef] [PubMed]
- Talih, S.; Salman, R.; El-Hage, R.; Karam, E.; Karaoghlanian, N.; El-Hellani, A.; Saliba, N.; Shihadeh, A. Characteristics and toxicant emissions of JUUL electronic cigarettes. Tob. Control. 2019, 28, 678–680. [Google Scholar] [CrossRef]
- Son, Y.; Bhattarai, C.; Samburova, V.; Khlystov, A. Carbonyls and Carbon Monoxide Emissions from Electronic Cigarettes Affected by Device Type and Use Patterns. Int. J. Environ. Res. Public Health 2020, 17, 2767. [Google Scholar] [CrossRef]
- Food & Drug Administration. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke: Established List. Available online: https://www.fda.gov/tobacco-products/rules-regulations-and-guidance/harmful-and-potentially-harmful-constituents-tobacco-products-and-tobacco-smoke-established-list (accessed on 22 October 2022).
- A Tierney, P.; Karpinski, C.D.; E Brown, J.; Luo, W.; Pankow, J.F. Flavour chemicals in electronic cigarette fluids. Tob. Control. 2015, 25, e10–e15. [Google Scholar] [CrossRef]
- Hutzler, C.; Paschke, M.; Kruschinski, S.; Henkler, F.; Hahn, J.; Luch, A. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch. Toxicol. 2014, 88, 1295–1308. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Voudris, V.; Poulas, K. E-cigarettes generate high levels of aldehydes only in ‘dry puff’ conditions. Addiction 2015, 110, 1352–1356. [Google Scholar] [CrossRef]
- Flora, J.W.; Meruva, N.; Huang, C.B.; Wilkinson, C.T.; Ballentine, R.; Smith, D.C.; Werley, M.S.; McKinney, W.J. Characterization of potential impurities and degradation products in electronic cigarette formulations and aerosols. Regul. Toxicol. Pharmacol. 2016, 74, 1–11. [Google Scholar] [CrossRef]
- Jensen, R.P.; Luo, W.; Pankow, J.F.; Strongin, R.M.; Peyton, D.H. Hidden Formaldehyde in E-Cigarette Aerosols. N. Engl. J. Med. 2015, 372, 392–394. [Google Scholar] [CrossRef]
- Gillman, I.G.; Pennington, A.S.; Humphries, K.E.; Oldham, M.J. Determining the impact of flavored e-liquids on aldehyde production during Vaping. Regul. Toxicol. Pharmacol. 2020, 112, 104588. [Google Scholar] [CrossRef]
- Behar, R.Z.; Luo, W.; McWhirter, K.J.; Pankow, J.F.; Talbot, P. Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci. Rep. 2018, 8, 8288. [Google Scholar] [CrossRef]
- Barhdadi, S.; Rogiers, V.; Deconinck, E.; Vanhaecke, T. Toxicity assessment of flavour chemicals used in e-cigarettes: Current state and future challenges. Arch. Toxicol. 2021, 95, 2879–2881. [Google Scholar] [CrossRef]
- Bengalli, R.; Ferri, E.; Labra, M.; Mantecca, P. Lung Toxicity of Condensed Aerosol from E-CIG Liquids: Influence of the Flavor and the In Vitro Model Used. Int. J. Environ. Res. Public Health 2017, 14, 1254. [Google Scholar] [CrossRef]
- Gholap, V.V.; Kosmider, L.; Golshahi, L.; Halquist, M.S. Nicotine forms: Why and how do they matter in nicotine delivery from electronic cigarettes? Expert Opin. Drug Deliv. 2020, 17, 1727–1736. [Google Scholar] [CrossRef]
- Ranpara, A.; Stefaniak, A.B.; Fernandez, E.; LeBouf, R.F. Effect of Puffing Behavior on Particle Size Distributions and Respiratory Depositions from Pod-Style Electronic Cigarette, or Vaping, Products. Front. Public Health 2021, 9. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Emergency and Continuous Exposure Guidance Levels for Selected Subma-rine Contaminants. Acetaldehyde. In Emergency and Continuous Exposure Guidance Levels for Selected Submarine Contaminants: Volume 3; National Academies Press (US): Washington, DC, USA, 2009; p. 2. Available online: https://www.ncbi.nlm.nih.gov/books/NBK219914/ (accessed on 10 December 2022).
- United States Department of Labor. Occupational Safety and Health Administration (OSHA). Available online: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1000TABLEZ1 (accessed on 10 December 2022).
- Krüsemann, E.J.; Pennings, J.L.; Cremers, J.W.; Bakker, F.; Boesveldt, S.; Talhout, R. GC–MS analysis of e-cigarette refill solutions: A comparison of flavoring composition between flavor categories. J. Pharm. Biomed. Anal. 2020, 188, 113364. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Jabba, S.V.; Dewinter, T.M.; Mendizabal, M.; Anastas, P.T.; Jordt, S.E.; Zimmerman, J.B. Formation of flavorant–propylene Glycol Adducts with Novel Toxicological Properties in Chemically Unstable E-Cigarette Liquids. Nicotine Tob. Res. 2018, 21, 1248–1258. [Google Scholar] [CrossRef]
- Jabba, S.V.; Diaz, A.N.; Erythropel, H.C.; Zimmerman, J.B.; Jordt, S.-E. Chemical Adducts of Reactive Flavor Aldehydes Formed in E-Cigarette Liquids Are Cytotoxic and Inhibit Mitochondrial Function in Respiratory Epithelial Cells. Nicotine Tob. Res. 2020, 22, S25–S34. [Google Scholar] [CrossRef]
- Kosmider, L.; Sobczak, A.; Prokopowicz, A.; Kurek, J.; Zaciera, M.; Knysak, J.; Smith, D.; Goniewicz, M.L. Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde. Thorax 2016, 71, 376–377. [Google Scholar] [CrossRef] [PubMed]
- Effah, F.; Taiwo, B.; Baines, D.; Bailey, A.; Marczylo, T. Pulmonary effects of e-liquid flavors: A systematic review. J. Toxicol. Environ. Health Part B 2022, 25, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.K.; Whitted, L.; Mason, T.B.; Pang, R.D.; Tackett, A.P.; Leventhal, A.M. Characterizing different-flavored e-cigarette solutions from user-reported sensory attributes and appeal. Exp. Clin. Psychopharmacol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, J.; Sundar, I.K.; Freter, R.; Sekera, E.R.; Friedman, A.E.; Robinson, R.; Pagano, T.; Rahman, I. Inflammatory Response and Barrier Dysfunction by Different e-Cigarette Flavoring Chemicals Identified by Gas Chromatography–Mass Spectrometry in e-Liquids and e-Vapors on Human Lung Epithelial Cells and Fibroblasts. Appl. Vitr. Toxicol. 2017, 3, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, P.J.; McDonald, J.D.; Weber, D.T.; Khlystov, A.; Nystoriak, M.A.; Conklin, D.J. Composition of aerosols from thermal degradation of flavors used in ENDS and tobacco products. Inhal. Toxicol. 2022, 34, 319–328. [Google Scholar] [CrossRef]
- Lucas, J.H.; Muthumalage, T.; Wang, Q.; Friedman, M.R.; Friedman, A.E.; Rahman, I. E-Liquid Containing a Mixture of Coconut, Vanilla, and Cookie Flavors Causes Cellular Senescence and Dysregulated Repair in Pulmonary Fibroblasts: Implications on Premature Aging. Front. Physiol. 2020, 11, 924. [Google Scholar] [CrossRef]
- Sundar, I.K.; Javed, F.; Romanos, G.E.; Rahman, I. E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget 2016, 7, 77196–77204. [Google Scholar] [CrossRef]
- Carnevale, R.; Sciarretta, S.; Violi, F.; Nocella, C.; Loffredo, L.; Perri, L.; Peruzzi, M.; Marullo, A.G.; De Falco, E.; Chimenti, I.; et al. Acute Impact of Tobacco vs Electronic Cigarette Smoking on Oxidative Stress and Vascular Function. Chest 2016, 150, 606–612. [Google Scholar] [CrossRef]
- Bitzer, Z.T.; Goel, R.; Reilly, S.M.; Elias, R.J.; Silakov, A.; Foulds, J.; Muscat, J.; Richie, J.P., Jr. Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free Radic. Biol. Med. 2018, 120, 72–79. [Google Scholar] [CrossRef]
- Lerner, C.A.; Sundar, I.K.; Yao, H.; Gerloff, J.; Ossip, D.J.; McIntosh, S.; Robinson, R.; Rahman, I. Vapors Produced by Electronic Cigarettes and E-Juices with Flavorings Induce Toxicity, Oxidative Stress, and Inflammatory Response in Lung Epithelial Cells and in Mouse Lung. PLoS ONE 2015, 10, e0116732. [Google Scholar] [CrossRef]
- Sussan, T.E.; Gajghate, S.; Thimmulappa, R.K.; Ma, J.; Kim, J.-H.; Sudini, K.; Consolini, N.; Cormier, S.; Lomnicki, S.; Hasan, F.; et al. Exposure to Electronic Cigarettes Impairs Pulmonary Anti-Bacterial and Anti-Viral Defenses in a Mouse Model. PLoS ONE 2015, 10, e0116861. [Google Scholar] [CrossRef]
- Rowell, T.R.; Reeber, S.L.; Lee, S.L.; Harris, R.A.; Nethery, R.C.; Herring, A.H.; Glish, G.L.; Tarran, R. Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Am. J. Physiol. Cell Mol. Physiol. 2017, 313, L52–L66. [Google Scholar] [CrossRef]
- Berkelhamer, S.K.; Helman, J.M.; Gugino, S.F.; Leigh, N.J.; Lakshminrusimha, S.; Goniewicz, M.L. In Vitro Consequences of Electronic-Cigarette Flavoring Exposure on the Immature Lung. Int. J. Environ. Res. Public Health 2019, 16, 3635. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Effects of E-Cigarette Refill Liquid Flavorings with and without Nicotine on Human Retinal Pigment Epithelial Cells: A Preliminary Study. Int. J. Environ. Res. Public Health 2021, 18, 11655. [Google Scholar] [CrossRef]
- Chatterjee, S.; Tao, J.-Q.; Johncola, A.; Guo, W.; Caporale, A.; Langham, M.C.; Wehrli, F.W. Acute exposure to e-cigarettes causes inflammation and pulmonary endothelial oxidative stress in nonsmoking, healthy young subjects. Am. J. Physiol. Cell Mol. Physiol. 2019, 317, L155–L166. [Google Scholar] [CrossRef]
- Sassano, M.F.; Davis, E.; Keating, J.E.; Zorn, B.T.; Kochar, T.K.; Wolfgang, M.C.; Glish, G.L.; Tarran, R. Evaluation of e-liquid toxicity using an open-source high-throughput screening assay. PLoS Biol. 2018, 16, e2003904. [Google Scholar] [CrossRef]
- Truman, P.; Stanfill, S.; Heydari, A.; Silver, E.; Fowles, J. Monoamine oxidase inhibitory activity of flavoured e-cigarette liquids. Neurotoxicology 2019, 75, 123–128. [Google Scholar] [CrossRef]
- Bahl, V.; Lin, S.; Xu, N.; Davis, B.; Wang, Y.-H.; Talbot, P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod. Toxicol. 2012, 34, 529–537. [Google Scholar] [CrossRef]
- Sherwood, C.L.; Boitano, S. Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine. Respir. Res. 2016, 17, 57. [Google Scholar] [CrossRef]
- Omaiye, E.E.; McWhirter, K.J.; Luo, W.; Pankow, J.F.; Talbot, P. High-Nicotine Electronic Cigarette Products: Toxicity of JUUL Fluids and Aerosols Correlates Strongly with Nicotine and Some Flavor Chemical Concentrations. Chem. Res. Toxicol. 2019, 32, 1058–1069. [Google Scholar] [CrossRef]
- Pearce, K.; Gray, N.; Gaur, P.; Jeon, J.; Suarez, A.; Shannahan, J.; Pappas, R.; Watson-Wright, C. Toxicological analysis of aerosols derived from three electronic nicotine delivery systems using normal human bronchial epithelial cells. Toxicol. Vitr. 2020, 69, 104997. [Google Scholar] [CrossRef] [PubMed]
- Abou-Assali, O.; Chang, M.; Chidipi, B.; Martinez-De-Juan, J.L.; Reiser, M.; Kanithi, M.; Soni, R.; McDonald, T.V.; Herweg, B.; Saiz, J.; et al. In vitro and in vivo cardiac toxicity of flavored electronic nicotine delivery systems. Am. J. Physiol. Circ. Physiol. 2021, 320, H133–H143. [Google Scholar] [CrossRef] [PubMed]

| Genes | Gene Primers (5′–3′) |
|---|---|
| ACTB | Forward–GGACCTGACTGACTACCTCAT Reverse–CGTAGCACAGCTTCTCCTTAAT |
| ALDH-3A1 | Forward–TGTTCTCCAGCAACGACAAGG Reverse–AGGGCAGAGAGTGCAAGGT |
| CYP-1A1 | Forward–TCGGCCACGGAGTTTCTTC Reverse–GGTCAGCATGTGCCCAATCA |
| HPRT | Forward–CGAGATGTGATGAAGGAGATGG Reverse–TTGATGTAATCCAGCAGGTCAG |
| IL-6 | Forward–ACTCACCTCTTCAGAACGAATTG Reverse–CCATCTTTGGAAGGTTCAGGTTG |
| IL-8 | Forward–ACTGAGAGTGATTGAGAGTGGAC Reverse– AACCCTCTGCACCCAGTTTTC |
| MMP-9 | Forward–TGTACCGCTATGGTTACACTCG Reverse–GGCAGGGACAGTTGCTTCT |
| MMP-12 | Forward–GCATGGGCTAGGATTCCACC Reverse–CATGAACCGTGAGGATGTTGA |
| NQO-1 | Forward–GGATACTGAAAGTTCGCAGGG Reverse–GAAGAGCACTGATCGTACTGGC |
| SOD-1 | Forward–GGTGGGCCAAAGGATGAAGAG Reverse–CCACAAGCCAAACGACTTCC |
| Device Type & Flavor | E-Liquid Nicotine Concentration | ENDS Aerosol–Nicotine (mg/puff) | ENDS Aerosol–Propylene Glycol (PG) (mg/puff) | ENDS Aerosol–Glycerin (G) (mg/puff) |
|---|---|---|---|---|
| JUUL menthol | 3% nicotine salt | 0.0735 | 2.13 | 0.614 |
| JUUL menthol | 5% nicotine salt | 0.0987 | 1.55 | 0.515 |
| JUUL Virginia tobacco | 5% nicotine salt | 0.112 | 1.66 | 0.544 |
| JUUL Mango | 5% nicotine salt | 0.145 | 2.09 | 0.639 |
| Puff Bar Cool mint | 5% nicotine salt | 0.378 | 4.05 | 3.26 |
| Puff Bar Café latte | 5% nicotine salt | 0.148 | 1.45 | 1.19 |
| Puff Bar OMG | 5% nicotine salt | 0.358 | 3.76 | 2.56 |
| Posh Vape Crème brûlée | 6% nicotine salt | 0.28 | 4.58 | 3.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noël, A.; Ghosh, A. Carbonyl Profiles of Electronic Nicotine Delivery System (ENDS) Aerosols Reflect Both the Chemical Composition and the Numbers of E-Liquid Ingredients–Focus on the In Vitro Toxicity of Strawberry and Vanilla Flavors. Int. J. Environ. Res. Public Health 2022, 19, 16774. https://doi.org/10.3390/ijerph192416774
Noël A, Ghosh A. Carbonyl Profiles of Electronic Nicotine Delivery System (ENDS) Aerosols Reflect Both the Chemical Composition and the Numbers of E-Liquid Ingredients–Focus on the In Vitro Toxicity of Strawberry and Vanilla Flavors. International Journal of Environmental Research and Public Health. 2022; 19(24):16774. https://doi.org/10.3390/ijerph192416774
Chicago/Turabian StyleNoël, Alexandra, and Arpita Ghosh. 2022. "Carbonyl Profiles of Electronic Nicotine Delivery System (ENDS) Aerosols Reflect Both the Chemical Composition and the Numbers of E-Liquid Ingredients–Focus on the In Vitro Toxicity of Strawberry and Vanilla Flavors" International Journal of Environmental Research and Public Health 19, no. 24: 16774. https://doi.org/10.3390/ijerph192416774
APA StyleNoël, A., & Ghosh, A. (2022). Carbonyl Profiles of Electronic Nicotine Delivery System (ENDS) Aerosols Reflect Both the Chemical Composition and the Numbers of E-Liquid Ingredients–Focus on the In Vitro Toxicity of Strawberry and Vanilla Flavors. International Journal of Environmental Research and Public Health, 19(24), 16774. https://doi.org/10.3390/ijerph192416774





