Structural and Functional Neural Correlates in Individuals with Excessive Smartphone Use: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Eligibility
2.2. Electronic Searches
2.3. Data Extraction
2.4. Effect Size Analysis
2.5. ALE Analysis
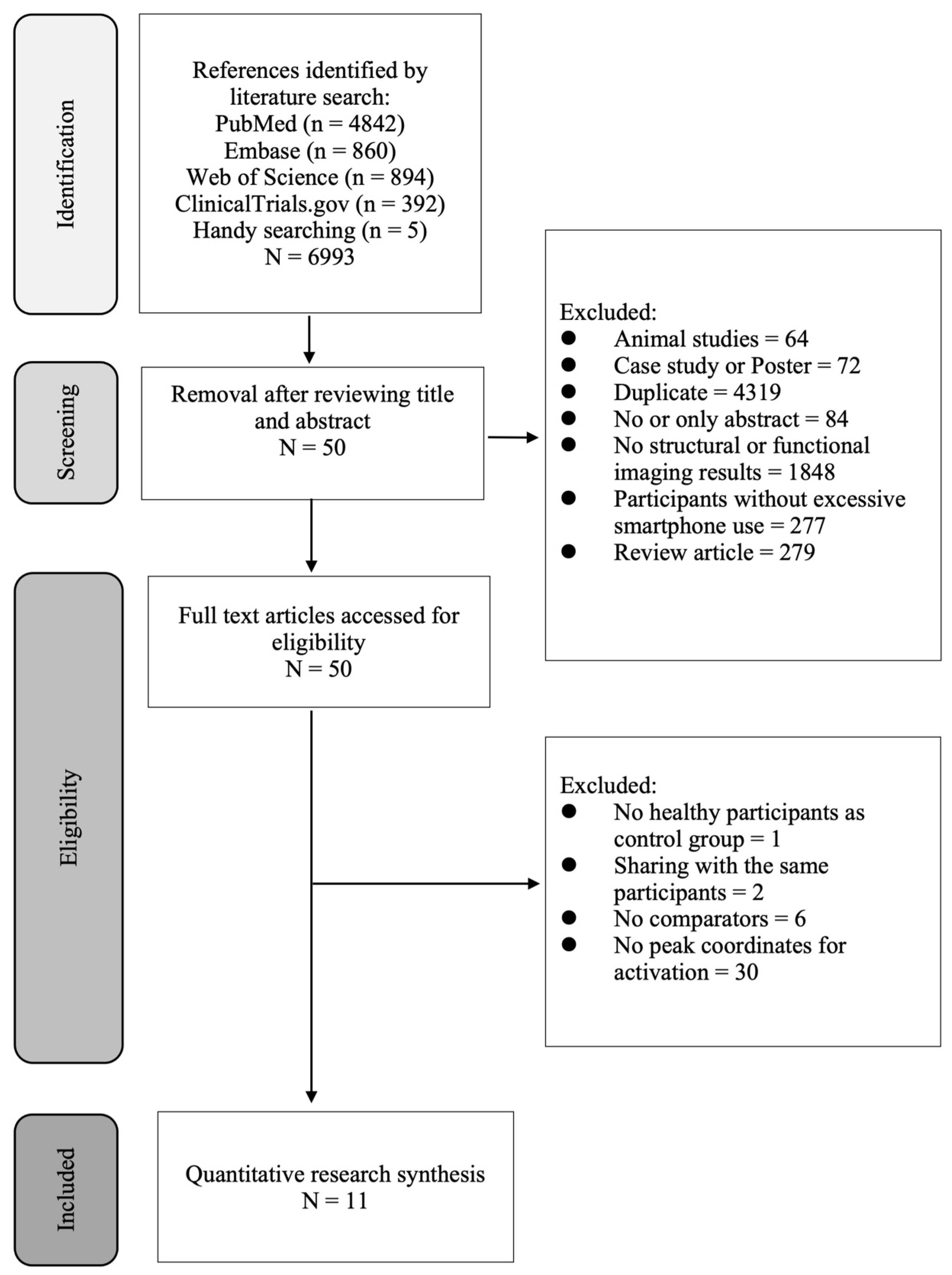
3. Results
3.1. Study Characteristics and Participants
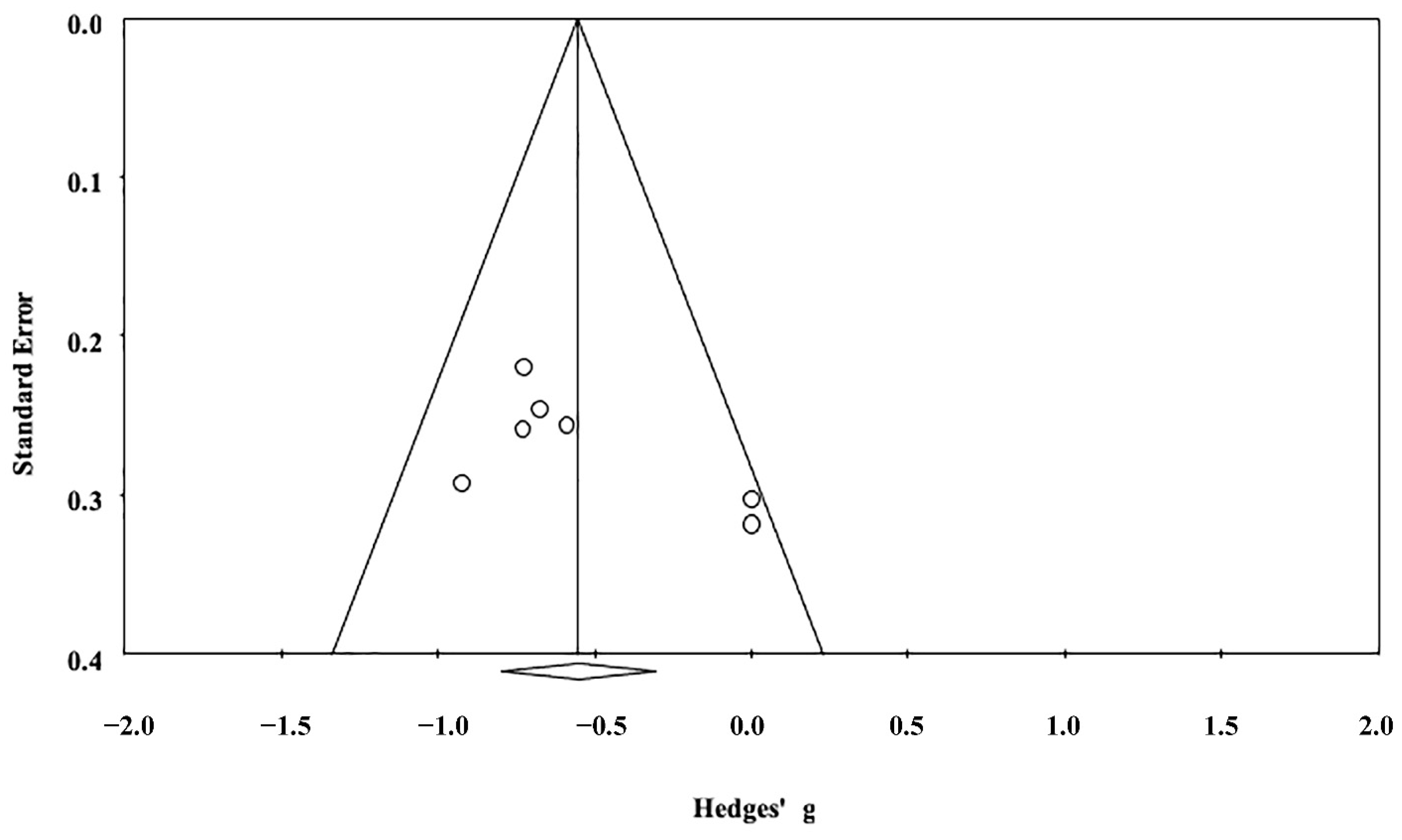
3.2. Quantitative Brain Volume Data Synthesis
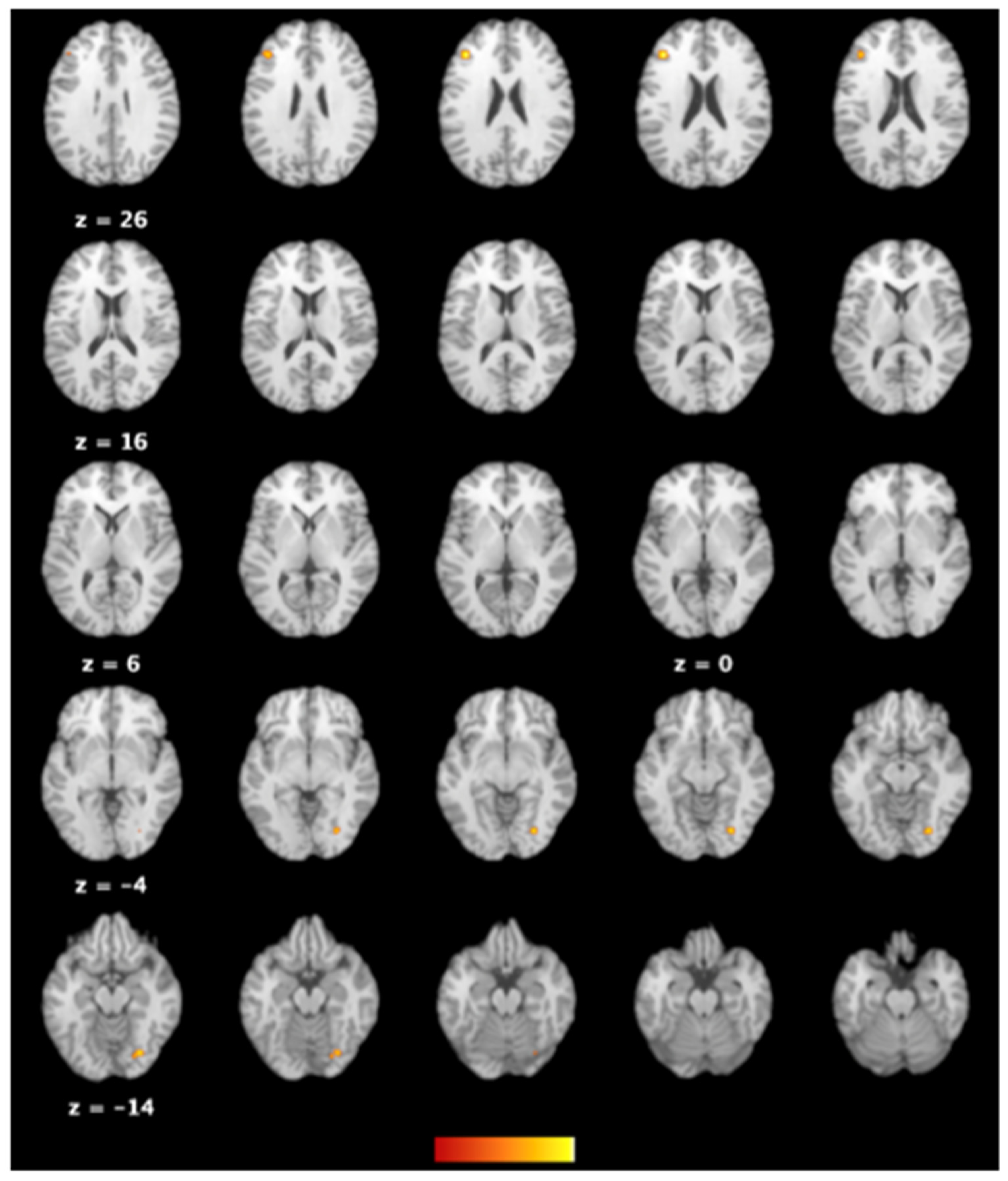
3.3. The Difference in Brain Activations between Individuals with Excessive Smartphone Use and Their Counterparts with Regular Use
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Anterior cingulate cortex |
| ADHD | Attention deficit hyperactivity disorder |
| ALE | Activation likelihood estimation |
| BDI | Beck depression inventory |
| BIS | Barratt impulsiveness scale |
| CBA | Cluster-based approach |
| CIUS | Compulsive internet use scale |
| CSF | Cerebrospinal fluid |
| ES | Effect size |
| fMRI | Functional magnetic resonance imaging |
| FEW | Family-wise error |
| GMD | Gray matter density |
| GMV | Gray matter volume |
| ICD | International Classification of Disease |
| LORETA | Low-resolution electromagnetic tomography |
| MA | Modeled Activation |
| MPAI | Mobile phone addiction index |
| MRI | Magnetic resonance imaging |
| NF | Neurofeedback |
| SAPS | Smartphone addiction proneness scale |
| SAS | Smartphone addiction scale |
| VBA | Voxel-based approach |
References
- WHO Gaming Disorder. Available online: https://www.who.int/news/item/14-09-2018-inclusion-of-gaming-disorder-in-icd-11 (accessed on 5 November 2022).
- Alina, R.; Alexinschi, O. Gambling disorder in ICD-11—A review of the neurobiological mechanisms behind the inclusion in behavioral addictions. Psihiatru Ro 2019, 56, 22–23. [Google Scholar]
- Lin, Y.-H.; Lin, S.-H.; Yang, C.C.H.; Kuo, T.B.J. Psychopathology of Everyday Life in the 21st Century: Smartphone Addiction. In Internet Addiction: Neuroscientific Approaches and Therapeutical Implications Including Smartphone Addiction; Montag, C., Reuter, M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 339–358. [Google Scholar]
- Cerniglia, L.; Zoratto, F.; Cimino, S.; Laviola, G.; Ammaniti, M.; Adriani, W. Internet Addiction in adolescence: Neurobiological, psychosocial and clinical issues. Neurosci. Biobehav. Rev. 2017, 76, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M. Brain change in addiction as learning, not disease. N. Engl. J. Med. 2018, 379, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.M. Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology 2011, 61, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Ratan, Z.A.; Parrish, A.-M.; Zaman, S.B.; Alotaibi, M.S.; Hosseinzadeh, H. Smartphone addiction and associated health outcomes in adult populations: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 12257. [Google Scholar] [CrossRef] [PubMed]
- Bankmycell How Many Smartphones Are in the World? Available online: https://www.bankmycell.com/blog/how-many-phones-are-in-the-world (accessed on 26 November 2022).
- Solly, J.E.; Hook, R.W.; Grant, J.E.; Cortese, S.; Chamberlain, S.R. Structural gray matter differences in Problematic Usage of the Internet: A systematic review and meta-analysis. Mol. Psychiatry 2022, 27, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Lerch, J.P.; Van Der Kouwe, A.J.; Raznahan, A.; Paus, T.; Johansen-Berg, H.; Miller, K.L.; Smith, S.M.; Fischl, B.; Sotiropoulos, S.N. Studying neuroanatomy using MRI. Nat. Neurosci. 2017, 20, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.-J.; Deng, W.; Wang, H.-Y.; Guo, W.-J.; Li, T.; Lam, C.; Lin, X. Reward pathway dysfunction in gambling disorder: A meta-analysis of functional magnetic resonance imaging studies. Behav. Brain Res. 2014, 275, 243–251. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Voxel-Based Morphometry—The Methods. NeuroImage 2000, 11, 805–821. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Bzdok, D.; Laird, A.R.; Kurth, F.; Fox, P.T. Activation likelihood estimation meta-analysis revisited. NeuroImage 2012, 59, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: Oxfordshire, UK, 2013. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Riley, R.D.; Higgins, J.P.; Deeks, J.J. Interpretation of random effects meta-analyses. BMJ 2011, 342, d549. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Higgins, J. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int. J. Epidemiol. 2008, 37, 1158–1160. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Laird, A.R.; Grefkes, C.; Wang, L.E.; Zilles, K.; Fox, P.T. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009, 30, 2907–2926. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Eickhoff, S.B.; Laird, A.R.; Fox, M.; Wiener, M.; Fox, P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 2012, 33, 1–13. [Google Scholar] [CrossRef]
- Heller, R.; Stanley, D.; Yekutieli, D.; Rubin, N.; Benjamini, Y. Cluster-based analysis of FMRI data. NeuroImage 2006, 33, 599–608. [Google Scholar] [CrossRef]
- Woo, C.-W.; Krishnan, A.; Wager, T.D. Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. NeuroImage 2014, 91, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Nichols, T.E.; Laird, A.R.; Hoffstaedter, F.; Amunts, K.; Fox, P.T.; Bzdok, D.; Eickhoff, C.R. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. NeuroImage 2016, 137, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.L.; Tordesillas-Gutiérrez, D.; Martinez, M.; Salinas, F.; Evans, A.; Zilles, K.; Mazziotta, J.C.; Fox, P.T. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 2007, 28, 1194–1205. [Google Scholar] [CrossRef]
- Hirjak, D.; Henemann, G.M.; Schmitgen, M.M.; Götz, L.; Wolf, N.D.; Kubera, K.M.; Sambataro, F.; Leménager, T.; Koenig, J.; Wolf, R.C. Cortical surface variation in individuals with excessive smartphone use. Dev. Neurobiol. 2022, 82, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Schmitgen, M.M.; Horvath, J.; Mundinger, C.; Wolf, N.D.; Sambataro, F.; Hirjak, D.; Kubera, K.M.; Koenig, J.; Wolf, R.C. Neural correlates of cue reactivity in individuals with smartphone addiction. Addict. Behav. 2020, 108, 106422. [Google Scholar] [CrossRef] [PubMed]
- Horvath, J.; Mundinger, C.; Schmitgen, M.M.; Wolf, N.D.; Sambataro, F.; Hirjak, D.; Kubera, K.M.; Koenig, J.; Wolf, R.C. Structural and functional correlates of smartphone addiction. Addict. Behav. 2020, 105, 106334. [Google Scholar] [CrossRef]
- Cho, I.H.; Yoo, J.H.; Chun, J.-W.; Cho, H.; Kim, J.-Y.; Choi, J.; Kim, D.-J. Reduced volume of a brainstem substructure in adolescents with problematic smartphone use. J. Korean Acad. Child Adolesc. Psychiatry 2021, 32, 137. [Google Scholar] [CrossRef]
- Choi, J.; Cho, H.; Choi, J.-S.; Choi, I.Y.; Chun, J.-W.; Kim, D.-J. The neural basis underlying impaired attentional control in problematic smartphone users. Transl. Psychiatry 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Rashid, A.A.; Suppiah, S.; Nasser, N.S.; Sharifat, H.; Mohamad, M.; Loh, J.L.; Ibrahim, B.; Ibrahim, N.S.N.; Azmi, N.H.M.; Rahim, E.A. The neurobiology of smartphone addiction in emerging adults evaluated using brain morphometry and resting-state functional MRI. Neurosci. Res. Notes 2021, 4, 19–28. [Google Scholar] [CrossRef]
- Yoo, J.H.; Chun, J.-W.; Choi, M.R.; Cho, H.; Kim, J.-Y.; Choi, J.; Kim, D.-J. Caudate nucleus volume mediates the link between glutamatergic neurotransmission and problematic smartphone use in youth. J. Behav. Addict. 2021, 10, 338–346. [Google Scholar] [CrossRef]
- Nasser, N.S.; Sharifat, H.; Rashid, A.A.; Hamid, S.A.; Rahim, E.A.; Loh, J.L.; Ching, S.M.; Hoo, F.K.; Ismail, S.I.F.; Tyagi, R.; et al. Cue-Reactivity among Young Adults With Problematic Instagram Use in Response to Instagram-Themed Risky Behavior Cues: A Pilot fMRI Study. Front. Psychol. 2020, 11, 556060. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Namkoong, K.; Lee, J.; Lee, B.O.; Jung, Y.-C. Lateral orbitofrontal gray matter abnormalities in subjects with problematic smartphone use. J. Behav. Addict. 2019, 8, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.-H.; Zhang, Z.-Z.; Guan, M.; Gao, L.; Ma, L.; Li, Z.; Wang, E.; Guo, H.-H.; Yan, B.; Tong, L.; et al. Altered default-mode network functional connectivity in college students with mobile phone addiction. Int. J. Clin. Exp. Med. 2019, 12, 1877–1887. [Google Scholar]
- Chun, J.W.; Choi, J.; Kim, J.Y.; Cho, H.; Ahn, K.J.; Nam, J.H.; Choi, J.S.; Kim, D.J. Altered brain activity and the effect of personality traits in excessive smartphone use during facial emotion processing. Sci. Rep. 2017, 7, 12156. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Turel, O.; Brevers, D.; Bechara, A. Excess social media use in normal populations is associated with amygdala-striatal but not with prefrontal morphology. Psychiatry Res. Neuroimaging 2017, 269, 31–35. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Z.; Song, H.; Xu, X.; Wang, H.; d’Oleire Uquillas, F.; Huang, X. Altered gray matter volume and white matter integrity in college students with mobile phone dependence. Front. Psychology 2016, 7, 597. [Google Scholar] [CrossRef]
- Patton, J.H.; Stanford, M.S.; Barratt, E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychology 1995, 51, 768–774. [Google Scholar] [CrossRef]
- Richter, P.; Werner, J.; Heerlein, A.; Kraus, A.; Sauer, H. On the validity of the Beck Depression Inventory. Psychopathology 1998, 31, 160–168. [Google Scholar] [CrossRef]
- Wacks, Y.; Weinstein, A.M. Excessive Smartphone Use Is Associated With Health Problems in Adolescents and Young Adults. Front. Psychiatry 2021, 12, 669042. [Google Scholar] [CrossRef]
- Lai, C.-H. Gray matter volume in major depressive disorder: A meta-analysis of voxel-based morphometry studies. Psychiatry Res. NeuroImaging 2013, 211, 37–46. [Google Scholar] [CrossRef]
- Gold, A.L.; Steuber, E.R.; White, L.K.; Pacheco, J.; Sachs, J.F.; Pagliaccio, D.; Berman, E.; Leibenluft, E.; Pine, D.S. Cortical thickness and subcortical gray matter volume in pediatric anxiety disorders. Neuropsychopharmacology 2017, 42, 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- McTeague, L.M.; Huemer, J.; Carreon, D.M.; Jiang, Y.; Eickhoff, S.B.; Etkin, A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am. J. Psychiatry 2017, 174, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.U.; Madathil, D.; Huang, C.M. Age-related and individual variations in altered prefrontal and cerebellar connectivity associated with the tendency of developing internet addiction. Hum. Brain Mapp. 2021, 42, 4525–4537. [Google Scholar] [CrossRef] [PubMed]
- Couvy-Duchesne, B.; Strike, L.T.; de Zubicaray, G.I.; McMahon, K.L.; Thompson, P.M.; Hickie, I.B.; Martin, N.G.; Wright, M.J. Lingual Gyrus Surface Area Is Associated with Anxiety-Depression Severity in Young Adults: A Genetic Clustering Approach. eNeuro 2018, 5, ENEURO.0153-17.2017. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.L.; Ladouceur, C.D.; Drevets, W.C. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol. Psychiatry 2008, 13, 833–857. [Google Scholar] [CrossRef]
- Hwang, Y.; Park, N. Is smartphone addiction comparable between adolescents and adults? Examination of the degree of smartphone use, type of smartphone activities, and addiction levels among adolescents and adults. Int. Telecommun. Policy Rev. 2017, 24, 17. [Google Scholar]
- Madden, M.; Lenhart, A.; Duggan, M.; Cortesi, S.; Gasser, U. Teens and Technology; Pew Internet & American Life Project: Washington, DC, USA, 2005. [Google Scholar]
- Yuan, K.; Qin, W.; Wang, G.; Zeng, F.; Zhao, L.; Yang, X.; Liu, P.; Liu, J.; Sun, J.; von Deneen, K.M.; et al. Microstructure abnormalities in adolescents with internet addiction disorder. PLoS ONE 2011, 6, e20708. [Google Scholar] [CrossRef]
- Hong, S.-B.; Kim, J.-W.; Choi, E.-J.; Kim, H.-H.; Suh, J.-E.; Kim, C.-D.; Klauser, P.; Whittle, S.; Yűcel, M.; Pantelis, C.; et al. Reduced orbitofrontal cortical thickness in male adolescents with internet addiction. Behav. Brain Funct. 2013, 9, 11. [Google Scholar] [CrossRef]
- Gennatas, E.D.; Avants, B.B.; Wolf, D.H.; Satterthwaite, T.D.; Ruparel, K.; Ciric, R.; Hakonarson, H.; Gur, R.E.; Gur, R.C. Age-Related Effects and Sex Differences in Gray Matter Density, Volume, Mass, and Cortical Thickness from Childhood to Young Adulthood. J. Neurosci. 2017, 37, 5065–5073. [Google Scholar] [CrossRef]
- Manninen, S.; Karjalainen, T.; Tuominen, L.J.; Hietala, J.; Kaasinen, V.; Joutsa, J.; Rinne, J.; Nummenmaa, L. Cerebral grey matter density is associated with neuroreceptor and neurotransporter availability: A combined PET and MRI study. NeuroImage 2021, 235, 117968. [Google Scholar] [CrossRef]
- Breukelaar, I.A.; Antees, C.; Grieve, S.M.; Foster, S.L.; Gomes, L.; Williams, L.M.; Korgaonkar, M.S. Cognitive control network anatomy correlates with neurocognitive behavior: A longitudinal study. Hum. Brain Mapp. 2017, 38, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-G.; Park, J.; Kim, H.-T.; Pan, Z.; Lee, Y.; McIntyre, R.S. The relationship between smartphone addiction and symptoms of depression, anxiety, and attention-deficit/hyperactivity in South Korean adolescents. Ann. Gen. Psychiatry 2019, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ghaziri, J.; Tucholka, A.; Larue, V.; Blanchette-Sylvestre, M.; Reyburn, G.; Gilbert, G.; Lévesque, J.; Beauregard, M. Neurofeedback training induces changes in white and gray matter. Clin. EEG Neurosci. 2013, 44, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Misaki, M.; Mulyana, B.; Zotev, V.; Wurfel, B.E.; Krueger, F.; Feldner, M.; Bodurka, J. Hippocampal volume recovery with real-time functional MRI amygdala neurofeedback emotional training for posttraumatic stress disorder. J. Affect Disord. 2021, 283, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Singh, P.; Sahni, S. Towards efficacy of EEG neurofeedback from traditional to advanced approach: A review. Biomed. Pharmacol. J. 2019, 12, 619–627. [Google Scholar] [CrossRef]
- Walter, H.; Berger, M.; Schnell, K. Neuropsychotherapy: Conceptual, empirical and neuroethical issues. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 259, 173–182. [Google Scholar] [CrossRef]

| Studies | EXP | CON | EVAL of EXP | MPA Definition | Brain Volume | Functional Connectivity | Country | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % F | Avg. Age (SD) | N | % F | Avg. Age (SD) | ANAT Meas. | EXP < CON | EXP > CON | CM | EXP < CON | EXP > CON | ||||
| Cho et al. [33] | 20 | 60.00 | 16.20 (1.11) | 67 | 28.36 | 15.27(1.69) | SAPS | ≥42 on SAPS as EXP | GMV and WMV | SCP | N/A | N/A | Korea | ||
| Choi et al. [34] | 33 | 54.55 | 25.21 (5.54) | 33 | 54.55 | 24.85(4.49) | SAPS | EXP score > CON score | N/A | BOLD signal | Left MOOC |
| Korea | ||
| Rashid et al. [35] | 20 | N/A | 18–25 | 20 | N/A | 18–25 | SAS-M | Subjects with scores of >98 were considered as EXP | GMD and GMV | Left IPL | Right insula and right PreCG | BOLD signal | N/A |
| Malaysia |
| Yoo et al. [36] | 20 | 60.00 | 16.20 (1.11) | 68 | 27.94 | 15.26 (1.68) | SAPS | A SAPS score of 42 and higher as EXP | GMV and WMV | Bilateral CN | N/A | N/A | Korea | ||
| Horvath et al. [32] | 22 | 68.18 | 22.5 (3.0) | 26 | 69.23 | 23.0 (3.2) | SAS-SV | scoring > 31 (M) and >33 (F) on SAS-SV | GMV | Left anterior insula, IT, and PHC | Left SMG | BOLD signal | Right ACC | N/A | Germany |
| Nasser et al. [37] | 15 | 33.00 | 22.2 (0.86) | 15 | 53.00 | 21.67 (1.18) | SAS-M and IGAT | A cut-off score of ≥98 as EXP | N/A | BOLD signal |
|
| Malaysia | ||
| Lee et al. [38] | 39 | 25.64 | 22.9 (2.2) | 49 | 34.69 | 22.4 (2.7) | SAPS | Total SAPS score > 40, or subscale score > 14 for disturbance of adaptive function as EXP | GMV | Right OPFC | N/A | N/A | Korea | ||
| Lou et al. [39] | 24 | 54.17 | 23.25 (1.33) | 16 | 75.0 | 23.88 (0.86) | YIAT | Over 5 “yes” to the 8 questions as EXP | N/A | BOLD signal | N/A |
| China | ||
| Chun et al. [40] | 25 | 48.00 | 27.76 (5.97) | 27 | 33.30 | 28.93 (6.93) | SAPS | Total > 44, or subscale > 15 (disturbance of adaptive function), >13(withdrawal), >13 (tolerance) | N/A | BOLD signal |
| N/A | Korea | ||
| He et al. [41] | 25 | 32.0 | 24.12 (6.15) | 25 | 32.0 | 29.80 (10.9) | FACIUI | EXP > CON | GMV | Right VS, bilateral amygdala | N/A | N/A | China | ||
| Wang et al. [42] | 34 | 61.76 | 21.60 (2.10) | 34 | 61.76 | 21.73 (1.94) | MPAI | Over 51 as EXP | GMV& DTI |
| N/A | N/A | China | ||
| Subgroup Analysis | NComp | ga | 95% CI | Z | Qb | pc |
|---|---|---|---|---|---|---|
| Age group | ||||||
| Adolescents | 2 | −0.66 | −1.01 to −0.30 | −3.61 ** | 0.41 | 0.00 |
| Adults | 5 | −0.49 | −0.85 to −0.14 | −2.74 ** | ||
| Location | ||||||
| Cerebral cortex d | 4 | −0.14 | −0.92 to 0.64 | −0.34 | 2.35 | 0.00 |
| Subcortical structure e | 5 | −0.77 | −1.01 to −0.54 | −6.42 ** | ||
| Variable (Continuous) | Coefficient (95% CI) | p |
|---|---|---|
| Prevalence of female | 0.01 (−0.002 to 0.03) (n = 6) | 0.09 |
| Age | −0.006 (−0.07 to 0.06) (n = 6) | 0.86 |
| Intelligence quotient | −0.006 (−0.07 to 0.06) (n = 3) | 0.85 |
| Mean Beck depression inventory score | −0.08 (−0.36 to 0.19) (n = 3) | 0.55 |
| Mean Barratt impulsiveness scale score | 0.03 (0.004 to 0.06) (n = 4) | 0.03 |
| Cluster | Side | Brain Area | BA | Volume (mm3) | ALE | x | y | z |
|---|---|---|---|---|---|---|---|---|
| 1 | R | Declive of posterior lobe | 568 | 0.014351934 | 28 | −76 | −14 | |
| 1 | R | Lingual gyrus | 18 | 0.014068902 | 28 | −76 | −8 | |
| 2 | L | Middle frontal gyrus | 46 | 472 | 0.016162576 | −38 | 30 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-M.; Chang, Y.-T.; Chen, M.-H.; Liu, S.-T.; Chen, B.-S.; Li, L.; Lee, C.-Y.; Sue, Y.-R.; Sung, T.-M.; Sun, C.-K.; et al. Structural and Functional Neural Correlates in Individuals with Excessive Smartphone Use: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 16277. https://doi.org/10.3390/ijerph192316277
Lin H-M, Chang Y-T, Chen M-H, Liu S-T, Chen B-S, Li L, Lee C-Y, Sue Y-R, Sung T-M, Sun C-K, et al. Structural and Functional Neural Correlates in Individuals with Excessive Smartphone Use: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(23):16277. https://doi.org/10.3390/ijerph192316277
Chicago/Turabian StyleLin, Hsiu-Man, Yu-Tzu Chang, Meng-Hsiang Chen, Shu-Tsen Liu, Bo-Shen Chen, Lin Li, Chiao-Yu Lee, Yu-Ru Sue, Tsai-Mei Sung, Cheuk-Kwan Sun, and et al. 2022. "Structural and Functional Neural Correlates in Individuals with Excessive Smartphone Use: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 23: 16277. https://doi.org/10.3390/ijerph192316277
APA StyleLin, H.-M., Chang, Y.-T., Chen, M.-H., Liu, S.-T., Chen, B.-S., Li, L., Lee, C.-Y., Sue, Y.-R., Sung, T.-M., Sun, C.-K., & Yeh, P.-Y. (2022). Structural and Functional Neural Correlates in Individuals with Excessive Smartphone Use: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(23), 16277. https://doi.org/10.3390/ijerph192316277







