Bismuth-Decorated Beta Zeolites Catalysts for Highly Selective Catalytic Oxidation of Cellulose to Biomass-Derived Glycolic Acid
Abstract
1. Introduction
2. Materials and Method
2.1. Chemicals
2.2. Catalyst Preparation
2.3. Catalyst Characterization
2.4. Catalyst Experiments
2.5. Product Analysis
3. Results and Discussion
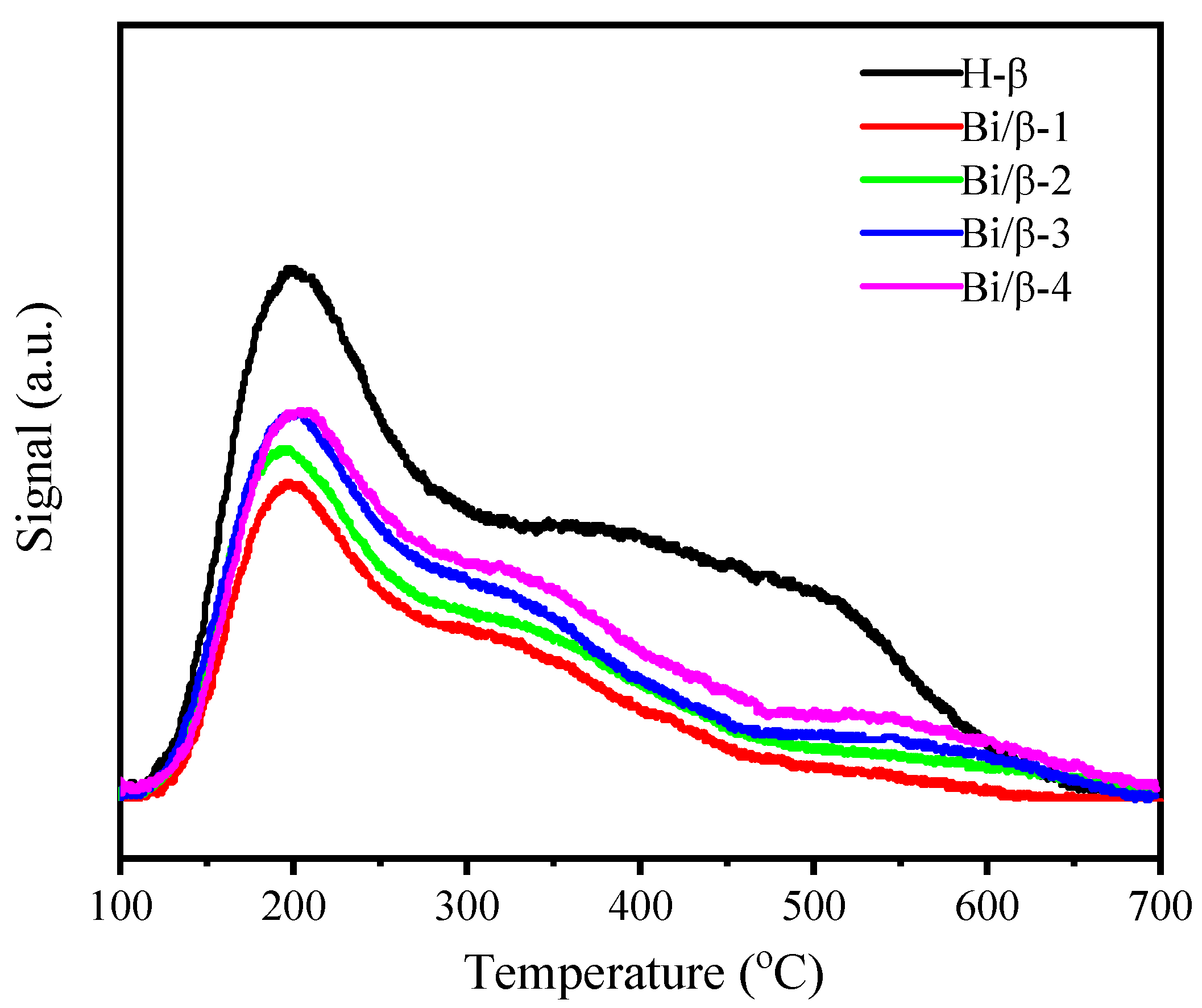
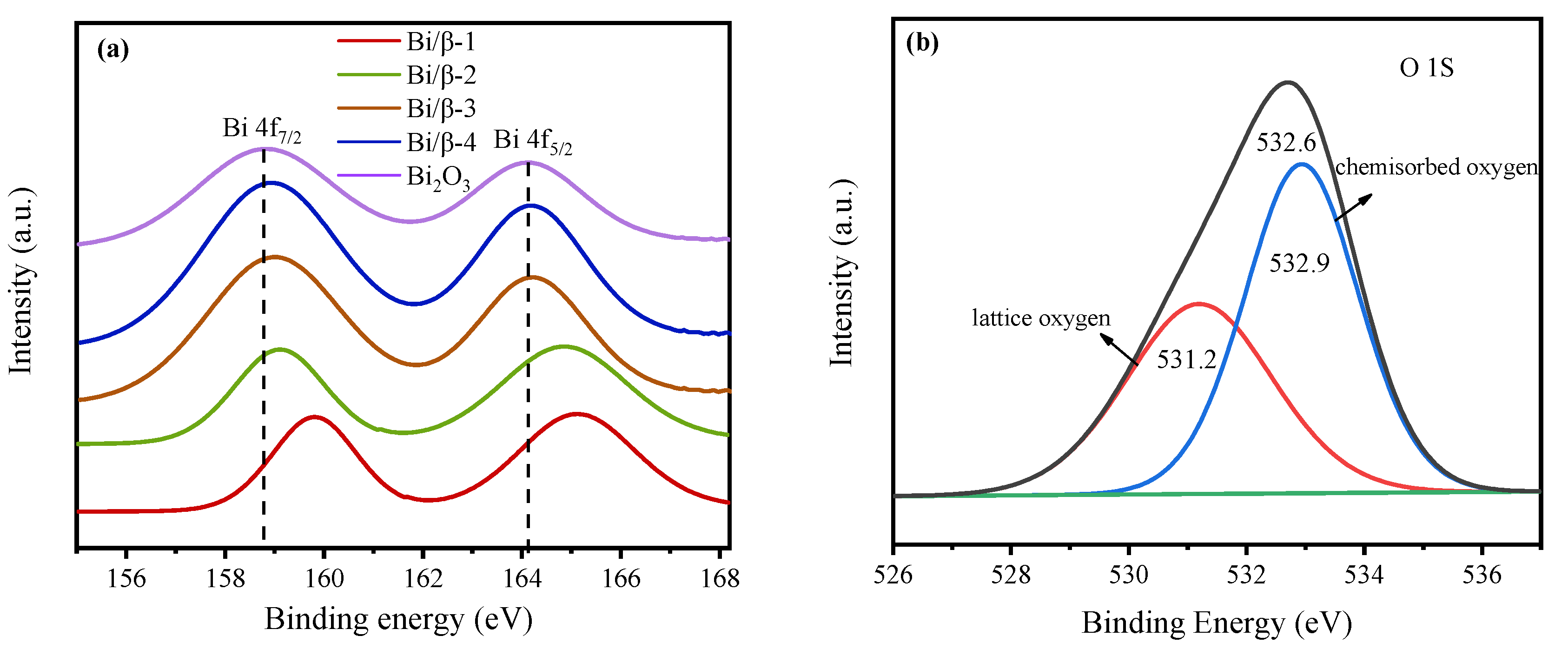
3.1. Characterization of Catalysts
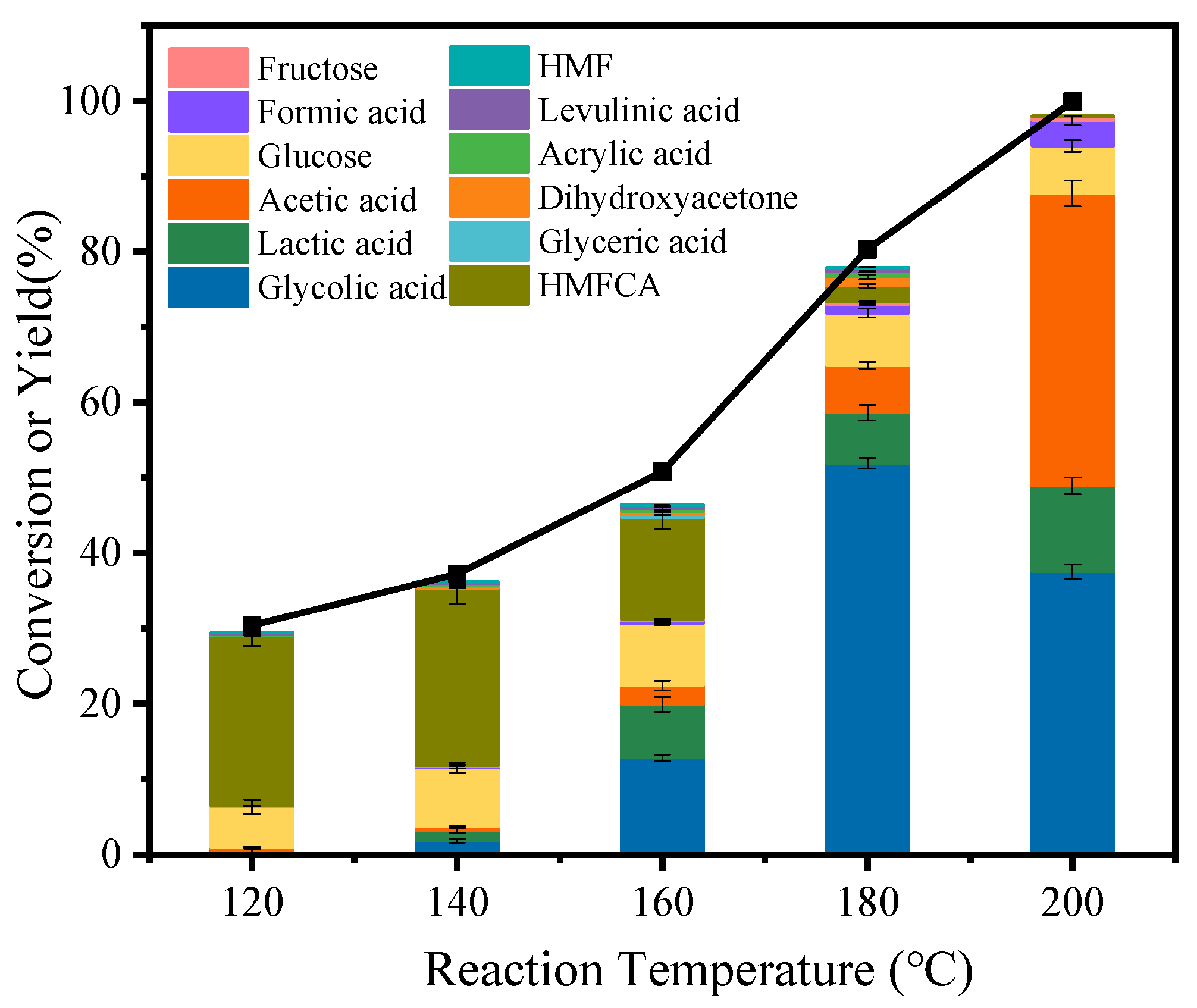
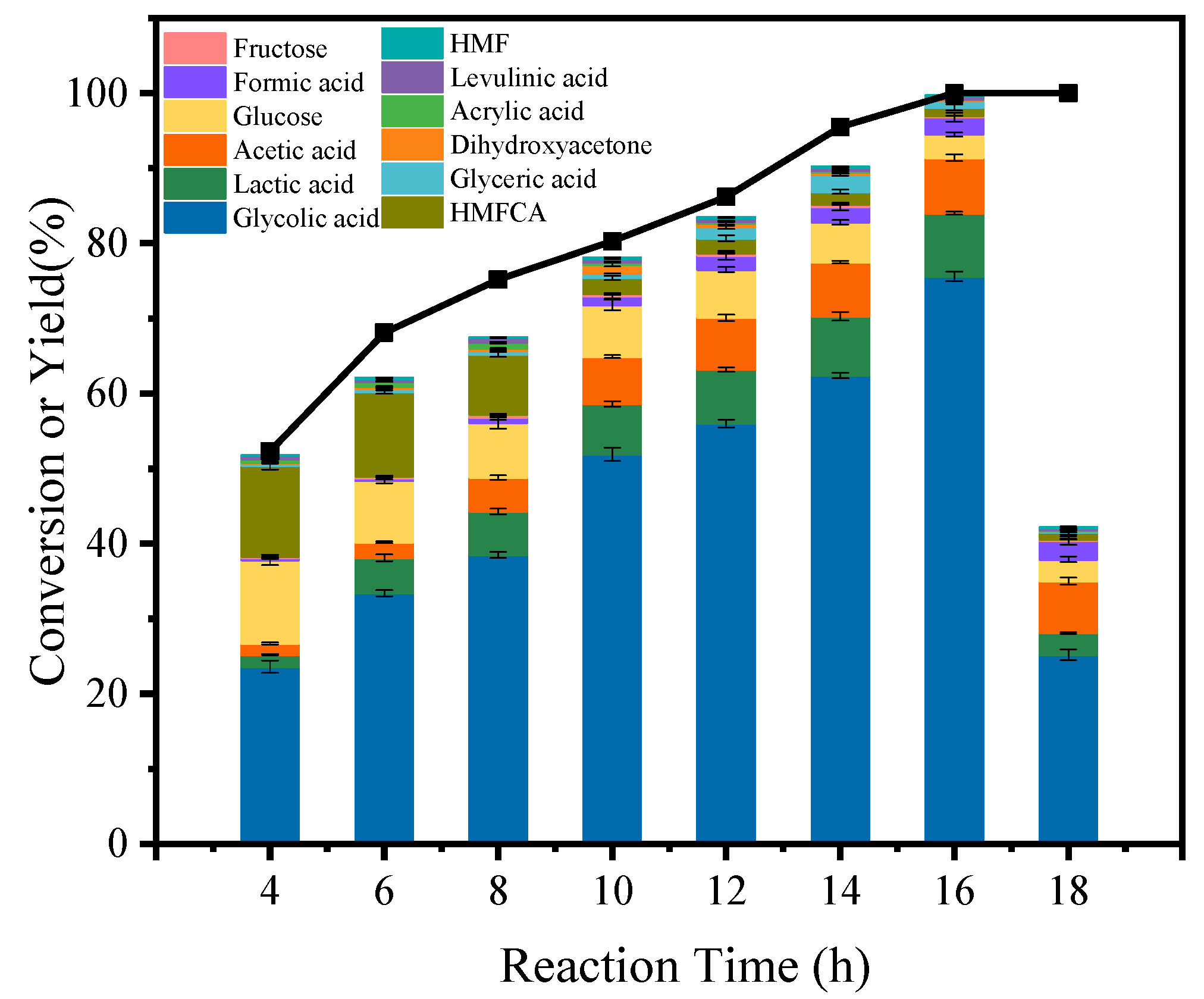
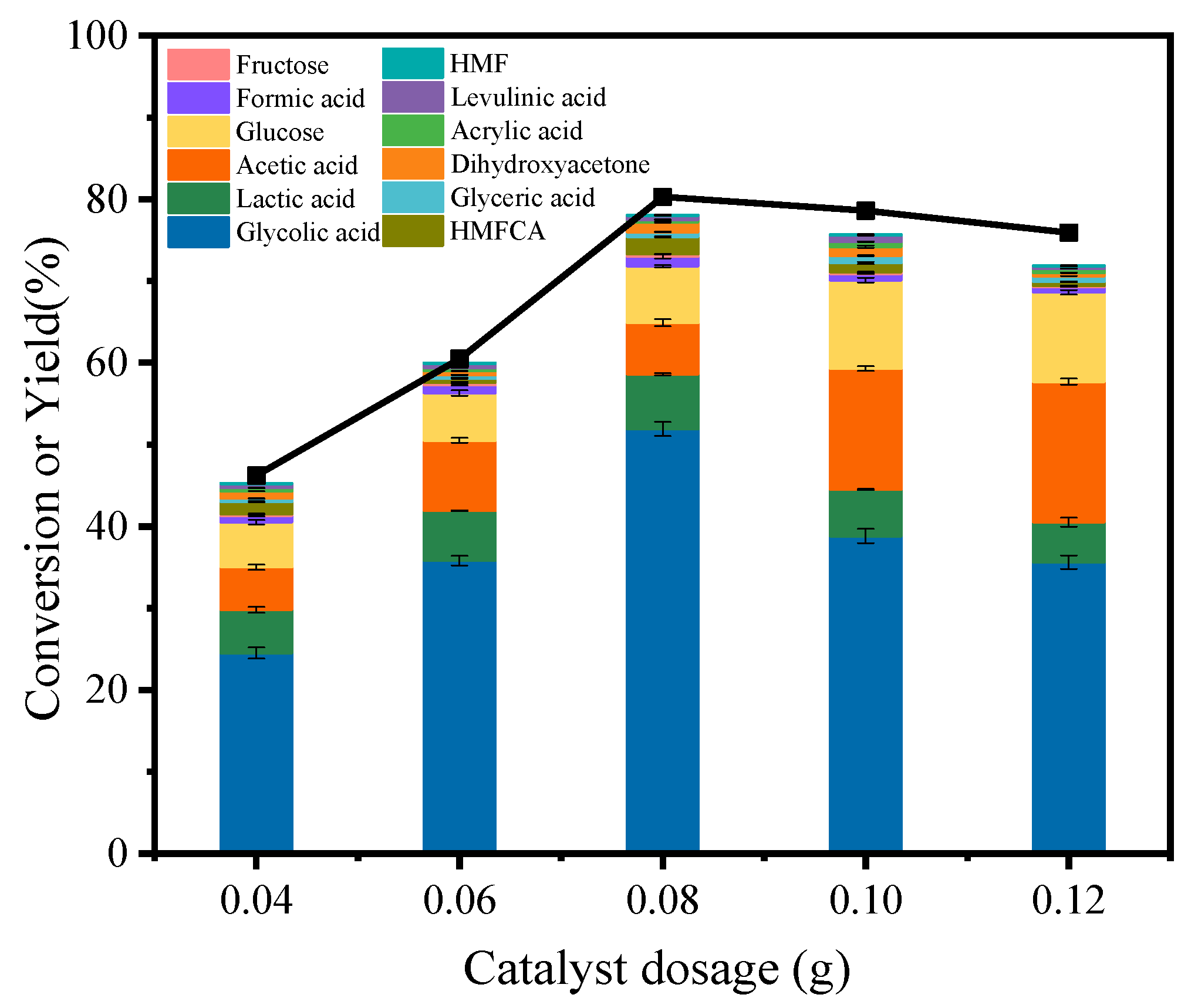
3.2. Catalytic Conversion of Cellulose to GA over Various Catalysts
3.3. Reaction Mechanism
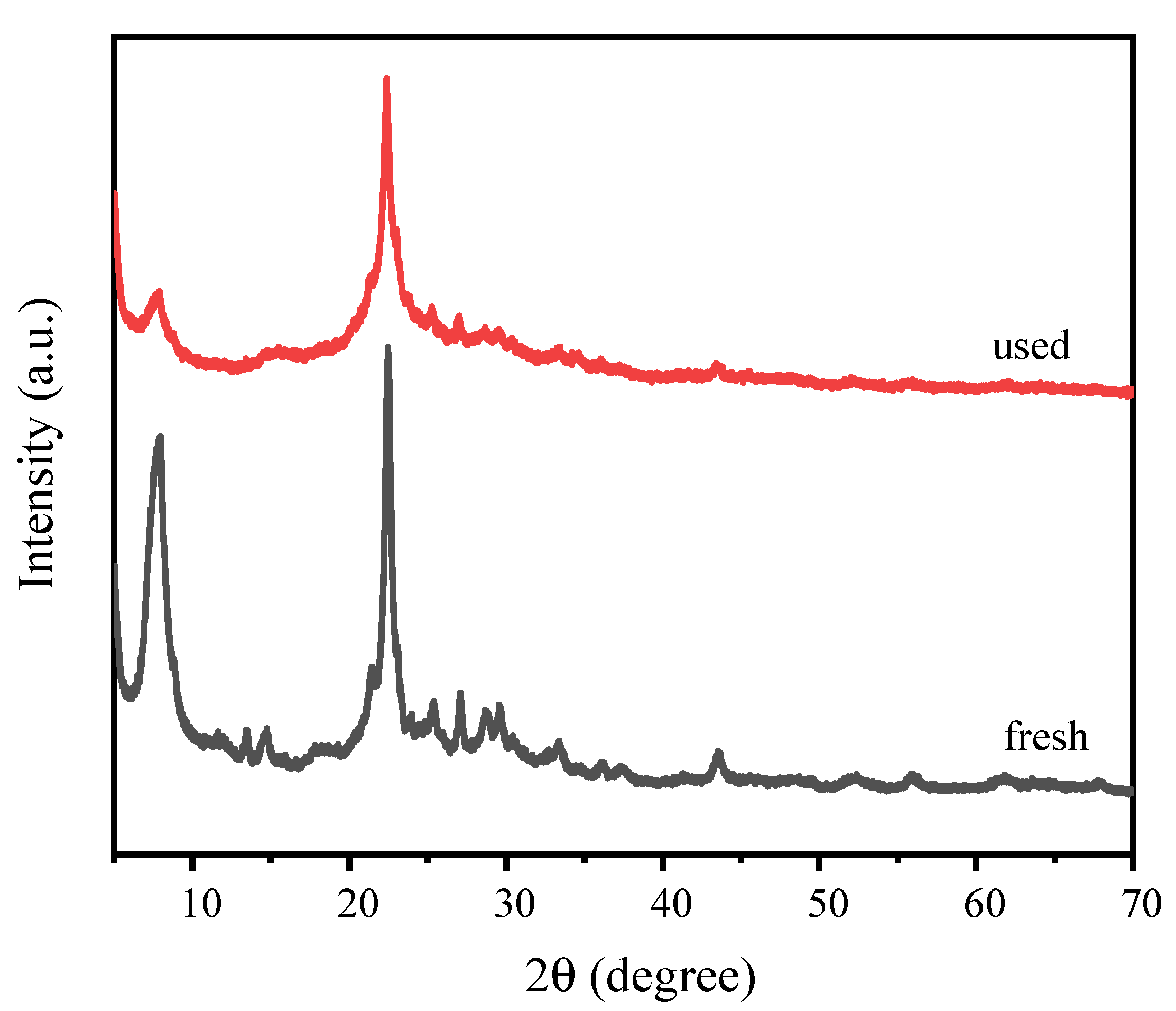
3.4. The Reusability of Catalysts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, L.; Han, X.; Han, B.; Yang, S. Emerging heterogeneous catalysts for biomass conversion: Studies of the reaction mechanism. Chem. Soc. Rev. 2021, 50, 11270–11292. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Gallezot, P. Process options for converting renewable feedstocks to bioproducts. Green Chem. 2007, 9, 295–302. [Google Scholar] [CrossRef]
- McCallum, C.S.; Strachan, N.; Bennett, S.C.; Forsythe, W.G.; Garrett, M.D.; Hardacre, C.; Morgan, K.; Sheldrake, G.N. Catalytic depolymerisation of suberin rich biomass with precious metal catalysts. Green Chem. 2018, 20, 2702–2705. [Google Scholar] [CrossRef]
- Taarning, E.; Osmundsen, C.M.; Yang, X.; Voss, B.; Andersen, S.I.; Christensen, C.H. Zeolite-catalyzed biomass conversion to fuels and chemicals. Energy Environ. Sci. 2011, 4, 793–804. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, G.W. Catalytic oxidation of carbohydrates into organic acids and furan chemicals. Chem. Soc. Rev. 2018, 47, 1351–1390. [Google Scholar] [CrossRef]
- Wang, F.-F.; Liu, C.-L.; Dong, W.-S. Highly efficient production of lactic acid from cellulose using lanthanide triflate catalysts. Green Chem. 2013, 15, 2091–2095. [Google Scholar] [CrossRef]
- Yan, X.; Jin, F.; Tohji, K.; Kishita, A.; Enomoto, H. Hydrothermal conversion of carbohydrate biomass to lactic acid. AIChE J. 2010, 56, 2727–2733. [Google Scholar] [CrossRef]
- Wang, H.; Lv, J.; Zhu, X.; Liu, X.; Han, J.; Ge, Q. Efficient Hydrolytic Hydrogenation of Cellulose on Mesoporous HZSM-5 Supported Ru Catalysts. Top. Catal. 2015, 58, 623–632. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Li, N.; Ma, P.; Zhang, Y. Direct synthesis of hexitols from microcrystalline cellulose and birch over zirconium(iv) phosphate supported nickel catalysts and the mechanism study. Green Chem. 2021, 23, 1353–1360. [Google Scholar] [CrossRef]
- Dong, W.; Ou, M.; Qu, D.; Shi, X.; Guo, M.; Liu, G.; Wang, S.; Wang, F.; Chen, Y. Rare-Earth Metal Yttrium-Modified Composite Metal Oxide Catalysts for High Selectivity Synthesis of Biomass-Derived Lactic Acid from Cellulose. ChemCatChem 2022, 14, e202200265. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Hedhili, M.N.; Zhu, Y.; Han, Y. Highly selective and complete conversion of cellobiose to gluconic acid over Au/Cs2HPW12O40 nanocomposite catalyst. ChemCatChem 2011, 3, 1294–1298. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Sun, M.; Ma, X.; Han, Y. Direct Conversion of Cellulose to Glycolic Acid with a Phosphomolybdic Acid Catalyst in a Water Medium. ACS Catal. 2012, 2, 1698–1702. [Google Scholar] [CrossRef]
- Li, J.; Yang, R.; Xu, S.; Zhou, C.; Xiao, Y.; Hu, C.; Tsang, D.C. Biomass-derived polyols valorization towards glycolic acid production with high atom-economy. Appl. Catal. B Environ. 2022, 317, 121785. [Google Scholar] [CrossRef]
- She, Z.; Wang, J.G.; Ni, J.P.; Liu, X.Q.; Zhang, R.Y.; Na, H.N.; Zhu, J. Direct conversion of cellulose into glycolic acid by a zinc-stabilized UV-Fenton reaction. RSC Adv. 2015, 5, 5741–5744. [Google Scholar] [CrossRef]
- Zhan, Y.; Hou, W.; Li, G.; Shen, Y.; Zhang, Y.; Tang, Y. Oxidant-Free Transformation of Ethylene Glycol toward Glycolic Acid in Water. ACS Sustain. Chem. Eng. 2019, 7, 17559–17564. [Google Scholar] [CrossRef]
- Zhou, X.; Zha, M.; Cao, J.; Yan, H.; Feng, X.; Chen, D.; Yang, C. Glycolic Acid Production from Ethylene Glycol via Sustainable Biomass Energy: Integrated Conceptual Process Design and Comparative Techno-economic–Society–Environment Analysis. ACS Sustain. Chem. Eng. 2021, 9, 10948–10962. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Jin, F.; Yao, G.; Huo, Z.; Zeng, X.; Jing, Z. One-Pot Hydrothermal Conversion of Cellulose into Organic Acids with CuO as an Oxidant. Ind. Eng. Chem. Res. 2014, 53, 7939–7946. [Google Scholar] [CrossRef]
- Bayu, A.; Karnjanakom, S.; Yoshida, A.; Kusakabe, K.; Abudula, A.; Guan, G. Polyoxomolybdates catalysed cascade conversions of cellulose to glycolic acid with molecular oxygen via selective aldohexoses pathways (an epimerization and a [2 + 4] Retro-aldol reaction). Catal. Today 2019, 332, 28–34. [Google Scholar] [CrossRef]
- Dijkmans, J.; Gabriëls, D.; Dusselier, M.; de Clippel, F.; Vanelderen, P.; Houthoofd, K.; Malfliet, A.; Pontikes, Y.; Sels, B.F. Productive sugar isomerization with highly active Sn in dealuminated β zeolites. Green Chem. 2013, 15, 2777–2785. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, L.; Wang, H.; Miao, G.; Kong, L.; Li, S.; Sun, Y. Efficient production of lactic acid from sugars over Sn-Beta zeolite in water: Catalytic performance and mechanistic insights. Sustain. Energy Fuels 2019, 3, 1163–1171. [Google Scholar] [CrossRef]
- Wang, F.-F.; Wu, H.-Z.; Ren, H.-F.; Liu, C.-L.; Xu, C.-L.; Dong, W.-S. Er/β-zeolite-catalyzed one-pot conversion of cellulose to lactic acid. J. Porous Mater. 2016, 24, 697–706. [Google Scholar] [CrossRef]
- Li, J.; Smith, R.L.; Xu, S.; Li, D.; Yang, J.; Zhang, K.; Shen, F. Manganese oxide as an alternative to vanadium-based catalysts for effective conversion of glucose to formic acid in water. Green Chem. 2022, 24, 315–324. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, G.; Chen, H.; Zhang, G.; Han, Y.; Chang, Y.; Gong, P. Mn/beta and Mn/ZSM-5 for the low-temperature selective catalytic reduction of NO with ammonia: Effect of manganese precursors. Chin. J. Catal. 2018, 39, 118–127. [Google Scholar] [CrossRef]
- Jurković, D.L.; Puliyalil, H.; Pohar, A.; Likozar, B. Plasma-activated methane partial oxidation reaction to oxygenate platform chemicals over Fe, Mo, Pd and zeolite catalysts. Int. J. Energy Res. 2019, 43, 8085–8099. [Google Scholar] [CrossRef]
- Tian, P.; Liu, Z.; Wu, Z.; Xu, L.; He, Y. Characterization of metal-containing molecular sieves and their catalytic properties in the selective oxidation of cyclohexane. Catal. Today 2004, 93–95, 735–742. [Google Scholar] [CrossRef]
- Tan, P.; Au, C.T.; Lai, S.Y. Effects of acidification and basification of impregnating solution on the performance of Mo/HZSM-5 in methane aromatization. Appl. Catal. A Gen. 2007, 324, 36–41. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Nie, Q.; Hu, L.; Cui, Y.; Tan, Z.; Yu, H. One-step synthesis of metallic Bi deposited Bi2WO6 nanoclusters for enhanced photocatalytic performance: An experimental and DFT study. Appl. Surf. Sci. 2021, 559, 149970. [Google Scholar] [CrossRef]
- Reddy, J.K.; Lalitha, K.; Kumari, V.D.; Subrahmanyam, M. Photocatalytic study of Bi-ZSM-5 and Bi2O3/HZSM-5 for the treatment of phenolic wastes. Catal. Lett. 2007, 121, 131–136. [Google Scholar] [CrossRef]
- Wang, H.; Li, R.; Zheng, Y.; Chen, H.; Wang, F.; Ma, J. An Efficient and Reusable Catalyst of Bismuth-Containing SBA-15 Mesoporous Materials for Solvent-free Liquid Phase Oxidation of Cyclohexane by Oxygen. Catal. Lett. 2008, 122, 330–337. [Google Scholar] [CrossRef]
- Jin, D.; Ma, J.; Sun, R. Nitrogen-doped biochar nanosheets facilitate charge separation of a Bi/Bi2O3 nanosphere with a Mott–Schottky heterojunction for efficient photocatalytic reforming of biomass. J. Mater. Chem. C 2022, 10, 3500–3509. [Google Scholar] [CrossRef]
- Luo, L.; Chen, W.; Xu, S.-M.; Yang, J.; Li, M.; Zhou, H.; Xu, M.; Shao, M.; Kong, X.; Li, Z.; et al. Selective Photoelectrocatalytic Glycerol Oxidation to Dihydroxyacetone via Enhanced Middle Hydroxyl Adsorption over a Bi2O3-Incorporated Catalyst. J. Am. Chem. Soc. 2022, 144, 7720–7730. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.D.; Peralta, M.; Querini, C. Gas phase dehydration of glycerol over, lanthanum-modified beta-zeolite. Appl. Catal. A Gen. 2014, 472, 53–63. [Google Scholar] [CrossRef]
- Li, P.; Liu, G.; Wu, H.; Liu, Y.; Jiang, J.-G.; Wu, P. Postsynthesis and Selective Oxidation Properties of Nanosized Sn-Beta Zeolite. J. Phys. Chem. C 2011, 115, 3663–3670. [Google Scholar] [CrossRef]
- Xu, S.; Pan, D.; Hu, F.; Wu, Y.; Wang, H.; Chen, Y.; Yuan, H.; Gao, L.; Xiao, G. Highly efficient Cr/β zeolite catalyst for conversion of carbohydrates into 5-hydroxymethylfurfural: Characterization and performance. Fuel Process. Technol. 2019, 190, 38–46. [Google Scholar] [CrossRef]
- Xia, Y.; Zhan, W.; Guo, Y.; Guo, Y.; Lu, G. Fe-Beta zeolite for selective catalytic reduction of NOx with NH3: Influence of Fe content. Chin. J. Catal. 2016, 37, 2069–2078. [Google Scholar] [CrossRef]
- Xia, M.; Shen, Z.; Xiao, S.; Peng, B.-Y.; Gu, M.; Dong, W.; Zhang, Y. Synergistic effects and kinetic evidence of a transition metal-tin modified Beta zeolite on conversion of Miscanthus to lactic acid. Appl. Catal. A Gen. 2019, 583, 117126. [Google Scholar] [CrossRef]
- Wan, J.; Fu, L.; Yang, H.; Wang, K.; Xi, F.; Pan, L.; Li, Y.; Liu, Y. TiO2-ZrO2 composite oxide as an acid-base bifunctional catalyst for self-condensation of cyclopentanone. Ind. Eng. Chem. Res. 2020, 59, 19918–19928. [Google Scholar] [CrossRef]
- Wang, F.; Dong, W.; Qu, D.; Huang, Y.; Chen, Y. Synergistic Catalytic Conversion of Cellulose into Glycolic Acid over Mn-Doped Bismuth Oxyiodide Catalyst Combined with H-ZSM-5. Ind. Eng. Chem. Res. 2022, 61, 11382–11389. [Google Scholar] [CrossRef]
- Wang, F.-F.; Liu, J.; Li, H.; Liu, C.-L.; Yang, R.-Z.; Dong, W.-S. Conversion of cellulose to lactic acid catalyzed by erbium-exchanged montmorillonite K10. Green Chem. 2015, 17, 2455–2463. [Google Scholar] [CrossRef]
- Sadula, S.; Saha, B. Aerobic oxidation of xylose to yylaric acid in water over Pt catalysts. ChemSusChem 2018, 11, 2124–2129. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, R.; Taccardi, N.; Bösmann, A.; Wasserscheid, P. Selective catalytic conversion of biobased carbohydrates to formic acid using molecular oxygen. Green Chem. 2011, 13, 2759–2763. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, L.; Zhang, T.; Liu, H.; Zhang, X. Catalytic dehydration of lactic acid to acrylic acid over modified ZSM-5 catalysts. Chem. Eng. J. 2016, 284, 934–941. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, M.; Liu, X.; Han, Y. Catalytic oxidative conversion of cellulosic biomass to formic acid and acetic acid with exceptionally high yields. Catal. Today 2014, 233, 77–82. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, M.; Pan, X.; Zhang, J.; Gao, L.; Xiao, G. An Effective and Inexpensive Hf/ZSM-5 Catalyst for Efficient HMF Formation from Cellulose. Catal. Lett. 2020, 151, 1984–1992. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, X.; Dai, H. Experimental and kinetic study of the conversion of waste starch into glycolic acid over phosphomolybdic acid. RSC Adv. 2021, 11, 30961–30970. [Google Scholar] [CrossRef]
- Li, J.; Ding, D.; Deng, L.; Guo, Q.; Fu, Y. Catalytic Air Oxidation of Biomass-Derived Carbohydrates to Formic Acid. ChemSusChem 2012, 5, 1313–1318. [Google Scholar] [CrossRef]
- Wang, F.; Shi, A.-W.; Qin, X.-X.; Liu, C.-L.; Dong, W. Dehydration of fructose to 5-hydroxymethylfurfural by rare earth metal trifluoromethanesulfonates in organic solvents. Carbohydr. Res. 2011, 346, 982–985. [Google Scholar] [CrossRef]
- Tang, Z.; Deng, W.; Wang, Y.; Zhu, E.; Wan, X.; Zhang, Q.; Wang, Y. Transformation of Cellulose and its Derived Carbohydrates into Formic and Lactic Acids Catalyzed by Vanadyl Cations. ChemSusChem 2014, 7, 1557–1567. [Google Scholar] [CrossRef]
- Gorbanev, Y.Y.; Klitgaard, S.K.; Woodley, J.M.; Christensen, C.H.; Riisager, A. Gold-Catalyzed Aerobic Oxidation of 5-Hydroxymethylfurfural in Water at Ambient Temperature. ChemSusChem 2009, 2, 672–675. [Google Scholar] [CrossRef]
- Xu, S.; Tian, Q.; Xiao, Y.; Zhang, W.; Liao, S.; Li, J.; Hu, C. Regulating the competitive reaction pathway in glycerol conversion to lactic acid/glycolic acid selectively. J. Catal. 2022, 413, 407–416. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, X.; Xu, J.; Shi, M.; Wang, F.; Chen, C.; Xu, J. Depolymerization of cellulose to glucose by oxidation–hydrolysis. Green Chem. 2015, 17, 1519–1524. [Google Scholar] [CrossRef]
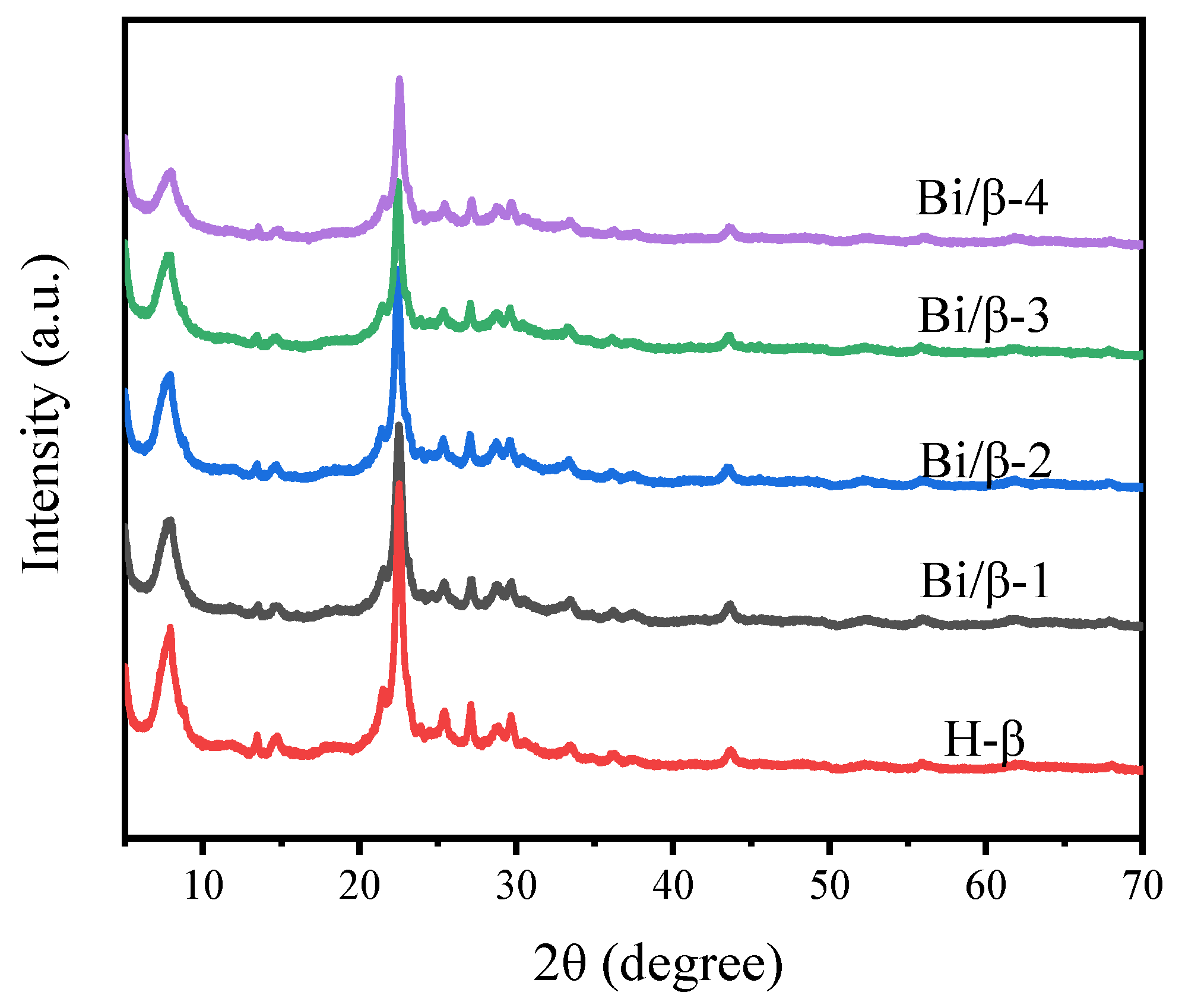
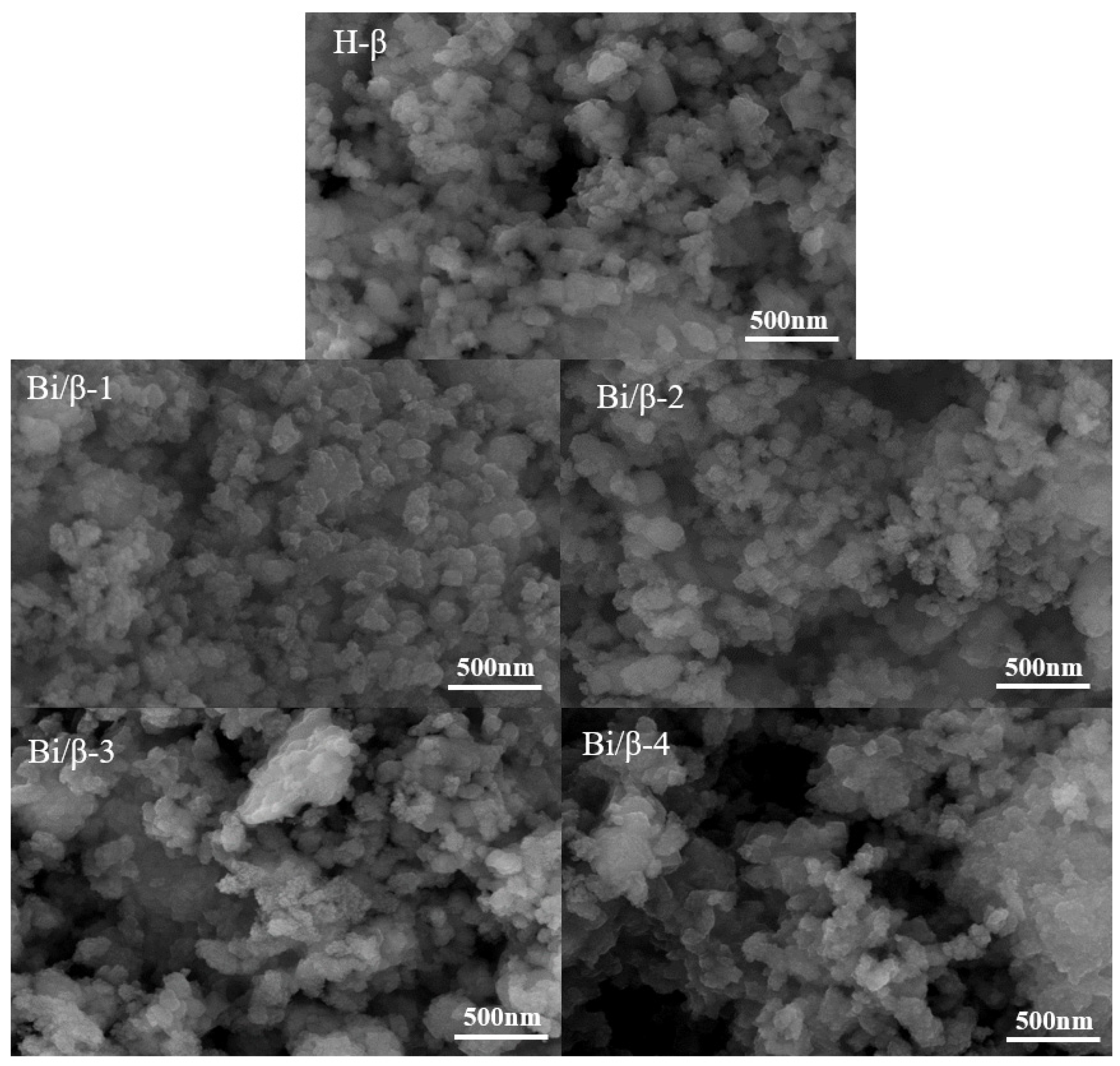
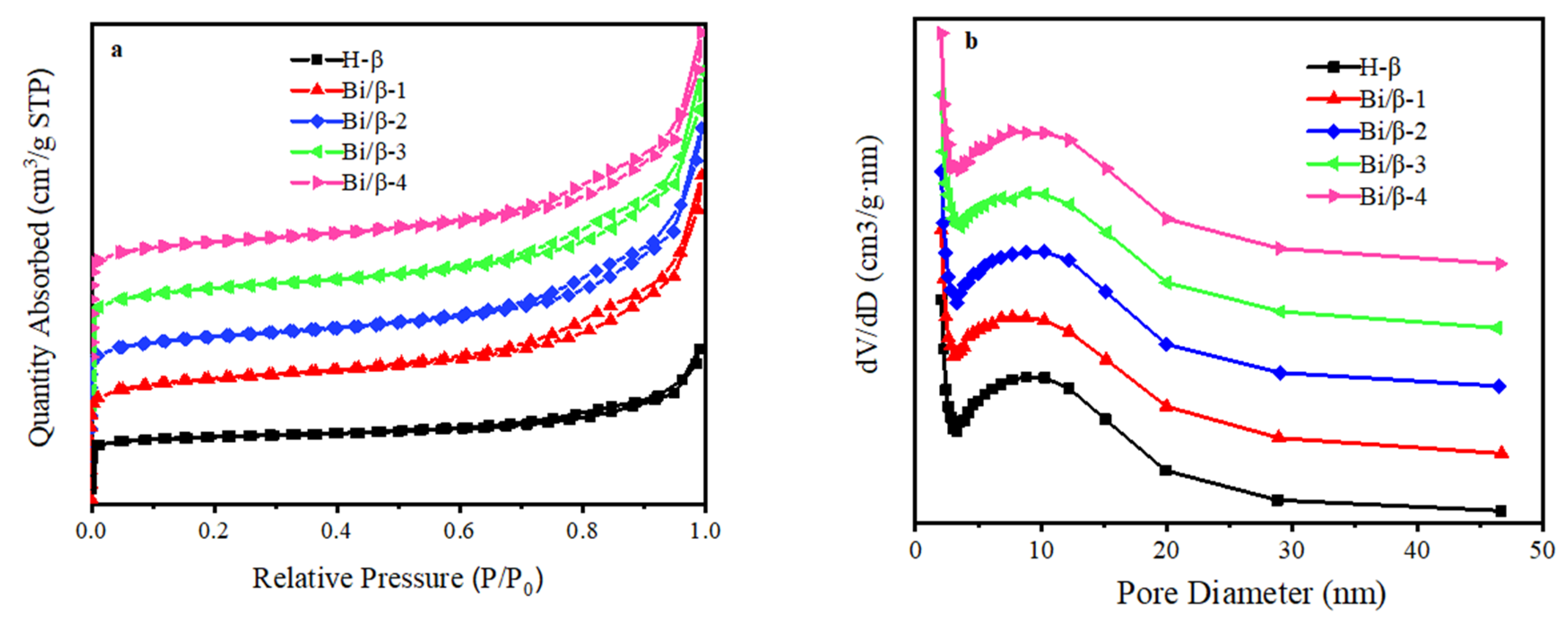

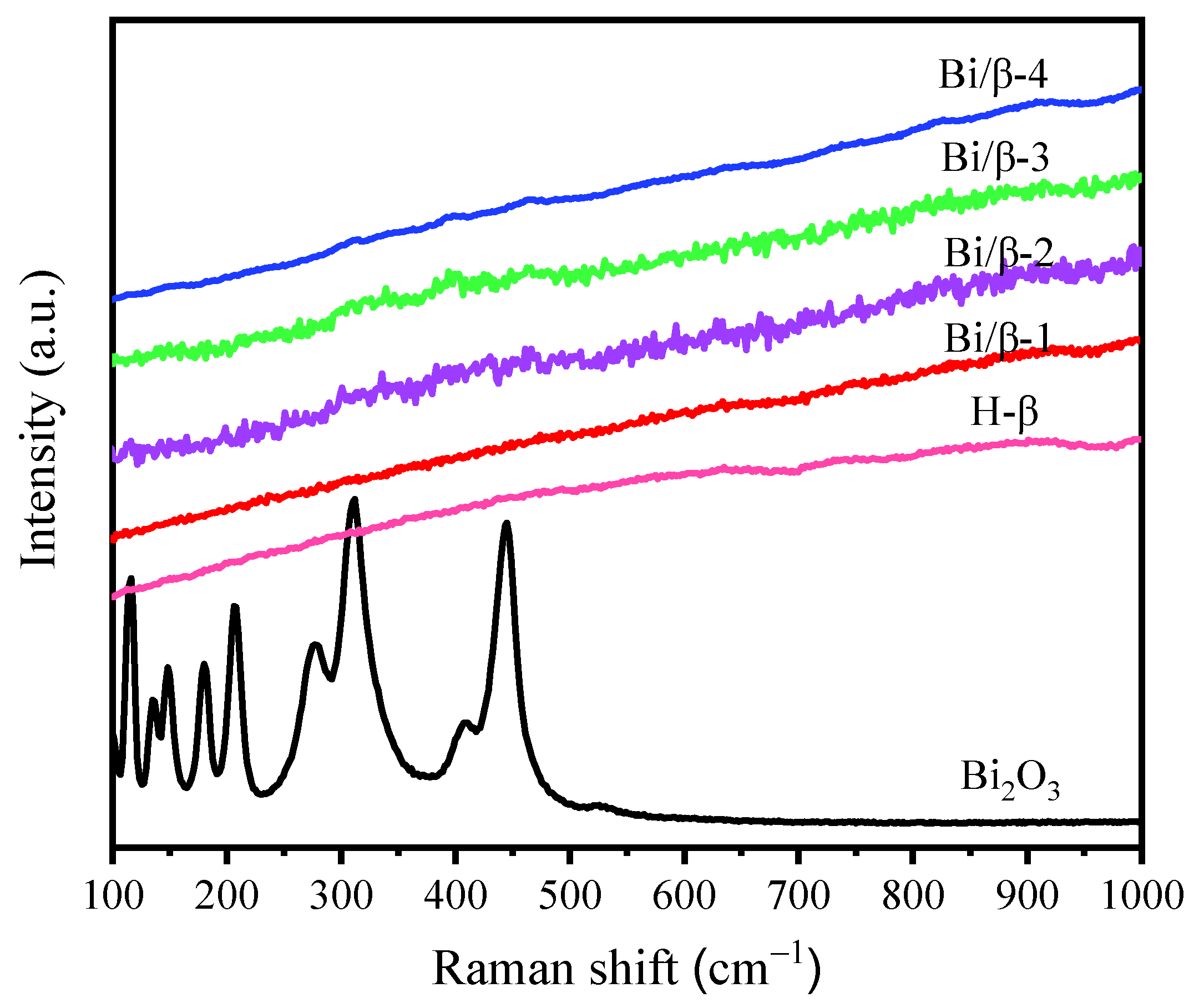





| Catalyst | SBET (m2/g) | SMicro (m2/g) | Vp (cm3/g) | VMicro (cm3/g) | D (nm) | Bi a (wt. %) |
|---|---|---|---|---|---|---|
| H-β | 556.62 | 449.51 | 0.575 | 0.181 | 4.13 | |
| Bi/β-1 | 533.89 | 424.40 | 0.553 | 0.171 | 4.09 | 1.26 |
| Bi/β-2 | 531.24 | 407.61 | 0.545 | 0.163 | 4.15 | 2.30 |
| Bi/β-3 | 510.60 | 403.99 | 0.544 | 0.162 | 4.40 | 3.40 |
| Bi/β-4 | 509.59 | 395.99 | 0.394 | 0.161 | 4.28 | 3.78 |
| Cu/β | 371.95 | 263.93 | 0.519 | 0.161 | 5.58 | |
| Co/β | 382.26 | 258.99 | 0.549 | 0.158 | 5.75 | |
| Mn/β | 379.84 | 263.07 | 0.552 | 0.161 | 5.82 |
| Catalyst | Lewis Acid Sites (mmol/g) | Brønsted Acid Sites (mmol/g) | Total Acidity (mmol/g) | Ratio of B to L |
|---|---|---|---|---|
| H-β | 38.16 | 33.40 | 71.55 | 0.87 |
| Bi/β-1 | 40.72 | 33.33 | 74.06 | 0.82 |
| Bi/β-2 | 52.26 | 50.36 | 102.62 | 0.96 |
| Bi/β-3 | 45.30 | 40.93 | 86.23 | 0.90 |
| Bi/β-4 | 34.63 | 30.46 | 65.19 | 0.88 |
| Entry | Catalyst | Con (%) | Yield (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GA | LA | AA | FA | Fru | Glu | HMFCA | GlyA | DHA | AcA | LeA | HMF | |||
| 1 | Blank | 25.8 | 7.7 ± 0.6 | 1.5 | 7.6 | 2.0 | 0.1 | 1.0 | 0.2 | 0.3 | 2.6 | 0.2 | 1.2 | 0.1 |
| 2 | H-β | 40.8 | 14.9 ± 0.3 | 3.7 | 2.5 | 0.6 | 0.1 | 11.0 | 6.0 | 1.3 | 0.5 | 0.1 | ||
| 3 | Bi/β-1 | 53.2 | 25.1 ± 0.4 | 3.2 | 3.5 | 0.9 | 0.2 | 8.2 | 4.2 | 0.3 | 1.4 | 0.1 | 0.2 | 0.1 |
| 4 | Bi/β-2 | 80.3 | 51.9 ± 0.2 | 6.7 | 6.3 | 1.2 | 0.3 | 6.9 | 2.1 | 0.6 | 1.2 | 0.3 | 0.5 | 0.1 |
| 5 | Bi/β-3 | 67.6 | 43.7 ± 0.3 | 4.4 | 6.1 | 0.5 | 0.2 | 6.6 | 2.7 | 0.4 | 1.1 | 0.1 | 0.2 | 0.1 |
| 6 | Bi/β-4 | 53.8 | 34.1 ± 0.2 | 3.5 | 5.0 | 0.6 | 0.2 | 5.7 | 4.1 | 0.4 | 0.8 | 0.1 | 0.3 | 0.1 |
| 7 | Mn/β | 40.0 | 8.7 ± 0.4 | 6.1 | 2.8 | 0.7 | 0.3 | 13.1 | 7.5 | 0.7 | 0.5 | 3.1 | 0.4 | |
| 8 | Co/β | 37.3 | 7.3 ± 0.5 | 1.7 | 6.4 | 1.2 | 0.2 | 13.4 | 7.1 | 0.7 | 0.1 | 0.4 | 0.3 | |
| 9 | Cu/β | 54.3 | 17.0 ± 0.1 | 8.0 | 7.8 | 1.5 | 0.5 | 12.6 | 6.9 | 1.3 | 0.6 | 4.3 | 0.1 | |
| 10 | Bi2O3 | 30.1 | 21.8 ± 0.3 | 1.4 | 1.0 | 1.4 | 0.1 | 0.5 | 0.1 | 1.7 | 0.1 | 0.2 | 0.1 | |
| 11 | Bi2O3 + H-β | 62.3 | 42.6 ± 0.3 | 3.5 | 2.7 | 1.1 | 0.2 | 7.3 | 1.6 | 3.9 | 0.3 | 0.3 | 0.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Qu, D.; Wang, S.; Liu, G.; Zhao, Q.; Hu, J.; Dong, W.; Huang, Y.; Xu, J.; Chen, Y. Bismuth-Decorated Beta Zeolites Catalysts for Highly Selective Catalytic Oxidation of Cellulose to Biomass-Derived Glycolic Acid. Int. J. Environ. Res. Public Health 2022, 19, 16298. https://doi.org/10.3390/ijerph192316298
Wang F, Qu D, Wang S, Liu G, Zhao Q, Hu J, Dong W, Huang Y, Xu J, Chen Y. Bismuth-Decorated Beta Zeolites Catalysts for Highly Selective Catalytic Oxidation of Cellulose to Biomass-Derived Glycolic Acid. International Journal of Environmental Research and Public Health. 2022; 19(23):16298. https://doi.org/10.3390/ijerph192316298
Chicago/Turabian StyleWang, Fenfen, Dongxue Qu, Shaoshuai Wang, Guojun Liu, Qiang Zhao, Jiaxue Hu, Wendi Dong, Yong Huang, Jinjia Xu, and Yuhui Chen. 2022. "Bismuth-Decorated Beta Zeolites Catalysts for Highly Selective Catalytic Oxidation of Cellulose to Biomass-Derived Glycolic Acid" International Journal of Environmental Research and Public Health 19, no. 23: 16298. https://doi.org/10.3390/ijerph192316298
APA StyleWang, F., Qu, D., Wang, S., Liu, G., Zhao, Q., Hu, J., Dong, W., Huang, Y., Xu, J., & Chen, Y. (2022). Bismuth-Decorated Beta Zeolites Catalysts for Highly Selective Catalytic Oxidation of Cellulose to Biomass-Derived Glycolic Acid. International Journal of Environmental Research and Public Health, 19(23), 16298. https://doi.org/10.3390/ijerph192316298







