Predictors for Upper-Limb Functional Recovery Trajectory in Individuals Receiving Stroke Rehabilitation: A Secondary Analysis of Data from Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Outcome Measures and Potential Predictors
2.3. Statistical Analysis
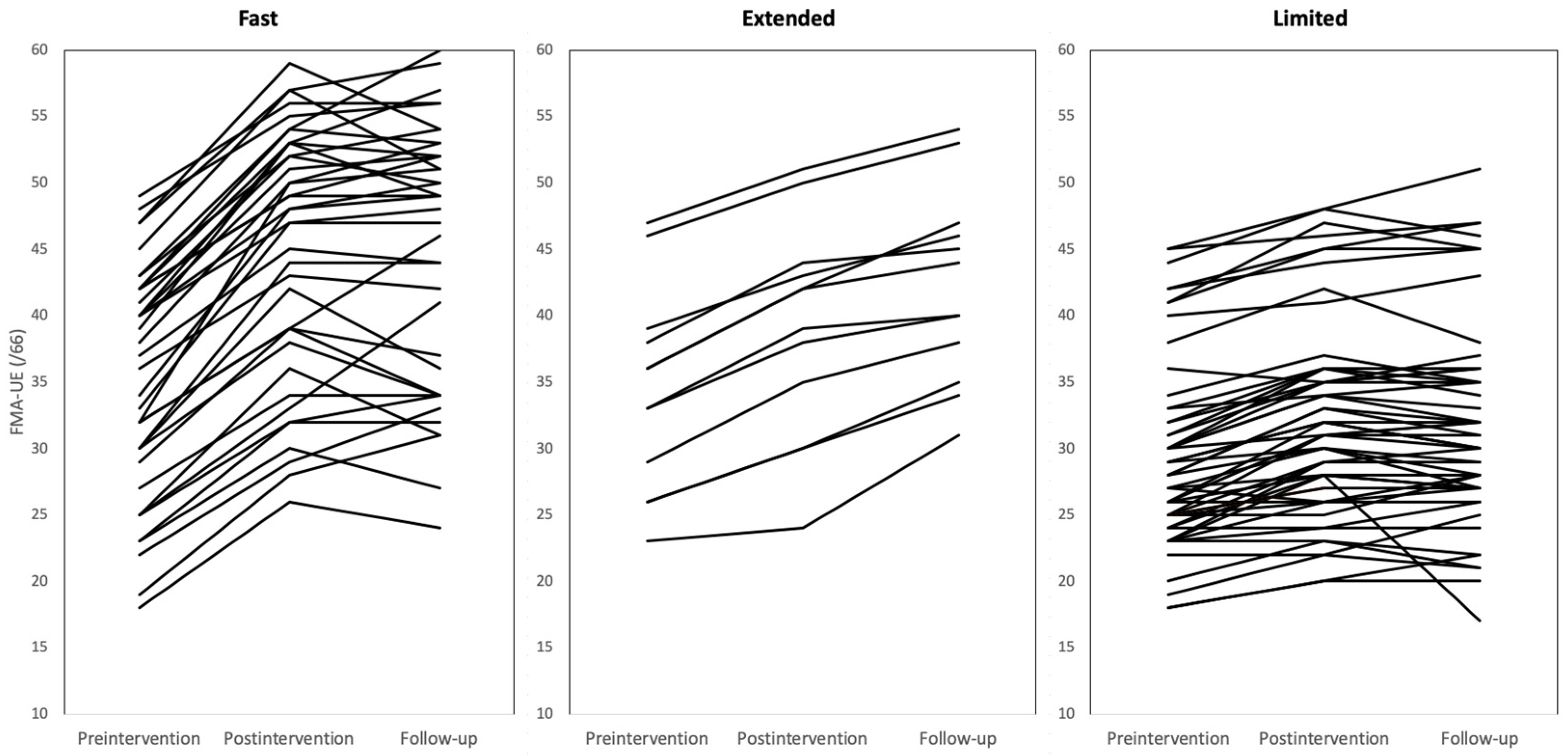
3. Results
3.1. Participant Characteristics
3.2. Comparisons of Baseline Measurement Scores among Recovery Groups
3.3. Results of Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collaborators GBDS. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef]
- Clarke, D.J. The role of multidisciplinary team care in stroke rehabilitation. Progress in Neurology and Psychiatry. Prog. Neurol. Psychiatry 2013, 17, 5–8. [Google Scholar] [CrossRef]
- Langhorne, P.; Baylan, S. Early Supported Discharge T. Early supported discharge services for people with acute stroke. Cochrane Database Syst. Rev. 2017, 7, CD000443. [Google Scholar] [CrossRef] [PubMed]
- Nylen, M.C.; Persson, H.C.; Abzhandadze, T.; Sunnerhagen, K.S. A register-based study comparing planned rehabilitation following acute stroke in 2011 and 2017. Sci. Rep. 2021, 11, 23001. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, K.; Kawakami, M.; Nakamura, T.; Oshima, O.; Hijikata, N.; Suda, M.; Yamada, Y.; Okuyama, K.; Tsuji, T. Do somatosensory deficits predict efficacy of neurorehabilitation using neuromuscular electrical stimulation for moderate to severe motor paralysis of the upper limb in chronic stroke? Ther. Adv. Neurol. Disord. 2021, 14, 17562864211039335. [Google Scholar] [CrossRef]
- Hawe, R.L.; Scott, S.H.; Dukelow, S.P. Taking Proportional Out of Stroke Recovery. Stroke 2019, 50, 204–211. [Google Scholar] [CrossRef]
- Prabhakaran, S.; Zarahn, E.; Riley, C.; Speizer, A.; Chong, J.Y.; Lazar, R.M.; Marshall, R.S.; Krakauer, J.W. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil. Neural. Repair 2008, 22, 64–71. [Google Scholar] [CrossRef]
- Winters, C.; van Wegen, E.E.; Daffertshofer, A.; Kwakkel, G. Generalizability of the Proportional Recovery Model for the Upper Extremity After an Ischemic Stroke. Neurorehabil. Neural. Repair 2015, 29, 614–622. [Google Scholar] [CrossRef]
- Byblow, W.D.; Stinear, C.M.; Barber, P.A.; Petoe, M.A.; Ackerley, S.J. Proportional recovery after stroke depends on corticomotor integrity. Ann. Neurol. 2015, 78, 848–859. [Google Scholar] [CrossRef]
- Gebruers, N.; Truijen, S.; Engelborghs, S.; De Deyn, P.P. Prediction of upper limb recovery, general disability, and rehabilitation status by activity measurements assessed by accelerometers or the Fugl-Meyer score in acute stroke. Am. J. Phys. Med. Rehabil. 2014, 93, 245–252. [Google Scholar] [CrossRef]
- Lee, J.J.; Shin, J.H. Predicting Clinically Significant Improvement After Robot-Assisted Upper Limb Rehabilitation in Subacute and Chronic Stroke. Front. Neurol. 2021, 12, 668923. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, H.K.; Liao, W.W.; Wu, C.Y.; Hsieh, Y.W.; Lee, T.H. Predicting clinically significant motor function improvement after contemporary task-oriented interventions using machine learning approaches. J. Neuroeng. Rehabil. 2020, 17, 131. [Google Scholar] [CrossRef] [PubMed]
- Tozlu, C.; Edwards, D.; Boes, A.; LaBar, D.; Tsagaris, K.Z.; Silverstein, J.; Lane, H.P.; Sabuncu, M.R.; Liu, C.; Kuceyeski, A. Machine Learning Methods Predict Individual Upper-Limb Motor Impairment Following Therapy in Chronic Stroke. Neurorehabil. Neural. Repair 2020, 34, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Yamada, N.; Hada, T.; Abo, M. Prediction of Motor Recovery in the Upper Extremity for Repetitive Transcranial Magnetic Stimulation and Occupational Therapy Goal Setting in Patients with Chronic Stroke: A Retrospective Analysis of Prospectively Collected Data. Front. Neurol. 2020, 11, 581186. [Google Scholar] [CrossRef] [PubMed]
- Kline, D.K.; Lin, D.J.; Cloutier, A.; Sloane, K.; Parlman, K.; Ranford, J.; Picard-Fraser, M.; Fox, A.B.; Hochberg, L.R.; Kimberley, T.J. Arm Motor Recovery After Ischemic Stroke: A Focus on Clinically Distinct Trajectory Groups. J. Neurol. Phys. Ther. 2021, 45, 70–78. [Google Scholar] [CrossRef]
- Li, Y.C.; Lin, K.C.; Chen, C.L.; Yao, G.; Chang, Y.-J.; Lee, Y.-Y.; Liu, C.-T. A Comparative Efficacy Study of Robotic Priming of Bilateral Approach in Stroke Rehabilitation. Front. Neurol. 2021, 12, 658567. [Google Scholar] [CrossRef]
- Lee, Y.C.; Li, Y.C.; Lin, K.C.; Yao, G.; Chang, Y.J.; Lee, Y.Y.; Liu, C.-T.; Hsu, W.-L.; Wu, Y.-H.; Chu, H.-T.; et al. Effects of robotic priming of bilateral arm training, mirror therapy, and impairment-oriented training on sensorimotor and daily functions in patients with chronic stroke: Study protocol of a single-blind, randomized controlled trial. Trials 2022, 23, 566. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.R.; Jaasko, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil. Neural. Repair 2002, 16, 232–240. [Google Scholar] [CrossRef]
- Sabari, J.S.; Woodbury, M.; Velozo, C.A. Rasch analysis of a new hierarchical scoring system for evaluating hand function on the motor assessment scale for stroke. Stroke Res. Treat. 2014, 2014, 730298. [Google Scholar] [CrossRef]
- Lin, K.C.; Hsieh, Y.W.; Wu, C.Y.; Chen, C.L.; Jang, Y.; Liu, J.S. Minimal detectable change and clinically important difference of the Wolf Motor Function Test in stroke patients. Neurorehabil. Neural. Repair. 2009, 23, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Bode, R.K.; Min Lai, S.; Perera, S. Glycine Antagonist in Neuroprotection Americans, I. Rasch analysis of a new stroke-specific outcome scale: The Stroke Impact Scale. Arch. Phys. Med. Rehabil. 2003, 84, 950–963. [Google Scholar] [CrossRef] [PubMed]
- Taub, E.M.; McCulloch, K.; Uswatte, G.; Morris, D.M. Motor Activity Log (MAL) Manual; UAB Training for CI Therapy: Birmingham, AL, USA, 2011. [Google Scholar]
- Nouri, F.; Lincoln, N. An extended activities of daily living scale for stroke patients. Clin. Rehabil. 1987, 1, 301–305. [Google Scholar] [CrossRef]
- Stinear, C.M.; Byblow, W.D.; Ackerley, S.J.; Barber, P.A.; Smith, M.C. Predicting Recovery Potential for Individual Stroke Patients Increases Rehabilitation Efficiency. Stroke 2017, 48, 1011–1019. [Google Scholar] [CrossRef]
- Hsieh, Y.W.; Lin, K.C.; Wu, C.Y.; Lien, H.-Y.; Chen, J.-L.; Chen, C.-C.; Chang, W.-H. Predicting clinically significant changes in motor and functional outcomes after robot-assisted stroke rehabilitation. Arch. Phys. Med. Rehabil. 2014, 95, 316–321. [Google Scholar] [CrossRef]
| Characteristics | Mean ± SD, n (%), or Median (Q1–Q3) |
|---|---|
| Age at time of stroke (years) | 55.68 ± 11.42 |
| Male sex | 70 (66.67) |
| Years of education | 12 (9–14) |
| Side of hemiplegia (right) | 61 (58.10) |
| Type of stroke (hemorrhagic) | 47 (44.76) |
| Time after stroke onset (month) | 14 (7–31) |
| NIHSS score | 4 (3–6) |
| Limited Recovery (n = 55) | Extended Recovery (n = 12) | Fast Recovery (n = 38) | p Value | |
|---|---|---|---|---|
| Age at stroke (years) | 54.11 ± 10.97 | 55.81 ± 11.98 | 57.90 ± 11.83 | 0.29 |
| Male sex | 39 (70.91) | 7 (58.33) | 24 (63.16) | 0.60 |
| Educated years | 12.00 (9.00–15.75) | 12.00 (8.25–14.25) | 12.00 (9.00–14.00) | 0.37 |
| Side of hemiplegia (right) | 32 (58.18) | 7 (58.33) | 22 (57.89) | 0.99 |
| Stroke diagnosis (hemorrhagic) | 28 (50.91) | 2 (16.67) | 17 (44.74) | 0.09 |
| Time after stroke (months) a | 20.00 (10.00–41.00) | 11.00 (3.75–44.00) | 11.50 (6.00–18.00) c | 0.005 * |
| NIHSS | 4.00 (3.00–6.75) | 3.50 (2.00–4.50) | 4.00 (3.00–6.00) | 0.45 |
| FMA-UE a | 27.50 (25.00–31.75) | 34.50 (28.25–38.25) b | 34.00 (27.00–42.00) c | 0.007 * |
| MAS | 0.89 (0.63–1.14) | 0.91 (0.62–1.01) | 0.82 (0.50–1.04) | 0.62 |
| WMFT (quality) a | 2.13 (1.88–2.38) | 2.70 (2.22–3.00) b | 2.80 (2.07–3.07) c | <0.001 * |
| WMFT (time) a | 14.22 (10.31–18.01) | 11.35 (6.66–13.42) b | 10.05 (5.13–15.14) c | 0.002 * |
| SIS-Hand a | 15.00 (5.00–35.00) | 35.00 (7.50–51.25) | 35.00 (15.00–55.00) c | 0.007 * |
| MAL-AOU a | 0.80 (0.53–1.04) | 1.40 (0.70–2.36) | 1.11 (0.72–1.83) c | 0.021 * |
| MAL-QOM a | 0.48 (0.25–0.77) | 1.24 (5.00–2.24) b | 0.81 (0.48–1.73) c | 0.004 * |
| NEADL | 28.00 (15.25–43.75) | 35.00 (24.25–45.75) | 28.00 (18.00–44.00) | 0.69 |
| Baseline Characteristics | β | p Value | Odds Ratio (95% CI) |
|---|---|---|---|
| Fast vs extended recovery | |||
| Time after stroke onset | −0.01 | 0.955 | 1.02 (0.96–1.02) |
| FMA-UE | 0.01 | 0.806 | 1.01 (0.93–1.09) |
| WMFT-Quality | −0.09 | 0.883 | 0.91 (0.28–3.03) |
| WMFT-Time | 0.03 | 0.689 | 1.03 (0.90–1.17) |
| SIS-Hand | 0.001 | 0.961 | 1.00 (0.98–1.03) |
| MAL-AOU | −0.28 | 0.390 | 0.75 (0.39–1.44) |
| MAL-QOM | −0.34 | 0.344 | 0.72 (0.36–1.43) |
| Fast vs limited recovery | |||
| Time after stroke onset | −0.03 | 0.024 | 0.97 (0.95–1.00) |
| FMA-UE | 0.08 | 0.003 | 1.09 (1.03–1.15) |
| WMFT-Quality | 1.68 | <0.001 | 5.37 (2.17–13.33) |
| WMFT-Time | −0.11 | 0.007 | 0.90 (0.83–0.97) |
| SIS-Hand | 0.03 | 0.002 | 1.03 (1.01–1.05) |
| MAL-AOU | 0.62 | 0.027 | 1.86 (1.07–3.24) |
| MAL-QOM | 0.84 | 0.006 | 2.32 (1.28–4.21) |
| Extended vs. limited recovery | |||
| Time after stroke onset | −0.01 | 0.37 | 0.99 (0.96–1.02) |
| FMA-UE | 0.08 | 0.065 | 1.08 (1.00–1.12) |
| WMFT-Quality | 1.77 | 0.006 | 5.88 (1.67–20.73) |
| WMFT-Time | −0.13 | 0.035 | 0.88 (0.77–0.99) |
| SIS-Hand | 0.03 | 0.034 | 1.03 (1.00–1.06) |
| MAL-AOU | 0.91 | 0.013 | 2.48 (1.21–5.05) |
| MAL-QOM | 1.18 | 0.003 | 3.24 (1.49–7.03) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buyandelger, B.; Chen, Y.-W.; Li, Y.-C.; Lin, C.-J.; Chen, C.-L.; Lin, K.-C. Predictors for Upper-Limb Functional Recovery Trajectory in Individuals Receiving Stroke Rehabilitation: A Secondary Analysis of Data from Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 16514. https://doi.org/10.3390/ijerph192416514
Buyandelger B, Chen Y-W, Li Y-C, Lin C-J, Chen C-L, Lin K-C. Predictors for Upper-Limb Functional Recovery Trajectory in Individuals Receiving Stroke Rehabilitation: A Secondary Analysis of Data from Randomized Controlled Trials. International Journal of Environmental Research and Public Health. 2022; 19(24):16514. https://doi.org/10.3390/ijerph192416514
Chicago/Turabian StyleBuyandelger, Batsaikhan, Yu-Wen Chen, Yi-Chun Li, Chia-Jung Lin, Chia-Ling Chen, and Keh-Chung Lin. 2022. "Predictors for Upper-Limb Functional Recovery Trajectory in Individuals Receiving Stroke Rehabilitation: A Secondary Analysis of Data from Randomized Controlled Trials" International Journal of Environmental Research and Public Health 19, no. 24: 16514. https://doi.org/10.3390/ijerph192416514
APA StyleBuyandelger, B., Chen, Y.-W., Li, Y.-C., Lin, C.-J., Chen, C.-L., & Lin, K.-C. (2022). Predictors for Upper-Limb Functional Recovery Trajectory in Individuals Receiving Stroke Rehabilitation: A Secondary Analysis of Data from Randomized Controlled Trials. International Journal of Environmental Research and Public Health, 19(24), 16514. https://doi.org/10.3390/ijerph192416514






