A Comprehensive Review on Social Inequalities and Pregnancy Outcome—Identification of Relevant Pathways and Mechanisms
Abstract
1. Introduction
- (a)
- to outline birth outcomes by social determinants and neighborhood deprivation and describe both methodological approaches and potential confounders.
- (b)
- to summarize the effect of socioeconomic characteristics (at individual and neighborhood levels) on pregnancy outcomes in selected studies.
- (c)
- to propose a theoretical model on the pathways and possible mechanisms through which social determinants may be related to adverse pregnancy outcomes.
2. Material and Methods
2.1. Search Strategy
2.2. Studies Selection Strategy
- General information: first author’s name, country of origin, and date of the study.
- Main study characteristics: study design, period, location, statistical methods, population size, and main findings (related to PTB, LBW, BW, and SGA outcome).
- Participant characteristics: information on confounders.
- Outcome measures.
3. Results
3.1. Location and Population
3.2. Adverse Pregnancy Outcomes
3.3. Methodological Approaches
3.4. Assessment of Social Inequalities
3.5. Various Confounders
3.6. Definition of Social Determinants
3.7. Findings in Terms of Social Determinants and Health Inequalities
3.8. Framework for Action on Social Determinants of Health
- (i).
- Structural determinants
- (ii).
- Intermediary determinants
- Public health prevention actions, may reduce inequalities by means of actions in various domains, such as nutrition, sanitation, housing, and working conditions
- Actions such as vaccination, empowerment, and social support as factors in building resistance to the health effects of unevenly distributed exposures
- Treatment and rehabilitation actions for those health problems that constitute the socioeconomic gap in the disease burden (rehabilitation of disabled people)
- Policy actions aimed at reproducing the contextual factors (e.g., social capital) capable of mitigating the effects of poverty on health
- Protective actions against the social and economic consequences of ill health by means of health insurance, sickness benefits, and labor market policies [63].
Gradients and Feedback Effect
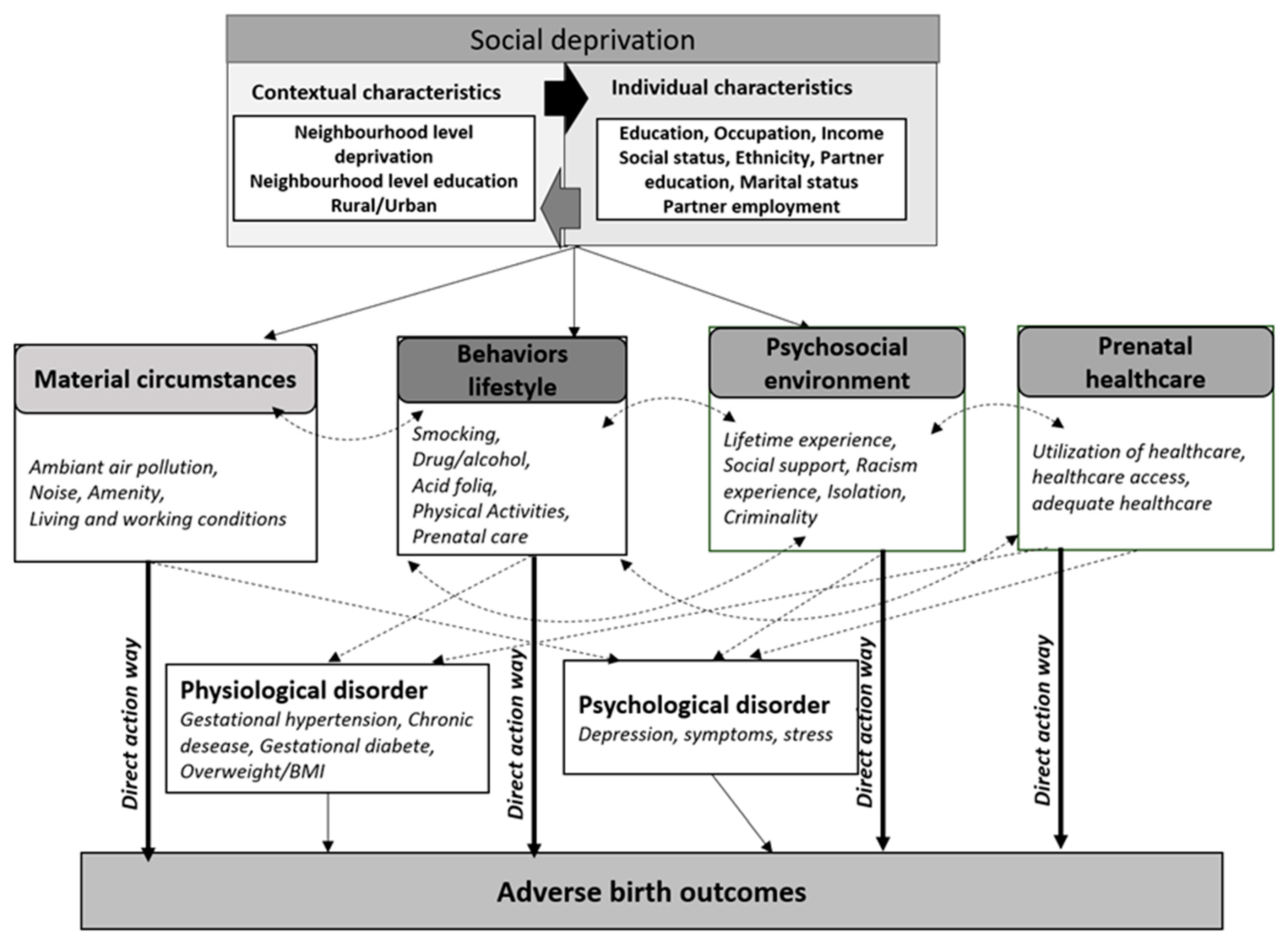
3.9. A Theoretical Contribution Aimed at Gaining a Better Understanding of the Potential Social Deprivation Effect on Maternal and Newborn Health
4. Pathway 1—The Mediating Role Played by Deprived Material Circumstances
4.1. The Effect of Deprived Material Circumstances via the Direct-Action Pathway
4.2. The Effect of Deprived Neighborhood via the Indirect-Action Pathway
5. Pathway 2—The Mediating Role Played by Healthy Behaviors and Living Conditions
6. Pathway 3—The Mediating Role Played by Psychosocial Environment
6.1. The Psychosocial Environment Effect: A Direct-Action Pathway
6.2. The Psychosocial Environment Effect: An Indirect-Action Pathway
7. Pathway 4—The Mediating Role of Access to Adequate Prenatal Healthcare Utilization
8. Public Health Intervention for Pregnant Women and Birth Health
- (i).
- Behaviors, lifestyle, and biological factors
- Diet and physical activity during pregnancy
- (ii).
- Psychosocial and psychological interventions
- (iii).
- Public policy
9. Recommendations
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ICD-11. Available online: https://icd.who.int/en (accessed on 6 October 2022).
- Huddy, C.L.J. Educational and Behavioural Problems in Babies of 32–35 Weeks Gestation. Arch. Dis. Child. Fetal Neonatal Ed. 2001, 85, 23F–28F. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Dorer, D.J.; Fleming, M.P.; Catlin, E.A. Clinical Outcomes of Near-Term Infants. Pediatrics 2004, 114, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Risnes, K.R.; Vatten, L.J.; Baker, J.L.; Jameson, K.; Sovio, U.; Kajantie, E.; Osler, M.; Morley, R.; Jokela, M.; Painter, R.C.; et al. Birthweight and Mortality in Adulthood: A Systematic Review and Meta-Analysis. Int. J. Epidemiol. 2011, 40, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Wojdyla, D.; Say, L.; Pilar Bertran, A.; Meraldi, M.; Harris Requejo, J.; Rubens, C.; Menon, R.; Van Look, P. The Worldwide Incidence of Preterm Birth: A Systematic Review of Maternal Mortality and Morbidity. Bull. World Health Org. 2010, 88, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P. The Developmental Origins of Adult Disease. J. Am. Coll. Nutr. 2004, 23, 588S–595S. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, B.; Baur, L.A.; Wang, J.J.; Teber, E.; Liew, G.; Cheung, N.; Wong, T.Y.; Mitchell, P. Smaller Birth Size Is Associated with Narrower Retinal Arterioles in Early Adolescence. Microcirculation 2010, 17, 660–668. [Google Scholar] [CrossRef]
- Howson, C.P.; Kinney, M.V.; McDougall, L.; Lawn, J.E.; The Born Too Soon Preterm Birth Action Group. Born Too Soon: Preterm Birth Matters. Reprod. Health 2013, 10, S1. [Google Scholar] [CrossRef]
- Mathewson, K.J.; Chow, C.H.T.; Dobson, K.G.; Pope, E.I.; Schmidt, L.A.; Van Lieshout, R.J. Mental Health of Extremely Low Birth Weight Survivors: A Systematic Review and Meta-Analysis. Psychol. Bull. 2017, 143, 347–383. [Google Scholar] [CrossRef]
- Osmond, C.; Barker, D.J. Fetal, Infant, and Childhood Growth Are Predictors of Coronary Heart Disease, Diabetes, and Hypertension in Adult Men and Women. Environ. Health Perspect. 2000, 108, 545–553. [Google Scholar] [CrossRef]
- Wilcox, A.J. On the Importance—And the Unimportance—of Birthweight. Int. J. Epidemiol. 2001, 30, 1233–1241. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm Birth: Causes, Consequences, and Prevention; Behrman, R.E., Butler, A.S., Eds.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 2007; ISBN 978-0-309-10159-2. [Google Scholar]
- Van Lieshout, R.J.; Boyle, M.H.; Saigal, S.; Morrison, K.; Schmidt, L.A. Mental Health of Extremely Low Birth Weight Survivors in Their 30s. Pediatrics 2015, 135, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Preterm Birth. Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 6 October 2022).
- Lawn, J.E.; Gravett, M.G.; Nunes, T.M.; Rubens, C.E.; Stanton, C.; The GAPPS Review Group. Global Report on Preterm Birth and Stillbirth (1 of 7): Definitions, Description of the Burden and Opportunities to Improve Data. BMC Pregnancy Childbirth 2010, 10, S1. [Google Scholar] [CrossRef]
- Coubert, F. Global Nutrition Targets 2025: Low Birth Weight Policy Brief. Available online: https://www.who.int/publications-detail-redirect/WHO-NMH-NHD-14.5 (accessed on 6 October 2022).
- Petrou, S. The Economic Consequences of Preterm Birth Duringthe First 10 Years of Life. BJOG Int. J. Obstet. Gynaecol. 2005, 112, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Petrou, S.; Mehta, Z.; Hockley, C.; Cook-Mozaffari, P.; Henderson, J.; Goldacre, M. The Impact of Preterm Birth on Hospital Inpatient Admissions and Costs During the First 5 Years of Life. Pediatrics 2003, 112, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Saigal, S.; Doyle, L.W. An Overview of Mortality and Sequelae of Preterm Birth from Infancy to Adulthood. Lancet 2008, 371, 261–269. [Google Scholar] [CrossRef]
- Niebler, A.; Mader, S.; Merialdi, M.; Keller, M. Too Little, Too Late? Why Europe Should Do More for Preterm Infants; EU Benchmarking Report 2009/2010; European Foundation for the Care of Newborn Infants: Munich, Germany, 2010. [Google Scholar]
- Lawn, J.E.; Cousens, S.N.; Darmstadt, G.L.; Bhutta, Z.A.; Martines, J.; Paul, V.; Knippenberg, R.; Fogstad, H. 1 Year after The Lancet Neonatal Survival Series—Was the Call for Action Heard? Lancet 2006, 367, 1541–1547. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and Causes of Preterm Birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Simoncic, V.; Enaux, C.; Deguen, S.; Kihal-Talantikite, W. Adverse Birth Outcomes Related to NO2 and PM Exposure: European Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8116. [Google Scholar] [CrossRef]
- Bibby, E.; Stewart, A. The Epidemiology of Preterm Birth. Neuro Endocrinol. Lett. 2004, 25 (Suppl. 1), 43–47. [Google Scholar]
- Du, M.; Ge, L.; Zhou, M.; Ying, J.; Qu, F.; Dong, M.; Chen, D. Effects of Pre-Pregnancy Body Mass Index and Gestational Weight Gain on Neonatal Birth Weight. J. Zhejiang Univ. Sci. B 2017, 18, 263–271. [Google Scholar] [CrossRef]
- Ensted, S.; Rankin, K.; Desisto, C.; Collins, J.W. Father’s Lifetime Socioeconomic Status, Small for Gestational Age Infants, and Infant Mortality: A Population-Based Study. Ethn. Dis. 2019, 29, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, J.C.; Kaciroti, N.; Retzloff, L.; Rosenblum, K.; Miller, A.L. Longitudinal Associations between Maternal Feeding and Overweight in Low-Income Toddlers. Appetite 2017, 113, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ospina, M.; Osornio-Vargas, Á.R.; Nielsen, C.C.; Crawford, S.; Kumar, M.; Aziz, K.; Serrano-Lomelin, J. Socioeconomic Gradients of Adverse Birth Outcomes and Related Maternal Factors in Rural and Urban Alberta, Canada: A Concentration Index Approach. BMJ Open 2020, 10, e033296. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.; Enquobahrie, D.A.; Peckham, T.; Seixas, N.; Hajat, A. Retrospective Cohort Study of the Association between Maternal Employment Precarity and Infant Low Birth Weight in Women in the USA. BMJ Open 2020, 10, e029584. [Google Scholar] [CrossRef] [PubMed]
- Elo, I.T.; Culhane, J.F.; Kohler, I.V.; O’Campo, P.; Burke, J.G.; Messer, L.C.; Kaufman, J.S.; Laraia, B.A.; Eyster, J.; Holzman, C. Neighbourhood Deprivation and Small-for-Gestational-Age Term Births in the United States. Paediatr. Perinat. Epidemiol. 2009, 23, 87–96. [Google Scholar] [CrossRef]
- Misra, D.; Strobino, D.; Trabert, B. Effects of Social and Psychosocial Factors on Risk of Preterm Birth in Black Women: Social and Psychosocial Risk Factors. Paediatr. Perinat. Epidemiol. 2010, 24, 546–554. [Google Scholar] [CrossRef]
- Sims, M.; Sims, T.H.; Bruce, M.A. Community Income, Smoking, and Birth Weight Disparities in Wisconsin. J. Natl. Black Nurse Assoc. 2016, 18, 14. [Google Scholar]
- Kaufman, J.S.; Alonso, F.T.; Pino, P. Multi-Level Modeling of Social Factors and Preterm Delivery in Santiago de Chile. BMC Pregnancy Childbirth 2008, 8, 46. [Google Scholar] [CrossRef]
- DeFranco, E.A.; Lian, M.; Muglia, L.J.; Schootman, M. Area-Level Poverty and Preterm Birth Risk: A Population-Based Multilevel Analysis. BMC Public Health 2008, 8, 316. [Google Scholar] [CrossRef]
- Young, R.L.; Weinberg, J.; Vieira, V.; Aschengrau, A.; Webster, T.F. A Multilevel Non-Hierarchical Study of Birth Weight and Socioeconomic Status. Int. J. Health. Geogr. 2010, 9, 36. [Google Scholar] [CrossRef]
- Urquia, M.L.; Frank, J.W.; Moineddin, R.; Glazier, R.H. Does Time Since Immigration Modify Neighborhood Deprivation Gradients in Preterm Birth? A Multilevel Analysis. J. Urban Health 2011, 88, 959–976. [Google Scholar] [CrossRef] [PubMed]
- Kozhimannil, K.B.; Attanasio, L.B.; McGovern, P.M.; Gjerdingen, D.K.; Johnson, P.J. Reevaluating the Relationship Between Prenatal Employment and Birth Outcomes: A Policy-Relevant Application of Propensity Score Matching. Women’s Health Issues 2013, 23, e77–e85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lamy Filho, F.; Assunção Júnior, A.N.; Silva, A.A.M.; Lamy, Z.C.; Barbieri, M.A.; Bettiol, H. Social Inequality and Perinatal Health: Comparison of Three Brazilian Cohorts. Braz. J. Med. Biol. Res. 2007, 40, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.; Harville, E.; Theall, K.; Webber, L.; Chen, W.; Berenson, G. Neighborhood Poverty, Allostatic Load, and Birth Outcomes in African American and White Women: Findings from the Bogalusa Heart Study. Health Place 2013, 24, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.M.; Kaufman, J.S.; Emch, M.E.; Hogan, V.K.; Savitz, D.A. Ethnic Density and Preterm Birth in African-, Caribbean-, and US-Born Non-Hispanic Black Populations in New York City. Am. J. Epidemiol. 2010, 172, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Colen, C.G.; Geronimus, A.T.; Bound, J.; James, S.A. Maternal Upward Socioeconomic Mobility and Black–White Disparities in Infant Birthweight. Am. J. Public Health 2006, 96, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, A.L.; Essalmi, A.G.; Alvalos, L.; Breton, C.; Camargo, C.A.; Cowell, W.J.; Dabelea, D.; Dager, S.R.; Duarte, C.; Elliott, A.; et al. Racial and Geographic Variation in Effects of Maternal Education and Neighborhood-Level Measures of Socioeconomic Status on Gestational Age at Birth: Findings from the ECHO Cohorts. PLoS ONE 2021, 16, e0245064. [Google Scholar] [CrossRef]
- Vang, Z.M.; Elo, I.T. Exploring the Health Consequences of Majority–Minority Neighborhoods: Minority Diversity and Birthweight among Native-Born and Foreign-Born Blacks. Soc. Sci. Med. 2013, 97, 56–65. [Google Scholar] [CrossRef]
- Bracken, M.B.; Thomas, J.; Thomas, J. Intergenerational effects of high socioeconomic status on low birthweight and preterm birth in african americans. J. Natl. Med. Assoc. 2000, 92, 9. [Google Scholar]
- Huang, J.Y.; Gavin, A.R.; Richardson, T.S.; Rowhani-Rahbar, A.; Siscovick, D.S.; Enquobahrie, D.A. Are Early-Life Socioeconomic Conditions Directly Related to Birth Outcomes? Grandmaternal Education, Grandchild Birth Weight, and Associated Bias Analyses. Am. J. Epidemiol. 2015, 182, 568–578. [Google Scholar] [CrossRef]
- Shankardass, K.; O’Campo, P.; Dodds, L.; Fahey, J.; Joseph, K.; Morinis, J.; Allen, V.M. Magnitude of Income-Related Disparities in Adverse Perinatal Outcomes. BMC Pregnancy Childbirth 2014, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.M.; Galea, S. Temporal Changes in Socioeconomic Influences on Health: Maternal Education and Preterm Birth. Am. J. Public Health 2012, 102, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Crespo, M.R.; Narla, N.P.; Williams, A.R.; Beebe, T.J.; Sloan, J.; Yawn, B.P.; Wheeler, P.H.; Juhn, Y.J. Comparison of Individual-Level versus Area-Level Socioeconomic Measures in Assessing Health Outcomes of Children in Olmsted County, Minnesota. J. Epidemiol. Community Health 2013, 67, 305–310. [Google Scholar] [CrossRef] [PubMed]
- d’Orsi, E.; Carvalho, M.S.; Cruz, O.G. Similarity between Neonatal Profile and Socioeconomic Index: A Spatial Approach. Cad. Saúde Pública 2005, 21, 786–794. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, G.; van Eijsden, M.; Vrijkotte, T.G.M.; Gemke, R.J.B.J. Educational Inequalities in Perinatal Outcomes: The Mediating Effect of Smoking and Environmental Tobacco Exposure. PLoS ONE 2012, 7, e37002. [Google Scholar] [CrossRef]
- Glinianaia, S.V.; Ghosh, R.; Rankin, J.; Pearce, M.S.; Parker, L.; Pless-Mulloli, T. No Improvement in Socioeconomic Inequalities in Birthweight and Preterm Birth over Four Decades: A Population-Based Cohort Study. BMC Public Health 2013, 13, 345. [Google Scholar] [CrossRef]
- Mortensen, L.H.; Diderichsen, F.; Arntzen, A.; Gissler, M.; Cnattingius, S.; Schnor, O.; Davey-Smith, G.; Nybo Andersen, A.-M. Social Inequality in Fetal Growth: A Comparative Study of Denmark, Finland, Norway and Sweden in the Period 1981–2000. J. Epidemiol. Community Health 2008, 62, 325–331. [Google Scholar] [CrossRef]
- Majdan, M.; Plančíková, D.; Melichová, J.; Dudáková, K.; Rechtoríková, V.; Kačmariková, M. Comparison of Birthweight Patterns in Rural Municipalities with and without a Roma Community: A Cross-Sectional Analysis in Slovakia 2009–2013. Cent. Eur. J. Public Health 2018, 26, 278–283. [Google Scholar] [CrossRef]
- Raab, R.; Hoffmann, J.; Spies, M.; Geyer, K.; Meyer, D.; Günther, J.; Hauner, H. Are Pre- and Early Pregnancy Lifestyle Factors Associated with the Risk of Preterm Birth? A Secondary Cohort Analysis of the Cluster-Randomised GeliS Trial. BMC Pregnancy Childbirth 2022, 22, 230. [Google Scholar] [CrossRef]
- Dickutė, J.; Padaiga, Z.; Grabauskas, V.; Nadisauskiene, R.J.; Basys, V.; Gaizauskiene, A. Maternal Socio-Economic Factors and the Risk of Low Birth Weight in Lithuania. Medicina 2004, 40, 475–482. [Google Scholar]
- Pei, L.; Kang, Y.; Zhao, Y.; Cheng, Y.; Yan, H. Changes in Socioeconomic Inequality of Low Birth Weight and Macrosomia in Shaanxi Province of Northwest China, 2010–2013: A Cross-Sectional Study. Medicine 2016, 95, e2471. [Google Scholar] [CrossRef] [PubMed]
- Martinson, M.L.; Reichman, N.E. Socioeconomic Inequalities in Low Birth Weight in the United States, the United Kingdom, Canada, and Australia. Am. J. Public Health 2016, 106, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Wilding, S.; Ziauddeen, N.; Roderick, P.; Smith, D.; Chase, D.; Macklon, N.; McGrath, N.; Hanson, M.; Alwan, N.A. Are Socioeconomic Inequalities in the Incidence of Small-for-Gestational-Age Birth Narrowing? Findings from a Population-Based Cohort in the South of England. BMJ Open 2019, 9, e026998. [Google Scholar] [CrossRef] [PubMed]
- Ryu, E.; Wi, C.-I.; Crow, S.S.; Armasu, S.M.; Wheeler, P.H.; Sloan, J.A.; Yawn, B.P.; Beebe, T.J.; Williams, A.R.; Juhn, Y.J. Assessing Health Disparities in Children Using a Modified Housing-Related Socioeconomic Status Measure: A Cross-Sectional Study. BMJ Open 2016, 6, e011564. [Google Scholar] [CrossRef]
- World Health Organization. A Conceptual Framework for Action on the Social Determinants of Health; WHO: Geneva, Switzerland, 2010; Volume 76. [Google Scholar]
- Mackenbach, J.P.; Kunst, A.E. Measuring the Magnitude of Socio-Economic Inequalities in Health: An Overview of Available Measures Illustrated with Two Examples from Europe. Soc. Sci. Med. 1997, 44, 757–771. [Google Scholar] [CrossRef]
- Krieger, N.; Rowley, D.L.; Herman, A.A.; Avery, B.; Phillips, M.T. Racism, Sexism, and Social Class: Implications for Studies of Health, Disease, and Well-Being. Am. J. Prev. Med. 1993, 9, 82–122. [Google Scholar] [CrossRef]
- Diderichsen, F.; Evans, T.; Whitehead, M. The Social Basis of Disparities in Health. In Challenging Inequities in Health: From Ethics to Action; Evans, T., Whitehead, M., Diderichsen, F., Bhuiya, A., Wirth, M., Eds.; Oxford University Press: Oxford, UK, 2001; ISBN 978-0-19-513740-8. [Google Scholar]
- Putnam, R.D. Bowling Alone: The Collapse and Revival of American Community; Simon & Schuster: New York, NY, USA, 2000. [Google Scholar]
- Kawachi, I.; Kennedy, B.P.; Lochner, K.; Prothrow-Stith, D. Social Capital, Income Inequality, and Mortality. Am. J. Public Health 1997, 87, 1491–1498. [Google Scholar] [CrossRef]
- POPAY, J. Social Capital: The Role of Narrative and Historical Research. J. Epidemiol. Community Health 2000, 54, 401. [Google Scholar] [CrossRef][Green Version]
- Marmot, M.G.; Shipley, M.J.; Rose, G. Inequalities in Death--Specific Explanations of a General Pattern? Lancet 1984, 1, 1003–1006. [Google Scholar] [CrossRef]
- Kihal-Talantikite, W.; Deguen, S. Environmental Amenities and the Fetal and Infant Health during the FIrst 1000 Days of Life: A Literature Review and Theoretical Contribution. Méd. Reprod. 2020, 22, 16. [Google Scholar]
- Kihal-Talantikite, W.; Padilla, C.M.; Lalloué, B.; Gelormini, M.; Zmirou-Navier, D.; Deguen, S. Green Space, Social Inequalities and Neonatal Mortality in France. BMC Pregnancy Childbirth 2013, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.; Timperio, A.; Giles-Corti, B.; Ball, K.; Hume, C.; Roberts, R.; Andrianopoulos, N.; Salmon, J. Do Features of Public Open Spaces Vary According to Neighbourhood Socio-Economic Status? Health Place 2008, 14, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Koohsari, M.J. Access to Public Open Space: Is Distribution Equitable across Different Socio-Economic Areas. J. Urban Environ. Eng. 2011, 5, 67–72. [Google Scholar] [CrossRef]
- Richardson, E.; Pearce, J.; Mitchell, R.; Day, P.; Kingham, S. The Association between Green Space and Cause-Specific Mortality in Urban New Zealand: An Ecological Analysis of Green Space Utility. BMC Public Health 2010, 10, 240. [Google Scholar] [CrossRef]
- Estabrooks, P.A.; Lee, R.E.; Gyurcsik, N.C. Resources for Physical Activity Participation: Does Availability and Accessibility Differ by Neighborhood Socioeconomic Status? Ann. Behav. Med. 2003, 25, 100–104. [Google Scholar] [CrossRef]
- Talen, E. The Social Equity of Urban Service Distribution: An Exploration of Park Access in Pueblo, Colorado, and Macon, Georgia. Urban Geogr. 1997, 18, 521–541. [Google Scholar] [CrossRef]
- Timperio, A.; Ball, K.; Salmon, J.; Roberts, R.; Crawford, D. Is Availability of Public Open Space Equitable across Areas? Health Place 2007, 13, 335–340. [Google Scholar] [CrossRef]
- Jones, A.; Hillsdon, M.; Coombes, E. Greenspace Access, Use, and Physical Activity: Understanding the Effects of Area Deprivation. Prev. Med. 2009, 49, 500–505. [Google Scholar] [CrossRef]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C. Air Pollution Removal by Urban Trees and Shrubs in the United States. Urban For. Urban Green. 2006, 4, 115–123. [Google Scholar] [CrossRef]
- Paoletti, E.; Bardelli, T.; Giovannini, G.; Pecchioli, L. Air Quality Impact of an Urban Park over Time. Procedia Environ. Sci. 2011, 4, 10–16. [Google Scholar] [CrossRef]
- Health Issues in Planning: Best Practice Guidance; Greater London Authority: London, UK, 2007; ISBN 978-1-84781-030-4.
- Klepac, P.; Locatelli, I.; Korošec, S.; Künzli, N.; Kukec, A. Ambient Air Pollution and Pregnancy Outcomes: A Comprehensive Review and Identification of Environmental Public Health Challenges. Environ. Res. 2018, 167, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Okun, M.L.; Schetter, C.D.; Glynn, L.M. Poor Sleep Quality Is Associated with Preterm Birth. Sleep 2011, 34, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Okun, M.L.; Luther, J.F.; Wisniewski, S.R.; Sit, D.; Prairie, B.A.; Wisner, K.L. Disturbed Sleep, a Novel Risk Factor for Preterm Birth? J. Womens Health 2012, 21, 54–60. [Google Scholar] [CrossRef]
- Strange, L.B.; Parker, K.P.; Moore, M.L.; Strickland, O.L.; Bliwise, D.L. Disturbed Sleep and Preterm Birth: A Potential Relationship? Clin. Exp. Obstet. Gynecol. 2009, 36, 166–168. [Google Scholar] [PubMed]
- Micheli, K.; Komninos, I.; Bagkeris, E.; Roumeliotaki, T.; Koutis, A.; Kogevinas, M.; Chatzi, L. Sleep Patterns in Late Pregnancy and Risk of Preterm Birth and Fetal Growth Restriction. Epidemiology 2011, 22, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Hartikainen-Sorri, A.L.; Sorri, M.; Anttonen, H.P.; Tuimala, R.; Läärä, E. Occupational Noise Exposure during Pregnancy: A Case Control Study. Int. Arch. Occup. Environ. Health 1988, 60, 279–283. [Google Scholar] [CrossRef]
- Mamelle, N.; Laumon, B.; Lazar, P. Prematurity and Occupational Activity during Pregnancy. Am. J. Epidemiol. 1984, 119, 309–322. [Google Scholar] [CrossRef]
- Hartikainen, A.L.; Sorri, M.; Anttonen, H.; Tuimala, R.; Läärä, E. Effect of Occupational Noise on the Course and Outcome of Pregnancy. Scand. J. Work Environ. Health 1994, 20, 444–450. [Google Scholar] [CrossRef]
- Knipschild, P.; Meijer, H.; Sallé, H. Aircraft Noise and Birth Weight. Int. Arch. Occup. Environ. Health 1981, 48, 131–136. [Google Scholar] [CrossRef]
- Dadvand, P.; de Nazelle, A.; Figueras, F.; Basagaña, X.; Su, J.; Amoly, E.; Jerrett, M.; Vrijheid, M.; Sunyer, J.; Nieuwenhuijsen, M.J. Green Space, Health Inequality and Pregnancy. Environ. Int. 2012, 40, 110–115. [Google Scholar] [CrossRef]
- Dadvand, P.; de Nazelle, A.; Triguero-Mas, M.; Schembari, A.; Cirach, M.; Amoly, E.; Figueras, F.; Basagaña, X.; Ostro, B.; Nieuwenhuijsen, M. Surrounding Greenness and Exposure to Air Pollution during Pregnancy: An Analysis of Personal Monitoring Data. Environ. Health Perspect. 2012, 120, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Dadvand, P.; Sunyer, J.; Basagaña, X.; Ballester, F.; Lertxundi, A.; Fernández-Somoano, A.; Estarlich, M.; García-Esteban, R.; Mendez, M.A.; Nieuwenhuijsen, M.J. Surrounding Greenness and Pregnancy Outcomes in Four Spanish Birth Cohorts. Environ. Health Perspect. 2012, 120, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Donovan, G.H.; Michael, Y.L.; Butry, D.T.; Sullivan, A.D.; Chase, J.M. Urban Trees and the Risk of Poor Birth Outcomes. Health Place 2011, 17, 390–393. [Google Scholar] [CrossRef]
- Kannan, S.; Misra, D.P.; Dvonch, J.T.; Krishnakumar, A. Exposures to Airborne Particulate Matter and Adverse Perinatal Outcomes: A Biologically Plausible Mechanistic Framework for Exploring Potential Effect Modification by Nutrition. Environ. Health Perspect. 2006, 114, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Hansel, N.N.; Tonorezos, E.S.; Williams, D.L.; Bilderback, A.; Breysse, P.N.; Diette, G.B.; McCormack, M.C. Indoor Particulate Matter Associated with Systemic Inflammation in COPD. J. Environ. Prot. 2015, 6, 566–572. [Google Scholar] [CrossRef]
- Clemente, D.B.P.; Casas, M.; Vilahur, N.; Begiristain, H.; Bustamante, M.; Carsin, A.-E.; Fernández, M.F.; Fierens, F.; Gyselaers, W.; Iñiguez, C.; et al. Prenatal Ambient Air Pollution, Placental Mitochondrial DNA Content, and Birth Weight in the INMA (Spain) and ENVIRONAGE (Belgium) Birth Cohorts. Environ. Health Perspect. 2016, 124, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Danielsen, P.H.; Karottki, D.G.; Jantzen, K.; Roursgaard, M.; Klingberg, H.; Jensen, D.M.; Christophersen, D.V.; Hemmingsen, J.G.; Cao, Y.; et al. Oxidative Stress and Inflammation Generated DNA Damage by Exposure to Air Pollution Particles. Mutat. Res. Rev. Mutat. Res. 2014, 762, 133–166. [Google Scholar] [CrossRef]
- Byun, H.-M.; Baccarelli, A.A. Environmental Exposure and Mitochondrial Epigenetics: Study Design and Analytical Challenges. Hum. Genet. 2014, 133, 247–257. [Google Scholar] [CrossRef]
- Hou, L.; Zhu, Z.-Z.; Zhang, X.; Nordio, F.; Bonzini, M.; Schwartz, J.; Hoxha, M.; Dioni, L.; Marinelli, B.; Pegoraro, V.; et al. Airborne Particulate Matter and Mitochondrial Damage: A Cross-Sectional Study. Environ. Health 2010, 9, 48. [Google Scholar] [CrossRef]
- Wick, P.; Malek, A.; Manser, P.; Meili, D.; Maeder-Althaus, X.; Diener, L.; Diener, P.-A.; Zisch, A.; Krug, H.F.; von Mandach, U. Barrier Capacity of Human Placenta for Nanosized Materials. Environ. Health Perspect. 2010, 118, 432–436. [Google Scholar] [CrossRef]
- Myllynen, P.; Pasanen, M.; Pelkonen, O. Human Placenta: A Human Organ for Developmental Toxicology Research and Biomonitoring. Placenta 2005, 26, 361–371. [Google Scholar] [CrossRef]
- Weldy, C.S.; Liu, Y.; Liggitt, H.D.; Chin, M.T. In Utero Exposure to Diesel Exhaust Air Pollution Promotes Adverse Intrauterine Conditions, Resulting in Weight Gain, Altered Blood Pressure, and Increased Susceptibility to Heart Failure in Adult Mice. PLoS ONE 2014, 9, e88582. [Google Scholar] [CrossRef] [PubMed]
- Headen, I.; Laraia, B.; Coleman-Phox, K.; Vieten, C.; Adler, N.; Epel, E. Neighborhood Typology and Cardiometabolic Pregnancy Outcomes in the Maternal Adiposity Metabolism and Stress Study. Obesity 2019, 27, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.; Kaplan, S. The Experience of Nature: A Psychological Perspective; Cambridge University Press: New York, NY, USA, 1989; pp. xii, 340; ISBN 978-0-521-34139-4. [Google Scholar]
- Hartig, T.; Evans, G.W.; Jamner, L.D.; Davis, D.S.; Gärling, T. Tracking Restoration in Natural and Urban Field Settings. J. Environ. Psychol. 2003, 23, 109–123. [Google Scholar] [CrossRef]
- Ulrich, R.S. Aesthetic and Affective Response to Natural Environment. In Behavior and the Natural Environment; Altman, I., Wohlwill, J.F., Eds.; Human Behavior and Environment; Springer: Boston, MA, USA, 1983; pp. 85–125. ISBN 978-1-4613-3539-9. [Google Scholar]
- Ulrich, R.S.; Simons, R.F.; Losito, B.D.; Fiorito, E.; Miles, M.A.; Zelson, M. Stress Recovery during Exposure to Natural and Urban Environments. J. Environ. Psychol. 1991, 11, 201–230. [Google Scholar] [CrossRef]
- Giurgescu, C.; Misra, D.P.; Sealy-Jefferson, S.; Howard-Caldwell, C.; Templin, T.N.; Slaughter, J.C.; Osypuk, T.L. The Impact of Neighborhood Quality, Perceived Stress, and Social Support on Depressive Symptoms during Pregnancy in African American Women. Soc. Sci. Med. 2015, 130, 172–180. [Google Scholar] [CrossRef]
- Schell, L.M.; Gallo, M.V.; Denham, M.; Ravenscroft, J. Effects of Pollution on Human Growth and Development: An Introduction. J. Physiol. Anthropol. 2006, 25, 103–112. [Google Scholar] [CrossRef]
- Christian, L.M. Psychoneuroimmunology in Pregnancy: Immune Pathways Linking Stress with Maternal Health, Adverse Birth Outcomes, and Fetal Development. Neurosci. Biobehav. Rev. 2012, 36, 350–361. [Google Scholar] [CrossRef]
- Sudo, A.; Nguyen, A.L.; Jonai, H.; Matsuda, S.; Villanueva, M.B.; Sotoyama, M.; Nguyen, T.C.; Le, V.T.; Hoang, M.H.; Nguyen, D.T.; et al. Effects of Earplugs on Catecholamine and Cortisol Excretion in Noise-Exposed Textile Workers. Ind. Health 1996, 34, 279–286. [Google Scholar] [CrossRef]
- Ising, H.; Babisch, W.; Kruppa, B. Noise-Induced Endocrine Effects and Cardiovascular Risk. Noise Health 1999, 1, 37–48. [Google Scholar]
- Melamed, S.; Bruhis, S. The Effects of Chronic Industrial Noise Exposure on Urinary Cortisol, Fatigue and Irritability: A Controlled Field Experiment. J. Occup. Environ. Med. 1996, 38, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Rojas-González, L.; Martínez-Leal, R.; Paz-Araviche, V.; Chacín-Almarza, B.; Corzo-Alvarez, G.; Sanabria-Vera, C.; Montiel-López, M. Serum cortisol levels in pre and post journal labor and non auditory manifestations in noise exposed workers of a brewer industry. Investig. Clin. 2004, 45, 297–307. [Google Scholar]
- Morishima, H.O.; Yeh, M.N.; James, L.S. Reduced Uterine Blood Flow and Fetal Hypoxemia with Acute Maternal Stress: Experimental Observation in the Pregnant Baboon. Am. J. Obstet. Gynecol. 1979, 134, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.E. Maternal Psychological Stress and Fetal Asphyxia: A Study in the Monkey. Am. J. Obstet. Gynecol. 1975, 122, 47–59. [Google Scholar] [CrossRef]
- Myers, R.E. Production of Fetal Asphyxia by Maternal Psychological Stress. Pavlov. J. Biol. Sci. 1977, 12, 51–62. [Google Scholar] [CrossRef]
- Hobel, C.J. Stress and Preterm Birth. Clin. Obstet. Gynecol. 2004, 47, 856–880; discussion 881#x2013;882. [Google Scholar] [CrossRef]
- Guski, R.; Schreckenberg, D.; Schuemer, R. WHO Environmental Noise Guidelines for the European Region: A Systematic Review on Environmental Noise and Annoyance. Int. J. Environ. Res. Public Health 2017, 14, E1539. [Google Scholar] [CrossRef]
- Clark, C.; Paunovic, K. WHO Environmental Noise Guidelines for the European Region: A Systematic Review on Environmental Noise and Quality of Life, Wellbeing and Mental Health. Int. J. Environ. Res Public Health 2018, 15, E2400. [Google Scholar] [CrossRef]
- Basner, M.; McGuire, S. WHO Environmental Noise Guidelines for the European Region: A Systematic Review on Environmental Noise and Effects on Sleep. Int. J. Environ. Res. Public Health 2018, 15, E519. [Google Scholar] [CrossRef]
- van Kempen, E.; Casas, M.; Pershagen, G.; Foraster, M. WHO Environmental Noise Guidelines for the European Region: A Systematic Review on Environmental Noise and Cardiovascular and Metabolic Effects: A Summary. Int. J. Environ. Res. Public Health 2018, 15, E379. [Google Scholar] [CrossRef]
- Dzhambov, A.M.; Lercher, P. Road Traffic Noise Exposure and Birth Outcomes: An Updated Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 2522. [Google Scholar] [CrossRef] [PubMed]
- Kihal-Talantikite, W.; Padilla, C.M.; Lalloue, B.; Rougier, C.; Defrance, J.; Zmirou-Navier, D.; Deguen, S. An Exploratory Spatial Analysis to Assess the Relationship between Deprivation, Noise and Infant Mortality: An Ecological Study. Environ. Health 2013, 12, 109. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, X.; Wang, Y.; Li, J.; Xu, Y.; Song, X.; Su, S.; Zhu, X.; Vitiello, M.V.; Shi, J.; et al. Sleep Disturbances during Pregnancy and Adverse Maternal and Fetal Outcomes: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2021, 58, 101436. [Google Scholar] [CrossRef] [PubMed]
- Montagnoli, C.; Zanconato, G.; Cinelli, G.; Tozzi, A.E.; Bovo, C.; Bortolus, R.; Ruggeri, S. Maternal Mental Health and Reproductive Outcomes: A Scoping Review of the Current Literature. Arch. Gynecol. Obs. 2020, 302, 801–819. [Google Scholar] [CrossRef]
- Vinikoor-Imler, L.C.; Messer, L.C.; Evenson, K.R.; Laraia, B.A. Neighborhood Conditions Are Associated with Maternal Health Behaviors and Pregnancy Outcomes. Soc. Sci. Med. 2011, 73, 1302–1311. [Google Scholar] [CrossRef]
- Tobias, D.K.; Zhang, C.; van Dam, R.M.; Bowers, K.; Hu, F.B. Physical Activity before and during Pregnancy and Risk of Gestational Diabetes Mellitus: A Meta-Analysis. Diabetes Care 2011, 34, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.; Petrella, E.; Bertarini, V.; Pedrielli, G.; Neri, I.; Facchinetti, F. Adherence to a Lifestyle Programme in Overweight/Obese Pregnant Women and Effect on Gestational Diabetes Mellitus: A Randomized Controlled Trial. Matern. Child Nutr. 2017, 13, e12333. [Google Scholar] [CrossRef]
- Cnattingius, S.; Villamor, E.; Johansson, S.; Edstedt Bonamy, A.-K.; Persson, M.; Wikström, A.-K.; Granath, F. Maternal Obesity and Risk of Preterm Delivery. JAMA 2013, 309, 2362–2370. [Google Scholar] [CrossRef]
- Ogneva-Himmelberger, Y.; Dahlberg, T.; Kelly, K.; Simas, T.A.M. Using Geographic Information Science to Explore Associations between Air Pollution, Environmental Amenities, and Preterm Births. AIMS Public Health 2015, 2, 469–486. [Google Scholar] [CrossRef]
- Laraia, B.A.; Siega-Riz, A.M.; Kaufman, J.S.; Jones, S.J. Proximity of Supermarkets Is Positively Associated with Diet Quality Index for Pregnancy. Prev. Med. 2004, 39, 869–875. [Google Scholar] [CrossRef]
- Maas, J.; van Dillen, S.M.E.; Verheij, R.A.; Groenewegen, P.P. Social Contacts as a Possible Mechanism behind the Relation between Green Space and Health. Health Place 2009, 15, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, M.; Baumgartner, T.; Kirschbaum, C.; Ehlert, U. Social Support and Oxytocin Interact to Suppress Cortisol and Subjective Responses to Psychosocial Stress. Biol. Psychiatry 2003, 54, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.; Alramadhan, S.; Iniguez, C.; Duijts, L.; Jaddoe, V.W.V.; Den Dekker, H.T.; Crozier, S.; Godfrey, K.M.; Hindmarsh, P.; Vik, T.; et al. A Systematic Review of Maternal Smoking during Pregnancy and Fetal Measurements with Meta-Analysis. PLoS ONE 2017, 12, e0170946. [Google Scholar] [CrossRef] [PubMed]
- Jaddoe, V.W.V.; Troe, E.-J.W.M.; Hofman, A.; Mackenbach, J.P.; Moll, H.A.; Steegers, E.A.P.; Witteman, J.C.M. Active and Passive Maternal Smoking during Pregnancy and the Risks of Low Birthweight and Preterm Birth: The Generation R Study. Paediatr. Perinat. Epidemiol. 2008, 22, 162–171. [Google Scholar] [CrossRef]
- Huang, T.; Yeh, C.-Y.; Tsai, Y.-C. A Diet and Physical Activity Intervention for Preventing Weight Retention among Taiwanese Childbearing Women: A Randomised Controlled Trial. Midwifery 2011, 27, 257–264. [Google Scholar] [CrossRef]
- Rozi, S.; Butt, Z.A.; Zahid, N.; Wasim, S.; Shafique, K. Association of Tobacco Use and Other Determinants with Pregnancy Outcomes: A Multicentre Hospital-Based Case-Control Study in Karachi, Pakistan. BMJ Open 2016, 6, e012045. [Google Scholar] [CrossRef]
- Berlin, I.; Golmard, J.-L.; Jacob, N.; Tanguy, M.-L.; Heishman, S.J. Cigarette Smoking During Pregnancy: Do Complete Abstinence and Low Level Cigarette Smoking Have Similar Impact on Birth Weight? Nicotine Tob. Res. 2017, 19, 518–524. [Google Scholar] [CrossRef]
- Lanting, C.I.; van Wouwe, J.P.K.; van den Burg, I.; Segaar, D.; van der Pal-de Bruin, K.M. Smoking during pregnancy: Trends between 2001 and 2010. Ned. Tijdschr. Geneeskd. 2012, 156, A5092. [Google Scholar]
- Aurrekoetxea, J.J.; Murcia, M.; Rebagliato, M.; Fernández-Somoano, A.; Castilla, A.M.; Guxens, M.; López, M.J.; Lertxundi, A.; Espada, M.; Tardón, A.; et al. Factors Associated with Second-Hand Smoke Exposure in Non-Smoking Pregnant Women in Spain: Self-Reported Exposure and Urinary Cotinine Levels. Sci. Total Environ. 2014, 470–471, 1189–1196. [Google Scholar] [CrossRef]
- Baron, R.; Manniën, J.; de Jonge, A.; Heymans, M.W.; Klomp, T.; Hutton, E.K.; Brug, J. Socio-Demographic and Lifestyle-Related Characteristics Associated with Self-Reported Any, Daily and Occasional Smoking during Pregnancy. PLoS ONE 2013, 8, e74197. [Google Scholar] [CrossRef]
- Hemsing, N.; Greaves, L.; O’Leary, R.; Chan, K.; Okoli, C. Partner Support for Smoking Cessation during Pregnancy: A Systematic Review. Nicotine Tob. Res. 2012, 14, 767–776. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, C.M.; Bower, C. Guidelines for Pregnancy: What’s an Acceptable Risk, and How Is the Evidence (Finally) Shaping Up? Drug. Alcohol. Rev. 2012, 31, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Barger, M.K. Maternal Nutrition and Perinatal Outcomes. J. Midwifery Womens Health 2010, 55, 502–511. [Google Scholar] [CrossRef]
- Waterland, R.A.; Jirtle, R.L. Early Nutrition, Epigenetic Changes at Transposons and Imprinted Genes, and Enhanced Susceptibility to Adult Chronic Diseases. Nutrition 2004, 20, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Irner, T.B. Substance Exposure in Utero and Developmental Consequences in Adolescence: A Systematic Review. Child Neuropsychol. 2012, 18, 521–549. [Google Scholar] [CrossRef] [PubMed]
- Behnke, M.; Smith, V.C.; Committee on Substance Abuse; Committee on Fetus and Newborn. Prenatal Substance Abuse: Short- and Long-Term Effects on the Exposed Fetus. Pediatrics 2013, 131, e1009-1024. [Google Scholar] [CrossRef]
- Viteri, O.A.; Soto, E.E.; Bahado-Singh, R.O.; Christensen, C.W.; Chauhan, S.P.; Sibai, B.M. Fetal Anomalies and Long-Term Effects Associated with Substance Abuse in Pregnancy: A Literature Review. Am. J. Perinatol. 2015, 32, 405–416. [Google Scholar] [CrossRef]
- Mamluk, L.; Edwards, H.B.; Savović, J.; Leach, V.; Jones, T.; Moore, T.H.M.; Ijaz, S.; Lewis, S.J.; Donovan, J.L.; Lawlor, D.; et al. Low Alcohol Consumption and Pregnancy and Childhood Outcomes: Time to Change Guidelines Indicating Apparently “safe” Levels of Alcohol during Pregnancy? A Systematic Review and Meta-Analyses. BMJ Open 2017, 7, e015410. [Google Scholar] [CrossRef]
- Luke, S.; Hutcheon, J.; Kendall, T. Cannabis Use in Pregnancy in British Columbia and Selected Birth Outcomes. J. Obstet. Gynaecol. Can. 2019, 41, 1311–1317. [Google Scholar] [CrossRef]
- Corsi, D.J.; Walsh, L.; Weiss, D.; Hsu, H.; El-Chaar, D.; Hawken, S.; Fell, D.B.; Walker, M. Association Between Self-Reported Prenatal Cannabis Use and Maternal, Perinatal, and Neonatal Outcomes. JAMA 2019, 322, 145–152. [Google Scholar] [CrossRef]
- Gouin, K.; Murphy, K.; Shah, P.S.; Knowledge Synthesis group on Determinants of Low Birth Weight and Preterm Births. Effects of Cocaine Use during Pregnancy on Low Birthweight and Preterm Birth: Systematic Review and Metaanalyses. Am. J. Obstet. Gynecol. 2011, 204, 340.e1–e12. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, A.M.; Burke, S.A. Identification of Early Developmental Deficits in Infants with Prenatal Heroin, Methadone, and Other Opioid Exposure. Clin. Pediatr. 2015, 54, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Flenady, V.; Koopmans, L.; Middleton, P.; Frøen, J.F.; Smith, G.C.; Gibbons, K.; Coory, M.; Gordon, A.; Ellwood, D.; McIntyre, H.D.; et al. Major Risk Factors for Stillbirth in High-Income Countries: A Systematic Review and Meta-Analysis. Lancet 2011, 377, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Veisani, Y.; Jenabi, E.; Delpisheh, A.; Khazaei, S. Effect of Prenatal Smoking Cessation Interventions on Birth Weight: Meta-Analysis. J. Matern. Fetal. Neonatal. Med. 2019, 32, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.; Jaddoe, V.W.V.; Mackenbach, J.P.; Hofman, A.; Steegers-Theunissen, R.P.M.; Steegers, E.A.P. Determinants of Folic Acid Use in Early Pregnancy in a Multi-Ethnic Urban Population in The Netherlands: The Generation R Study. Prev. Med. 2008, 47, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Manniën, J.; Klomp, T.; Wiegers, T.; Pereboom, M.; Brug, J.; de Jonge, A.; van der Meijde, M.; Hutton, E.; Schellevis, F.; Spelten, E. Evaluation of Primary Care Midwifery in the Netherlands: Design and Rationale of a Dynamic Cohort Study (DELIVER). BMC Health Serv. Res. 2012, 12, 69. [Google Scholar] [CrossRef]
- Liu, C.; Liu, C.; Wang, Q.; Zhang, Z. Supplementation of Folic Acid in Pregnancy and the Risk of Preeclampsia and Gestational Hypertension: A Meta-Analysis. Arch. Gynecol. Obstet. 2018, 298, 697–704. [Google Scholar] [CrossRef]
- Ion, R.; Bernal, A.L. Smoking and Preterm Birth. Reprod. Sci 2015, 22, 918–926. [Google Scholar] [CrossRef]
- Goldstein, H.; Goldberg, I.D.; Frazier, T.M.; Davis, G.E. Cigarette Smoking and Prematurity: Review. Public Health Rep. 1964, 79, 553. [Google Scholar] [CrossRef]
- Hedderson, M.M.; Ferrara, A.; Sacks, D.A. Gestational Diabetes Mellitus and Lesser Degrees of Pregnancy Hyperglycemia: Association with Increased Risk of Spontaneous Preterm Birth. Obs. Gynecol. 2003, 102, 850–856. [Google Scholar] [CrossRef]
- Marcoux, S.; Brisson, J.; Fabia, J. The Effect of Leisure Time Physical Activity on the Risk of Pre-Eclampsia and Gestational Hypertension. J. Epidemiol. Community Health 1989, 43, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, T.K.; Williams, M.A.; Lee, I.-M.; Dashow, E.E.; Thompson, M.L.; Luthy, D.A. Recreational Physical Activity during Pregnancy and Risk of Preeclampsia. Hypertension 2003, 41, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Saftlas, A.F.; Logsden-Sackett, N.; Wang, W.; Woolson, R.; Bracken, M.B. Work, Leisure-Time Physical Activity, and Risk of Preeclampsia and Gestational Hypertension. Am. J. Epidemiol. 2004, 160, 758–765. [Google Scholar] [CrossRef]
- Dye, T.D.; Knox, K.L.; Artal, R.; Aubry, R.H.; Wojtowycz, M.A. Physical Activity, Obesity, and Diabetes in Pregnancy. Am. J. Epidemiol. 1997, 146, 961–965. [Google Scholar] [CrossRef]
- Dempsey, J.C.; Sorensen, T.K.; Williams, M.A.; Lee, I.-M.; Miller, R.S.; Dashow, E.E.; Luthy, D.A. Prospective Study of Gestational Diabetes Mellitus Risk in Relation to Maternal Recreational Physical Activity before and during Pregnancy. Am. J. Epidemiol. 2004, 159, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.C.; Butler, C.L.; Sorensen, T.K.; Lee, I.-M.; Thompson, M.L.; Miller, R.S.; Frederick, I.O.; Williams, M.A. A Case-Control Study of Maternal Recreational Physical Activity and Risk of Gestational Diabetes Mellitus. Diabetes Res. Clin. Pract. 2004, 66, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, V.A.; Smart, N.A. Exercise Training for Blood Pressure: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2013, 2, e004473. [Google Scholar] [CrossRef] [PubMed]
- Kyu, H.H.; Bachman, V.F.; Alexander, L.T.; Mumford, J.E.; Afshin, A.; Estep, K.; Veerman, J.L.; Delwiche, K.; Iannarone, M.L.; Moyer, M.L.; et al. Physical Activity and Risk of Breast Cancer, Colon Cancer, Diabetes, Ischemic Heart Disease, and Ischemic Stroke Events: Systematic Review and Dose-Response Meta-Analysis for the Global Burden of Disease Study 2013. BMJ 2016, 354, i3857. [Google Scholar] [CrossRef]
- Rodriguez-Ayllon, M.; Cadenas-Sánchez, C.; Estévez-López, F.; Muñoz, N.E.; Mora-Gonzalez, J.; Migueles, J.H.; Molina-García, P.; Henriksson, H.; Mena-Molina, A.; Martínez-Vizcaíno, V.; et al. Role of Physical Activity and Sedentary Behavior in the Mental Health of Preschoolers, Children and Adolescents: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 1383–1410. [Google Scholar] [CrossRef]
- Da Costa, D.; Rippen, N.; Dritsa, M.; Ring, A. Self-Reported Leisure-Time Physical Activity during Pregnancy and Relationship to Psychological Well-Being. J. Psychosom. Obstet. Gynaecol. 2003, 24, 111–119. [Google Scholar] [CrossRef]
- Poudevigne, M.S.; O’Connor, P.J. Physical Activity and Mood during Pregnancy. Med. Sci. Sports Exerc. 2005, 37, 1374–1380. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sugiyama, T.; Leslie, E.; Giles-Corti, B.; Owen, N. Associations of Neighbourhood Greenness with Physical and Mental Health: Do Walking, Social Coherence and Local Social Interaction Explain the Relationships? J. Epidemiol. Community Health 2008, 62, e9. [Google Scholar] [CrossRef] [PubMed]
- Séguin, L.; Potvin, L.; St-Denis, M.; Loiselle, J. Chronic Stressors, Social Support, and Depression during Pregnancy. Obstet. Gynecol. 1995, 85, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, L.; Odouli, R. Presence of Depressive Symptoms during Early Pregnancy and the Risk of Preterm Delivery: A Prospective Cohort Study. Hum. Reprod. 2009, 24, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.D.; Fraser, W.D.; Frasch, M.G.; Séguin, J.R. Psychosocial Stress in Pregnancy and Preterm Birth: Associations and Mechanisms. J. Perinat. Med. 2013, 41, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, S.N.; Sharma, A.J.; Kim, S.Y.; Schieve, L.A. Maternal Prepregnancy Weight Status and Associations with Children’s Development and Disabilities at Kindergarten. Int. J. Obes. 2013, 37, 1344–1351. [Google Scholar] [CrossRef]
- Luoma, I.; Kaukonen, P.; Mäntymaa, M.; Puura, K.; Tamminen, T.; Salmelin, R. A Longitudinal Study of Maternal Depressive Symptoms, Negative Expectations and Perceptions of Child Problems. Child Psychiatry Hum. Dev. 2004, 35, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Sebire, N.J.; Jolly, M.; Harris, J.; Regan, L.; Robinson, S. Is Maternal Underweight Really a Risk Factor for Adverse Pregnancy Outcome? A Population-Based Study in London. BJOG 2001, 108, 61–66. [Google Scholar] [CrossRef]
- Cedergren, M. Effects of Gestational Weight Gain and Body Mass Index on Obstetric Outcome in Sweden. Int. J. Gynaecol. Obstet. 2006, 93, 269–274. [Google Scholar] [CrossRef]
- Hedderson, M.M.; Weiss, N.S.; Sacks, D.A.; Pettitt, D.J.; Selby, J.V.; Quesenberry, C.P.; Ferrara, A. Pregnancy Weight Gain and Risk of Neonatal Complications: Macrosomia, Hypoglycemia, and Hyperbilirubinemia. Obstet. Gynecol. 2006, 108, 1153–1161. [Google Scholar] [CrossRef]
- Stotland, N.E.; Cheng, Y.W.; Hopkins, L.M.; Caughey, A.B. Gestational Weight Gain and Adverse Neonatal Outcome among Term Infants. Obstet. Gynecol. 2006, 108, 635–643. [Google Scholar] [CrossRef]
- Leddy, M.A.; Power, M.L.; Schulkin, J. The Impact of Maternal Obesity on Maternal and Fetal Health. Rev. Obstet. Gynecol. 2008, 1, 170–178. [Google Scholar]
- deCatanzaro, D.; Macniven, E. Psychogenic Pregnancy Disruptions in Mammals. Neurosci. Biobehav. Rev. 1992, 16, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Mulder, E.J.H.; Robles de Medina, P.G.; Huizink, A.C.; Van den Bergh, B.R.H.; Buitelaar, J.K.; Visser, G.H.A. Prenatal Maternal Stress: Effects on Pregnancy and the (Unborn) Child. Early Hum. Dev. 2002, 70, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Grote, N.K.; Bridge, J.A.; Gavin, A.R.; Melville, J.L.; Iyengar, S.; Katon, W.J. A Meta-Analysis of Depression during Pregnancy and the Risk of Preterm Birth, Low Birth Weight, and Intrauterine Growth Restriction. Arch. Gen. Psychiatry 2010, 67, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Mckee, M.D.; Cunningham, M.; Jankowski, K.R.; Zayas, L. Health-Related Functional Status in Pregnancy: Relationship to Depression and Social Support in a Multi-Ethnic Population. Obstet. Gynecol. 2001, 97, 988–993. [Google Scholar] [CrossRef]
- Orr, S.T.; James, S.A.; Blackmore Prince, C. Maternal Prenatal Depressive Symptoms and Spontaneous Preterm Births among African-American Women in Baltimore, Maryland. Am. J. Epidemiol. 2002, 156, 797–802. [Google Scholar] [CrossRef]
- Kurki, T.; Hiilesmaa, V.; Raitasalo, R.; Mattila, H.; Ylikorkala, O. Depression and Anxiety in Early Pregnancy and Risk for Preeclampsia. Obstet. Gynecol. 2000, 95, 487–490. [Google Scholar] [CrossRef]
- Zelkowitz, P.; Papageorgiou, A. Easing Maternal Anxiety: An Update. Womens Health 2012, 8, 205–213. [Google Scholar] [CrossRef]
- Catov, J.M.; Abatemarco, D.J.; Markovic, N.; Roberts, J.M. Anxiety and Optimism Associated with Gestational Age at Birth and Fetal Growth. Matern. Child Health J. 2010, 14, 758–764. [Google Scholar] [CrossRef]
- Barker, E.D.; Jaffee, S.R.; Uher, R.; Maughan, B. The Contribution of Prenatal and Postnatal Maternal Anxiety and Depression to Child Maladjustment. Depress. Anxiety 2011, 28, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Beijers, R.; Jansen, J.; Riksen-Walraven, M.; de Weerth, C. Maternal Prenatal Anxiety and Stress Predict Infant Illnesses and Health Complaints. Pediatrics 2010, 126, e401–e409. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-K.; Wen, S.W.; Fleming, N.; Demissie, K.; Rhoads, G.G.; Walker, M. Teenage Pregnancy and Adverse Birth Outcomes: A Large Population Based Retrospective Cohort Study. Int. J. Epidemiol. 2007, 36, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Oakley, L.; Maconochie, N.; Doyle, P.; Dattani, N.; Moser, K. Multivariate Analysis of Infant Death in England and Wales in 2005-06, with Focus on Socio-Economic Status and Deprivation. Health. Stat. Q. 2009, 42, 22–39. [Google Scholar] [CrossRef][Green Version]
- Gray, R.; Headley, J.; Oakley, L.; Kurinczuk, J.J.; Brocklehurst, P.; Hollowell, J. Towards an Understanding of Variations in Infant Mortality Rates between Different Ethnic Groups in England and Wales. 2010. Available online: https://www.semanticscholar.org/paper/Towards-an-understanding-of-variations-in-infant-in-Graya-Headleyb/d7a8fb6fb35f6b7516e7d6cfdbceb24f70cd080b (accessed on 20 August 2022).
- Boy, A.; Salihu, H.M. Intimate Partner Violence and Birth Outcomes: A Systematic Review. Int. J. Fertil. Womens Med. 2004, 49, 159–164. [Google Scholar]
- Arntzen, A.; Nybo Andersen, A.M. Social Determinants for Infant Mortality in the Nordic Countries, 1980–2001. Scand J. Public Health 2004, 32, 381–389. [Google Scholar] [CrossRef]
- Giurgescu, C.; Zenk, S.N.; Dancy, B.L.; Park, C.G.; Dieber, W.; Block, R. Relationships among Neighborhood Environment, Racial Discrimination, Psychological Distress, and Preterm Birth in African American Women. J. Obstet. Gynecol. Neonatal Nurs. 2012, 41, E51–E61. [Google Scholar] [CrossRef]
- Weinstock, M. The Potential Influence of Maternal Stress Hormones on Development and Mental Health of the Offspring. Brain Behav. Immun. 2005, 19, 296–308. [Google Scholar] [CrossRef]
- Nurminen, T. Female Noise Exposure, Shift Work, and Reproduction. J. Occup. Environ. Med. 1995, 37, 945–950. [Google Scholar] [CrossRef]
- Wadhwa, P.D.; Garite, T.J.; Porto, M.; Glynn, L.; Chicz-DeMet, A.; Dunkel-Schetter, C.; Sandman, C.A. Placental Corticotropin-Releasing Hormone (CRH), Spontaneous Preterm Birth, and Fetal Growth Restriction: A Prospective Investigation. Am. J. Obstet. Gynecol. 2004, 191, 1063–1069. [Google Scholar] [CrossRef]
- Hobel, C.J.; Dunkel-Schetter, C.; Roesch, S.C.; Castro, L.C.; Arora, C.P. Maternal Plasma Corticotropin-Releasing Hormone Associated with Stress at 20 Weeks’ Gestation in Pregnancies Ending in Preterm Delivery. Am. J. Obstet. Gynecol. 1999, 180, S257–S263. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.N.; Regan, J.A.; Norwitz, E.R. The Epidemiology of Preterm Labor. Semin. Perinatol. 2001, 25, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, H.M.; Di Quinzio, M.K.W.; Permezel, M.; Brennecke, S.P. Predicting Preterm Labour: Current Status and Future Prospects. Dis. Markers 2015, 2015, 435014. [Google Scholar] [CrossRef]
- Ding, X.-X.; Wu, Y.-L.; Xu, S.-J.; Zhu, R.-P.; Jia, X.-M.; Zhang, S.-F.; Huang, K.; Zhu, P.; Hao, J.-H.; Tao, F.-B. Maternal Anxiety during Pregnancy and Adverse Birth Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Affect Disord. 2014, 159, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Class, Q.A.; Lichtenstein, P.; Långström, N.; D’Onofrio, B.M. Timing of Prenatal Maternal Exposure to Severe Life Events and Adverse Pregnancy Outcomes: A Population Study of 2.6 Million Pregnancies. Psychosom. Med. 2011, 73, 234–241. [Google Scholar] [CrossRef]
- Priest, S.R.; Austin, M.-P.; Barnett, B.B.; Buist, A. A Psychosocial Risk Assessment Model (PRAM) for Use with Pregnant and Postpartum Women in Primary Care Settings. Arch. Womens Ment. Health 2008, 11, 307–317. [Google Scholar] [CrossRef]
- Lee, A.M.; Lam, S.K.; Sze Mun Lau, S.M.; Chong, C.S.Y.; Chui, H.W.; Fong, D.Y.T. Prevalence, Course, and Risk Factors for Antenatal Anxiety and Depression. Obstet. Gynecol. 2007, 110, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, B.M.; Gennaro, S.; Szalacha, L.A.; Hoying, J.; O’Connor, C.; Cooper, A.; Gibeau, A. Randomized Controlled Trial of the COPE-P Intervention to Improve Mental Health, Healthy Lifestyle Behaviors, Birth and Post-Natal Outcomes of Minority Pregnant Women: Study Protocol with Implications. Contemp. Clin. Trials 2020, 98, 106090. [Google Scholar] [CrossRef] [PubMed]
- Nielsen Forman, D.; Videbech, P.; Hedegaard, M.; Dalby Salvig, J.; Secher, N.J. Postpartum Depression: Identification of Women at Risk. BJOG 2000, 107, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Robertson, E.; Grace, S.; Wallington, T.; Stewart, D.E. Antenatal Risk Factors for Postpartum Depression: A Synthesis of Recent Literature. Gen. Hosp. Psychiatry 2004, 26, 289–295. [Google Scholar] [CrossRef]
- Paul, K.H.; Graham, M.L.; Olson, C.M. The Web of Risk Factors for Excessive Gestational Weight Gain in Low Income Women. Matern. Child Health J. 2013, 17, 344–351. [Google Scholar] [CrossRef] [PubMed]
- George, G.C.; Hanss-Nuss, H.; Milani, T.J.; Freeland-Graves, J.H. Food Choices of Low-Income Women during Pregnancy and Postpartum. J. Am. Diet Assoc. 2005, 105, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Hurley, K.M.; Caulfield, L.E.; Sacco, L.M.; Costigan, K.A.; Dipietro, J.A. Psychosocial Influences in Dietary Patterns during Pregnancy. J. Am. Diet. Assoc. 2005, 105, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Mezuk, B.; Rafferty, J.A.; Kershaw, K.N.; Hudson, D.; Abdou, C.M.; Lee, H.; Eaton, W.W.; Jackson, J.S. Reconsidering the Role of Social Disadvantage in Physical and Mental Health: Stressful Life Events, Health Behaviors, Race, and Depression. Am. J. Epidemiol. 2010, 172, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Lobel, M.; Cannella, D.L.; Graham, J.E.; DeVincent, C.; Schneider, J.; Meyer, B.A. Pregnancy-Specific Stress, Prenatal Health Behaviors, and Birth Outcomes. Health Psychol. 2008, 27, 604–615. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.D.; Han, Z.; Mulla, S.; Beyene, J.; Knowledge Synthesis Group. Overweight and Obesity in Mothers and Risk of Preterm Birth and Low Birth Weight Infants: Systematic Review and Meta-Analyses. BMJ 2010, 341, c3428. [Google Scholar] [CrossRef]
- Rogers, J.M. Tobacco and Pregnancy. Reprod. Toxicol. 2009, 28, 152–160. [Google Scholar] [CrossRef]
- Nucci, L.B.; Schmidt, M.I.; Duncan, B.B.; Fuchs, S.C.; Fleck, E.T.; Santos Britto, M.M. Nutritional Status of Pregnant Women: Prevalence and Associated Pregnancy Outcomes. Rev. Saude Publica 2001, 35, 502–507. [Google Scholar] [CrossRef]
- Zhang, C.; Solomon, C.G.; Manson, J.E.; Hu, F.B. A Prospective Study of Pregravid Physical Activity and Sedentary Behaviors in Relation to the Risk for Gestational Diabetes Mellitus. Arch. Intern. Med. 2006, 166, 543–548. [Google Scholar] [CrossRef]
- Petrou, S.; Kupek, E.; Vause, S.; Maresh, M. Antenatal Visits and Adverse Perinatal Outcomes: Results from a British Population-Based Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 106, 40–49. [Google Scholar] [CrossRef]
- Raatikainen, K.; Heiskanen, N.; Heinonen, S. Under-Attending Free Antenatal Care Is Associated with Adverse Pregnancy Outcomes. BMC Public Health 2007, 7, 268. [Google Scholar] [CrossRef] [PubMed]
- Gadson, A.; Akpovi, E.; Mehta, P.K. Exploring the Social Determinants of Racial/Ethnic Disparities in Prenatal Care Utilization and Maternal Outcome. Semin. Perinatol. 2017, 41, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Tumin, D.; Menegay, M.; Shrider, E.A.; Nau, M.; Tumin, R. Local Income Inequality, Individual Socioeconomic Status, and Unmet Healthcare Needs in Ohio, USA. Health Equity 2018, 2, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Origlia, P.; Jevitt, C.; Sayn-Wittgenstein, F.Z.; Cignacco, E. Experiences of Antenatal Care Among Women Who Are Socioeconomically Deprived in High-Income Industrialized Countries: An Integrative Review. J. Midwifery Women’s Health 2017, 62, 589–598. [Google Scholar] [CrossRef]
- Levesque, J.-F.; Harris, M.F.; Russell, G. Patient-Centred Access to Health Care: Conceptualising Access at the Interface of Health Systems and Populations. Int. J. Equity Health 2013, 12, 18. [Google Scholar] [CrossRef]
- Grand-Guillaume-Perrenoud, J.A.; Origlia, P.; Cignacco, E. Barriers and Facilitators of Maternal Healthcare Utilisation in the Perinatal Period among Women with Social Disadvantage: A Theory-Guided Systematic Review. Midwifery 2022, 105, 103237. [Google Scholar] [CrossRef]
- Downe, S.; Finlayson, K.; Walsh, D.; Lavender, T. ‘Weighing up and Balancing out’: A Meta-Synthesis of Barriers to Antenatal Care for Marginalised Women in High-Income Countries. BJOG Int. J. Obstet. Gynaecol. 2009, 116, 518–529. [Google Scholar] [CrossRef]
- Balaam, M.; Akerjordet, K.; Lyberg, A.; Kaiser, B.; Schoening, E.; Fredriksen, A.; Ensel, A.; Gouni, O.; Severinsson, E. A Qualitative Review of Migrant Women’s Perceptions of Their Needs and Experiences Related to Pregnancy and Childbirth. J. Adv. Nurs. 2013, 69, 1919–1930. [Google Scholar] [CrossRef]
- Heaman, M.I.; Gupton, A.L.; Moffatt, M.E. Prevalence and Predictors of Inadequate Prenatal Care: A Comparison of Aboriginal and Non-Aboriginal Women in Manitoba. J. Obstet. Gynaecol. Can. 2005, 27, 237–246. [Google Scholar] [CrossRef]
- Higginbottom, G.M.; Hadziabdic, E.; Yohani, S.; Paton, P. Immigrant Women’s Experience of Maternity Services in Canada: A Meta-Ethnography. Midwifery 2014, 30, 544–559. [Google Scholar] [CrossRef]
- Daoud, N.; O’Campo, P.; Anderson, K.; Agbaria, A.K.; Shoham-Vardi, I. The Social Ecology of Maternal Infant Care in Socially and Economically Marginalized Community in Southern Israel. Health Educ. Res. 2012, 27, 1018–1030. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hulsey, T.M.; Laken, M.; Miller, V.; Ager, J. The Influence of Attitudes about Unintended Pregnancy on Use of Prenatal and Postpartum Care. J. Perinatol. 2000, 20, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Kapaya, H.; Mercer, E.; Boffey, F.; Jones, G.; Mitchell, C.; Anumba, D. Deprivation and Poor Psychosocial Support Are Key Determinants of Late Antenatal Presentation and Poor Fetal Outcomes-a Combined Retrospective and Prospective Study. BMC Pregnancy Childbirth 2015, 15, 309. [Google Scholar] [CrossRef]
- Heaman, M.I.; Moffatt, M.; Elliott, L.; Sword, W.; Helewa, M.E.; Morris, H.; Gregory, P.; Tjaden, L.; Cook, C. Barriers, Motivators and Facilitators Related to Prenatal Care Utilization among Inner-City Women in Winnipeg, Canada: A Case-Control Study. BMC Pregnancy Childbirth 2014, 14, 227. [Google Scholar] [CrossRef]
- Gonthier, C.; Estellat, C.; Deneux-Tharaux, C.; Blondel, B.; Alfaiate, T.; Schmitz, T.; Oury, J.-F.; Mandelbrot, L.; Luton, D.; Ravaud, P.; et al. Association between Maternal Social Deprivation and Prenatal Care Utilization: The PreCARE Cohort Study. BMC Pregnancy Childbirth 2017, 17, 126. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Robertson Blackmore, E.; Stewart, D.E. Healthcare Worker’s Perceptions of Barriers to Care by Immigrant Women with Postpartum Depression: An Exploratory Qualitative Study. Arch. Womens Ment. Health 2007, 10, 93–101. [Google Scholar] [CrossRef]
- Davenport, M.H.; Ruchat, S.-M.; Poitras, V.J.; Jaramillo Garcia, A.; Gray, C.E.; Barrowman, N.; Skow, R.J.; Meah, V.L.; Riske, L.; Sobierajski, F.; et al. Prenatal Exercise for the Prevention of Gestational Diabetes Mellitus and Hypertensive Disorders of Pregnancy: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2018, 52, 1367–1375. [Google Scholar] [CrossRef]
- Du, M.-C.; Ouyang, Y.-Q.; Nie, X.-F.; Huang, Y.; Redding, S.R. Effects of Physical Exercise during Pregnancy on Maternal and Infant Outcomes in Overweight and Obese Pregnant Women: A Meta-Analysis. Birth 2019, 46, 211–221. [Google Scholar] [CrossRef]
- Muktabhant, B.; Lawrie, T.A.; Lumbiganon, P.; Laopaiboon, M. Diet or Exercise, or Both, for Preventing Excessive Weight Gain in Pregnancy. Cochrane Database Syst. Rev. 2015, 6, CD007145. [Google Scholar] [CrossRef]
- O’Brien, C.M.; Grivell, R.M.; Dodd, J.M. Systematic Review of Antenatal Dietary and Lifestyle Interventions in Women with a Normal Body Mass Index. Acta Obstet. Gynecol. Scand 2016, 95, 259–269. [Google Scholar] [CrossRef]
- Althuizen, E.; van der Wijden, C.L.; van Mechelen, W.; Seidell, J.C.; van Poppel, M.N.M. The Effect of a Counselling Intervention on Weight Changes during and after Pregnancy: A Randomised Trial. BJOG 2013, 120, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Asbee, S.M.; Jenkins, T.R.; Butler, J.R.; White, J.; Elliot, M.; Rutledge, A. Preventing Excessive Weight Gain during Pregnancy through Dietary and Lifestyle Counseling: A Randomized Controlled Trial. Obstet. Gynecol. 2009, 113, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Hui, A.; Back, L.; Ludwig, S.; Gardiner, P.; Sevenhuysen, G.; Dean, H.; Sellers, E.; McGavock, J.; Morris, M.; Bruce, S.; et al. Lifestyle Intervention on Diet and Exercise Reduced Excessive Gestational Weight Gain in Pregnant Women under a Randomised Controlled Trial. BJOG 2012, 119, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Hui, A.L.; Back, L.; Ludwig, S.; Gardiner, P.; Sevenhuysen, G.; Dean, H.J.; Sellers, E.; McGavock, J.; Morris, M.; Jiang, D.; et al. Effects of Lifestyle Intervention on Dietary Intake, Physical Activity Level, and Gestational Weight Gain in Pregnant Women with Different Pre-Pregnancy Body Mass Index in a Randomized Control Trial. BMC Pregnancy Childbirth 2014, 14, 331. [Google Scholar] [CrossRef]
- Hui, A.L.; Ludwig, S.M.; Gardiner, P.; Sevenhuysen, G.; Murray, R.; Morris, M.; Shen, G.X. Community-Based Exercise and Dietary Intervention during Pregnancy: A Pilot Study. Cochrane Library. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-00613161/full (accessed on 7 October 2022).
- Laitinen, K.; Poussa, T.; Isolauri, E.; Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota Group. Probiotics and Dietary Counselling Contribute to Glucose Regulation during and after Pregnancy: A Randomised Controlled Trial. Br. J. Nutr. 2009, 101, 1679–1687. [Google Scholar] [CrossRef]
- Phelan, S.; Phipps, M.G.; Abrams, B.; Darroch, F.; Schaffner, A.; Wing, R.R. Randomized Trial of a Behavioral Intervention to Prevent Excessive Gestational Weight Gain: The Fit for Delivery Study. Am. J. Clin. Nutr. 2011, 93, 772–779. [Google Scholar] [CrossRef]
- Polley, B.A.; Wing, R.R.; Sims, C.J. Randomized Controlled Trial to Prevent Excessive Weight Gain in Pregnant Women. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1494–1502. [Google Scholar] [CrossRef]
- Dodd, J.M.; Louise, J.; Deussen, A.R.; Grivell, R.M.; Dekker, G.; McPhee, A.J.; Hague, W. Effect of Metformin in Addition to Dietary and Lifestyle Advice for Pregnant Women Who Are Overweight or Obese: The GRoW Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Diabetes Endocrinol. 2019, 7, 15–24. [Google Scholar] [CrossRef]
- The International Weight Management in Pregnancy (i-WIP) Collaborative Group. Effect of Diet and Physical Activity Based Interventions in Pregnancy on Gestational Weight Gain and Pregnancy Outcomes: Meta-Analysis of Individual Participant Data from Randomised Trials. BMJ 2017, 358, j3119. [Google Scholar] [CrossRef]
- Catherine, N.L.A.; Boyle, M.; Zheng, Y.; McCandless, L.; Xie, H.; Lever, R.; Sheehan, D.; Gonzalez, A.; Jack, S.M.; Gafni, A.; et al. Nurse Home Visiting and Prenatal Substance Use in a Socioeconomically Disadvantaged Population in British Columbia: Analysis of Prenatal Secondary Outcomes in an Ongoing Randomized Controlled Trial. CMAJ Open 2020, 8, E667–E675. [Google Scholar] [CrossRef]
- Olds, D.L. Preventing Child Maltreatment and Crime with Prenatal and Infancy Support of Parents: The Nurse-Family Partnership. J. Scand Stud. Criminol. Crime. Prev. 2008, 9, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Catherine, N.L.A.; Gonzalez, A.; Boyle, M.; Sheehan, D.; Jack, S.M.; Hougham, K.A.; McCandless, L.; MacMillan, H.L.; Waddell, C.; For the British Columbia Healthy Connections Project Scientific Team. Improving Children’s Health and Development in British Columbia through Nurse Home Visiting: A Randomized Controlled Trial Protocol. BMC Health Serv. Res. 2016, 16, 349. [Google Scholar] [CrossRef] [PubMed]
- Catherine, N.L.A.; Lever, R.; Sheehan, D.; Zheng, Y.; Boyle, M.H.; McCandless, L.; Gafni, A.; Gonzalez, A.; Jack, S.M.; Tonmyr, L.; et al. The British Columbia Healthy Connections Project: Findings on Socioeconomic Disadvantage in Early Pregnancy. BMC Public Health 2019, 19, 1161. [Google Scholar] [CrossRef] [PubMed]
- Jack, S.M.; Catherine, N.; Gonzalez, A.; MacMillan, H.L.; Sheehan, D.; Waddell, D.; British Columbia Healthy Connections Project Scientific Team. Adapting, Piloting and Evaluating Complex Public Health Interventions: Lessons Learned from the Nurse-Family Partnership in Canadian Public Health Settings. Health Promot. Chronic Dis. Prev. Can. 2015, 35, 151–159. [Google Scholar] [CrossRef]
- Dennis, C.-L.; Dowswell, T. Psychosocial and Psychological Interventions for Preventing Postpartum Depression. Cochrane Database Syst. Rev. 2013, 2, CD001134. [Google Scholar] [CrossRef]
- Denktaş, S.; Poeran, J.; van Voorst, S.F.; Vos, A.A.; de Jong-Potjer, L.C.; Waelput, A.J.; Birnie, E.; Bonsel, G.J.; Steegers, E.A. Design and Outline of the Healthy Pregnancy 4 All Study. BMC Pregnancy Childbirth 2014, 14, 253. [Google Scholar] [CrossRef]
- Vos, A.A.; van Veen, M.J.; Birnie, E.; Denktaş, S.; Steegers, E.A.P.; Bonsel, G.J. An Instrument for Broadened Risk Assessment in Antenatal Health Care Including Non-Medical Issues. Int. J. Integr. Care 2015, 15, e002. [Google Scholar] [CrossRef]
- Rayment-Jones, H.; Murrells, T.; Sandall, J. An Investigation of the Relationship between the Caseload Model of Midwifery for Socially Disadvantaged Women and Childbirth Outcomes Using Routine Data--a Retrospective, Observational Study. Midwifery 2015, 31, 409–417. [Google Scholar] [CrossRef]
- Sandall, J.; Soltani, H.; Gates, S.; Shennan, A.; Devane, D. Midwife-Led Continuity Models versus Other Models of Care for Childbearing Women. Cochrane Database Syst. Rev. 2016, 4, CD004667. [Google Scholar] [CrossRef]
- Hadebe, R.; Seed, P.T.; Essien, D.; Headen, K.; Mahmud, S.; Owasil, S.; Fernandez Turienzo, C.; Stanke, C.; Sandall, J.; Bruno, M.; et al. Can Birth Outcome Inequality Be Reduced Using Targeted Caseload Midwifery in a Deprived Diverse Inner City Population? A Retrospective Cohort Study, London, UK. BMJ Open 2021, 11, e049991. [Google Scholar] [CrossRef]
- Kennedy, H.P. A Model of Exemplary Midwifery Practice: Results of a Delphi Study. J. Midwifery Womens Health 2000, 45, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.P. Midwives and Normalcy in Childbirth: A Phenomenologic Concept Development Study. J. Midwifery Womens Health 2010, 55, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Phillippi, J.C.; Avery, M.D. The 2012 American College of Nurse-Midwives Core Competencies for Basic Midwifery Practice: History and Revision. J. Midwifery Womens Health 2014, 59, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Rayment-Jones, H.; Silverio, S.A.; Harris, J.; Harden, A.; Sandall, J. Project 20: Midwives’ Insight into Continuity of Care Models for Women with Social Risk Factors: What Works, for Whom, in What Circumstances, and How. Midwifery 2020, 84, 102654. [Google Scholar] [CrossRef]
- ten Hoope-Bender, P.; de Bernis, L.; Campbell, J.; Downe, S.; Fauveau, V.; Fogstad, H.; Homer, C.S.E.; Kennedy, H.P.; Matthews, Z.; McFadden, A.; et al. Improvement of Maternal and Newborn Health through Midwifery. Lancet 2014, 384, 1226–1235. [Google Scholar] [CrossRef]
- McRae, D.N.; Muhajarine, N.; Stoll, K.; Mayhew, M.; Vedam, S.; Mpofu, D.; Janssen, P.A. Is Model of Care Associated with Infant Birth Outcomes among Vulnerable Women? A Scoping Review of Midwifery-Led versus Physician-Led Care. SSM Popul. Health 2016, 2, 182–193. [Google Scholar] [CrossRef]
- Bhandari, N.; Mazumder, S.; Bahl, R.; Martines, J.; Black, R.E.; Bhan, M.K. Use of Multiple Opportunities for Improving Feeding Practices in Under-Twos within Child Health Programmes. Health Policy Plan. 2005, 20, 328–336. [Google Scholar] [CrossRef][Green Version]
- Menon, P.; Saha, K.; Kennedy, A.; Khaled, A.; Tyagi, T.; Sanghvi, T.; Afsana, K.; Haque, R.; Frongillo, E.; Ruel, M.; et al. Social and Behavioral Change Interventions Delivered at Scale Have Large Impacts on Infant and Young Child Feeding (IYCF) Practices in Bangladesh. FASEB J. 2015, 29, 584.30. [Google Scholar] [CrossRef]
- Training in Complementary Feeding Counselling of Healthcare Workers and Its Influence on Maternal Behaviours and Child Growth: A Cluster-Randomized Controlled Trial in Lahore, Pakistan–PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/18686554/ (accessed on 7 October 2022).
- Lee, S.H.; Nurmatov, U.B.; Nwaru, B.I.; Mukherjee, M.; Grant, L.; Pagliari, C. Effectiveness of MHealth Interventions for Maternal, Newborn and Child Health in Low– and Middle–Income Countries: Systematic Review and Meta–Analysis. J. Glob. Health 2016, 6, 010401. [Google Scholar] [CrossRef]
- Sondaal, S.F.V.; Browne, J.L.; Amoakoh-Coleman, M.; Borgstein, A.; Miltenburg, A.S.; Verwijs, M.; Klipstein-Grobusch, K. Assessing the Effect of MHealth Interventions in Improving Maternal and Neonatal Care in Low- and Middle-Income Countries: A Systematic Review. PLoS ONE 2016, 11, e0154664. [Google Scholar] [CrossRef]
- Lunze, K.; Higgins-Steele, A.; Simen-Kapeu, A.; Vesel, L.; Kim, J.; Dickson, K. Innovative Approaches for Improving Maternal and Newborn Health--A Landscape Analysis. BMC Pregnancy Childbirth 2015, 15, 337. [Google Scholar] [CrossRef] [PubMed]

| Author | Study Design Period Localisation | Population | Outcome | Deprivation Scale | Level Zone | Confounders | Modèle/Analyse Stat |
|---|---|---|---|---|---|---|---|
| Enstad et al., 2019 [26] | Cross-sectional study, Infant from Illinois (1989–1991) from parents born in Chicago (1956–1976) | singleton births of African American (n = 8331), non-Latina White (n = 18,200), and Latina (n = 2637) women | SGA | Maternal SES - Maternal education, Paternal SES -contectuel median family income of father’s census tract residence during childhood and parenthood | Individual level and Census tract | Ethnicity Maternal age Marital status | Stratified and multilevel, multivariable logistic regression analyses |
| Ospina et al., 2020 [28] | Cross-sectional study, Alberta, Canada (2006–2012) | Women (n = 330,957) with singleton births | PTB, SGA; LGA, | 2006 SES index area-based socioeconomic gradients | Census block | Rural(CIdxR)/Urban(CIdxU), smoking and substance use during pregnancy and prepregnancy weight >91 kg gestational hypertension, gestational diabetes, maternal age at delivery | Prevalences comparison accross SES quintiles by calculating a absolute concentration index |
| Wilding et al., 2019 [58] | birth cohort Southampton, UK (2004–2016) | singleton births (n = 65,909) | SGA | Maternal SES - education - employment - partnership | Individuel level | Maternal smoking, BMI, parity. maternal age, parity, ethnicity, gestational diabetes, gestational hypertension and systolic blood pressure at booking | Multivariable logistic regression |
| Melissa et al., 2016 [57] | 4 nationally representative Cohorts study United states, United Kingdom, Canada and Australia (1998–2004) | singleton birth United States (n = 8400), the United Kingdom (n = 12,018), Canada (n = 5350), and Australia (n = 3452) | LBW | Maternal SES - maternal education Neighborhood SES Income quintile Income quintile calculated from total family income, available in each country, adjusted for family size | Individual level/country level | Maternal age Marital status Parity, Mother’s nativity, child’s sex, prenatal smoking race/ethnicity and region of origin | logistic regression models |
| van den Berg et al., 2012 [50] | Cohort study in the Netherlands, the Amsterdam (2003–2004) | Pregnant women (n = 3821) | PTB LBW SGA | Maternal SES maternal educational attainment The number of years of education after primary school was obtained by questionnaire, and categorized as low (less than 6 years of education after primary school), mid (6 to 10 years) and high (more than 10 years). | Individuel level | maternal smoking sex, maternal age, maternal height, parity maternal pre-pregnancy body mass index | logistic regression analysis |
| Patil et al., 2019 [29] | Cohort study USA (1979–2014) | Pregnant women (n = 2871) | LBW | Maternal SES Employment precarity scores evaluated using availability of employer sponsored insurance, income, long shifts, non-daytime shifts, availability of employer-sponsored training or educational benefits and membership in a union or collective bargaining unit | Individual level | maternal age, race/ethnicity, educational attainment, nativity, prepregnancy body mass index, alcohol consumption, smoking during pregnancy and infant year of birth | Modified Poisson regression models |
| Elo et al., 2009 [30] | The birth records based study Baltimore City, Baltimore County, Montgomery County and Prince Georges County in Maryland, 16 pooled cities in Michigan, Durham County and Wake County in North Carolina, and Philadelphia, Pennsylvania USA (1995–2001) | NA | SGA | neighborhood-level deprivation index (income/poverty, employment, education, housing, and occupation.) race/ethnicity | residential census tracts | Maternal age, maternal education, mother smoked during pregnancy, gestational and/ or chronic hypertension | Multilevel random intercept logistic regression models |
| Misra et al., 2010 [31] | a hybrid retrospective and prospective cohort Baltimore, Maryland USA (2001–2004) | women with singletons birth (n = 832) | PTB | Maternal SES Education level Family resource scale | Individual level | Stress depression symptoms pregnancy locus of control mastery anxiety and social support maternal age, education, income, and the Family Resources Scale cigarette smoking, alcohol and illicit drug use, and vaginal douching, parity, multiple gestation, initiation of prenatal care, number of prenatal visits, chronic diseases and complications of pregnancy | Cox proportional hazards analysis |
| Pei et al., 2015 [56] | cross-sectional study Shaanxi province China (2010–2013) | singleton births (n = 28,722) | LBW Macrosomia | Maternal SES education (primary, secondary and _ high education),employment (farming and other occupations which included teacher, official, commercial and service staff, and professional), Demographic and Health Survey household wealth index (HWI) (5 variables of family economic level: housing conditions, type of vehicle, income resources, and type and number of household appliances) | Individual level Household | sex, prematurity gestation (weeks) maternal age maternal health conditions negative (adverse) life events alcohol intake and passive (secondhand) exposure to smoke month antenatal care the number of ANC visits folic acid supplementation | generalized linear model |
| Sims et al., 2007 [32] | Vital Records Birth based study Wisconsin USA (1998–1999) | Pregnant women (n = 100,074). African-Americans (n = 11,313) Latinos (n = 6450) | LBW VLBW | Maternal SES Education level Neighborhood SES community-level income | Individual and community-level by zip code | individual-level characteristics individual-level factors | Multinomial logistic regression analysis |
| Glinianaia et al., 2013 [51] | hospital neonatal records based study Newcastle upon Tyne, North of England, (1961–2000) | singleton births (n = 113,182) | Birthweight LBW PTB | neighborhood SES Townsend Deprivation Score (proportion of home ownership, car ownership, unemployment and overcrowding) | Enumeration district (ED) | Gestational age Maternal age, parity and infant sex decades of birth | linear regression logistic regression |
| Kaufman et al., 2008 [33] | cross-sectional study Santiago Chile (2004) | Singleton births (n = 56,970) | PTB | Maternal SES Maternal Years of Education Neighborhood SES inverse density (i.e., number of domiciles per capita in the district) percentage of homes connected to a sewer system logarithm of the average total valuation per square meter percentage of the population that does not self-identify as indigenous percent of the population with formal schooling percent of the population that is not currently unemployed and seeking work percentage of the population that is classified as an owner or employer percent of domiciles that have concrete paving percent of domiciles that have indoor plumbing the percent of domiciles that have indoor heating. | Census district/individual level | parity, sex of child and maternal age | multilevel regression analyses logistic regression |
| DeFranco et al., 2008 [34] | Cross sectional study Missouri USA (1989–1997) | Singleton births (n = 634,994) | PTB | Individual-level SES maternal and paternal highest educational attainment and marital status (Mother’s education level < high school) The area-level SES County-level poverty rates included the percentage of the population within each county living below the poverty line | Individual/Counties level | Demographic factors Maternal age Reside inside city limits Black race Married Prenatal Care Inadequate prenatal care Behaviors Maternal tobacco use Maternal alcohol use Medical Risk Factors Medical risk factors | multilevel logistic regression analysis |
| Young et al., 2010 [35] | retrospective cohort study Cape Cod, Massachusetts (1969–1983) | singleton births (n = 1689) | Birthweight | Maternal SES maternal education; paternal occupation; Comunity level percent adults living in poverty; percent adults with a four years college degree; community mean family income; and percent adult unemployment | Individual, family- and community-levels (Enumeration district level) | child gender, gestational duration, birth order, year of birth, maternal age and race, adequate prenatal care, inadequate maternal weight gain, and cervical incompetence during pregnancy, any history of maternal diabetes or hypertension, prior low birth weight or preterm infants, and maternal smoking during pregnancy | multilevel models |
| Urquia et al., 2011 [36] | population-based study Ontario Canada (2002–2007) | singleton births (n = 474,614) | PTB | neighborhood deprivation index material-deprivation score: percent of population below the Statistics Canada low income cutoff, percent of population 20 years and over without high school diploma, percent of single-parent families, percent of income comprised of government transfer payments, percent of population unemployed and percent of homes needing major repairs) immigration Maternal SES Mother educational level | census tract level | Sex maternal age parity language knowledge maternal country of birth, age at arrival graduation marital status immigrant class knowledge of either official Canadian language | cross-classified random effect models |
| Kozhimannil et al., 2013 [37] | Cohort study USA (2005) | women with singleton birth (n = 1573) | LBW PTB | Maternal SES prenatal employment status (full-time, part-time, not employed) | Individual level | age, education, race, region, marital status, unintended pregnancy, mistimed pregnancy, fertility treatment, priorcesarean delivery, interaction between race and parity, interaction between parity and region, and interaction between age and marital status. | multivariable regression models. |
| Filho et al., 2007 [38] | Cohort study, Ribeirão Preto (1978–1979 and 1994) São Luís (1997–1998) Bresil | Singletons birth in Ribeirão Preto (n = 6747) in São Luís (n = 2839) | LBW PTB SGA | Individual SES family income, maternal schooling, occupation of the head of the family, paternal schooling, and marital status of the mother. | Individual level | gestational age were birth weight, parity, family income, and newborn infant sex | regression model |
| Wallace et al., 2013 [39] | Birth records based study Bogalusa USA (1987–2000) | women-with singleton births (n = 2743) | LBW PTB | Neighborhood SES poverty (percentage of households living below the federal poverty level + then categorized by their race and poverty level of the neighborhood) Individual level Maternal SES education at time of first birth (less than high school, high school, greater than high school) | Individuel/U.S.Census block group | Stratified by race// maternal, age, smoking during pregnancy, year of BHS examination and years between examination and conception | Generalized estimating equations |
| Mason et al., 2010 [40] | Birth records based study New york city USA (1995–2003) | singleton births (n = 925,277) | PTB | Maternal SES Education level education taking age into account (indicators for <12 years and age <20 years, <12 years and age _20 years, 12 years, 13–15 years, and _16 years), parity (indicators for 1, 2–5, and _6 previous births),Neighborhood SES residential stability and neighborhood deprivation the percentage of the neighborhood population residing in the same house from 1995 to 2000. Neighborhood deprivation was represented by using a standardized index arising from17 tract-level census variablesneighborhood immigrant or ethnic density in area of residence | Individual and census tracts (median area of 0.18 km2) | maternal age education taking age into account parity tobacco use prepregnancy weight payment type | logistic regression |
| Colen et al., 2006 [41] | Cross sectional study USA (1979–2002) | White Women with singletons birth (n = 574 = and Black Women with singletons birth (n = 1270) | LBW | Second-generation maternal SEP during adulthood. Maternal SEP Family income - Chronically poor was defined as living in a household during both childhood and adulthood where the income-to-needsRatio ≤ 200% of poverty. - Upwardly mobile is defined as living in a household during childhood, but not adulthood, where the income-to-needsratio≤200% of poverty Second-generation maternal SEP during childhood. The NLSY79 contains information concerning the occupation and educational attainment of first-generation individuals but does not provide measures of income | Households level | inflation using the Consumer Price Index for All Urban Consumers, Experimental Series, and reported in 2002 dollars race and gender | multivariate analyses logistic regression models. |
| Mortensen e at al., 2008 [52] | Cross sectional study Denmark, Finland, Norway and Sweden (1981–2000) | singleton birth (n = 1,077,584) Finland n = 400 442; Norway n = 929,458; Sweden n = 1,761,562). | Birthweight SGA LGA | Maternal SES - Mother education (years) - father’s education (years) | Individual level | r gestational age, parity, mother’s age, whether a father was known, father’s education and father’s age. | Linear and binomial linear regression |
| Dunlop et al., 2021 [42] | Cohort study USA (2018) | Women with singleton birth (n = 25,526) | PTB | Maternal SES Maternal level of education Neighborhood-level SES Urbanicity percentage black population d percentage population living below the 100% federal poverty line Maternal race and ethnicity | Individuel and Census tract | maternal age maternal parity marital status child sex maternal prenatal body mass index type 1 or type 2 diabetes mellitus; chronic hypertension; chronic infections (including HIV and hepatitis B or C); other chronic health conditions including asthma, other lung disease, cardiac disease other than hypertension prenatal tobacco use, alcohol use, and use of marijuana, stimulants, opiates pregnancy complications, gonorrhea, trichomoniasis, cervicitis, and pelvic inflammatory disease; receipt of prenatal care (yes/no) and prenatal health insurance | Multinomial logistic regression |
| Majdan et al., 2018 [53] | cross-sectional study Slovakia (2009–2013) | Municipalities (n = 2515) | LBW | Neighbodhood SES municipalities with minor Roma population municipalities with large Roma population Rate of unemployment | Municipalities level | Mean age of mothers at the date of birth and proportion of registered unemployed people in the respective municipality | square regression models multivariable models |
| Vang et al., 2013 [43] | Cross sectional study New Jersey USA (2002–2006) | Singleton births (n = 73,907) | Birthweight | - neighborhood minority diversity standardized entropy score - Neighborhood Deprivation - Residential instability Maternel SES educational level | Individuel and census tract | maternal health behaviors and conditions, and gestational age | linear regression |
| Huang et al. 2015 [45] | Cohort study USA (1995–2009) | Women with singleton birth (n = 1681) | Birthweight | Grandmaternal educational level Maternal education level | Individuel level | maternal life-course factors: maltreatment as a child, education and income as an adult, prepregnancy overweight, and prenatal smoking | marginal structural model (MSM) approaches |
| Shankardass et al., 2014 [46] | Cross sectional study Nova Scotia Canada (1988 and 2003) | singleton births (n = 117,734) | SGA LGA PTB perinatal death post-neonatal death | Maternal SESTotal family income Neighborhood deprivation index of neighborhood deprivation (None information) | Family (individuel) and Postal code | urban or rural place of residence and birth year marital status parity, pre-pregnancy weight, weight gain during pregnancy, maternal age, maternal smoking at delivery gestational diabetes and prenatal class attendance. | Multiple logistic regression |
| El-Sayed et al. 2012 [47] | Cross sectional study Michigan USA (1989 2006). | Pregnant women (n = 1,876,471) | PTB | Maternal SES maternal education | Individuel level | race maternal age at parturition ( parity stratified by year and analyzed births by year of occurrence. | bivariate and multivariable Poisson regression models |
| Ryu et al., 2016 [59] | cross-sectional study Olmsted County, Minnesota Jackson County, Missouri USA (2006) | parents (n = 728) with singleton birth (n = 701) | LBW | SES measure based on 4 housing-related characteristics (termed HOUSES) housing-related characteristics HOUSES index - parental education level - family annual income - Hollingshead index - Nakao-Treas index square footage of housing unit, (2) assessed housing value, (3) number of bathrooms, (4) number of bedrooms, (5) ownership of housing unit, (6) residential status and (7) lot size of housing unit in acres, and six neighborhood characteristics collected from census tract-level data, including (1) per cent of people speaking English as a second language, (2) per cent of foreignborn people, (3) per cent of households headed by a female, (4) per cent of households that are non-family households, (5) per cent of people with less than a high school education and (6) per cent of families with family income below poverty level. | Census tract level ? house level ?? | smoking exposure | gradient boosting machine (GBM) models underlogistic regression model framework |
| Raab et al. 2022 [54] | Cohort study Bavaria Germany (2013–2015) | Pregnant women (1738) | PTB | Educational level | Individuel level | maternal age, parity, and pre-pregnancy BMI. | Multivariate logistic regression models |
| Pardo-Crespo et al., 2013 [48] | Cross sectional study Olmsted County, Minnesota USA (2000) | Households (n = 750) | LBW Overweight | individual-level SES - Parents’ highest education level - annual family income Area-level SES - percentage of people with a bachelor degree or higher education - median family income at a census block-group level. | Individual and Census block group level | tobacco smoking status of household members | Cohen’s κ indexes using the categories of individual-level and arealevel SES measures logistic regression models |
| d’Orsi et al., 2005 [49] | Ecological study Rio de Janeiro Brasil (1991–1994) | singleton births (n = 97,519) | LBW | socioeconomic index Mean percentage of: - single-family buildings - sewerage access b - rented domiciles - income >10 x min. wage - slum or “favela” census tracts | Census tract level (as neighborhood) | Spatial analysis, multivariate cluster classification non-hierarchical clustering K-means algorithm Moran “I” statistics | |
| Dičkutė et al., 2004 [55] | Case-control study Lithuania (2001–2002) | 851 singleton births with LBW (n = 851) Singleton births control (n = 851) | Birthweight LBW | Maternal SES - Education level (primary.secondary, university) - Income - employment status (before and during pregnancy) | Smoking, alcohol consumption and drug use during pregnancy | univariate analysis logistic multivariable regression analysis |
 | ||||||
| LBW | Low maternal level of education | OR = 2.0 [1.49;2.71] [55] | Structural determinant:
Intermediary determinant:
| Four Pathways proposed with direct and indirect action:
| Example of some efficient interventions:
| Suggestions of a targeting intervention: Interventions to target various unhealthy behaviors and psychosocial conditions in early pregnancy. health policy must support:
|
| RR = 3.77 [2.26;6.30] [38] | ||||||
| Unemployment during pregnancy | OR = 1.7 [1.37;2.10] [55] | |||||
| Low maternal income | OR = 1.7 [1.58;2.84] [55] | |||||
| Bottom income quartile | In US: OR = 2.37 [1.80;3.11] [57] | |||||
| In UK: OR = 1.78 [1.30;2.44] [57] | ||||||
| In Australia OR = 2.11 [1.12;3.99] [57] | ||||||
| Low level of employment precarity | RR = 1.48 [1.11;1.98] [29] | |||||
| African-American women living in low-poverty | OR = 5.23 [2.26;12.10] [39] | |||||
| PTB | Highest quartile of poverty compared to the lowest quartile | OR = 1.18 [1.03,1.35] [34] | ||||
| Deprived neighborhoods | OR = 1.16 [1.06;1.27] [46] | |||||
| Most deprived groups | OR = 1.5 [1.3;1.7] [51] | |||||
| SGA | Most deprived neighborhoods | OR = 1.18 [1.07;1.30] [46] | ||||
| Lowest income decile | OR = 2.0 [1.78;2.26] [46] | |||||
| Head of family had a less skilled occupation | RR = 1.79 [1.29;2.47] [38] | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simoncic, V.; Deguen, S.; Enaux, C.; Vandentorren, S.; Kihal-Talantikite, W. A Comprehensive Review on Social Inequalities and Pregnancy Outcome—Identification of Relevant Pathways and Mechanisms. Int. J. Environ. Res. Public Health 2022, 19, 16592. https://doi.org/10.3390/ijerph192416592
Simoncic V, Deguen S, Enaux C, Vandentorren S, Kihal-Talantikite W. A Comprehensive Review on Social Inequalities and Pregnancy Outcome—Identification of Relevant Pathways and Mechanisms. International Journal of Environmental Research and Public Health. 2022; 19(24):16592. https://doi.org/10.3390/ijerph192416592
Chicago/Turabian StyleSimoncic, Valentin, Séverine Deguen, Christophe Enaux, Stéphanie Vandentorren, and Wahida Kihal-Talantikite. 2022. "A Comprehensive Review on Social Inequalities and Pregnancy Outcome—Identification of Relevant Pathways and Mechanisms" International Journal of Environmental Research and Public Health 19, no. 24: 16592. https://doi.org/10.3390/ijerph192416592
APA StyleSimoncic, V., Deguen, S., Enaux, C., Vandentorren, S., & Kihal-Talantikite, W. (2022). A Comprehensive Review on Social Inequalities and Pregnancy Outcome—Identification of Relevant Pathways and Mechanisms. International Journal of Environmental Research and Public Health, 19(24), 16592. https://doi.org/10.3390/ijerph192416592









