Development of a Cyclic Voltammetry-Based Method for the Detection of Antigens and Antibodies as a Novel Strategy for Syphilis Diagnosis
Abstract
1. Introduction
2. Materials and Methods
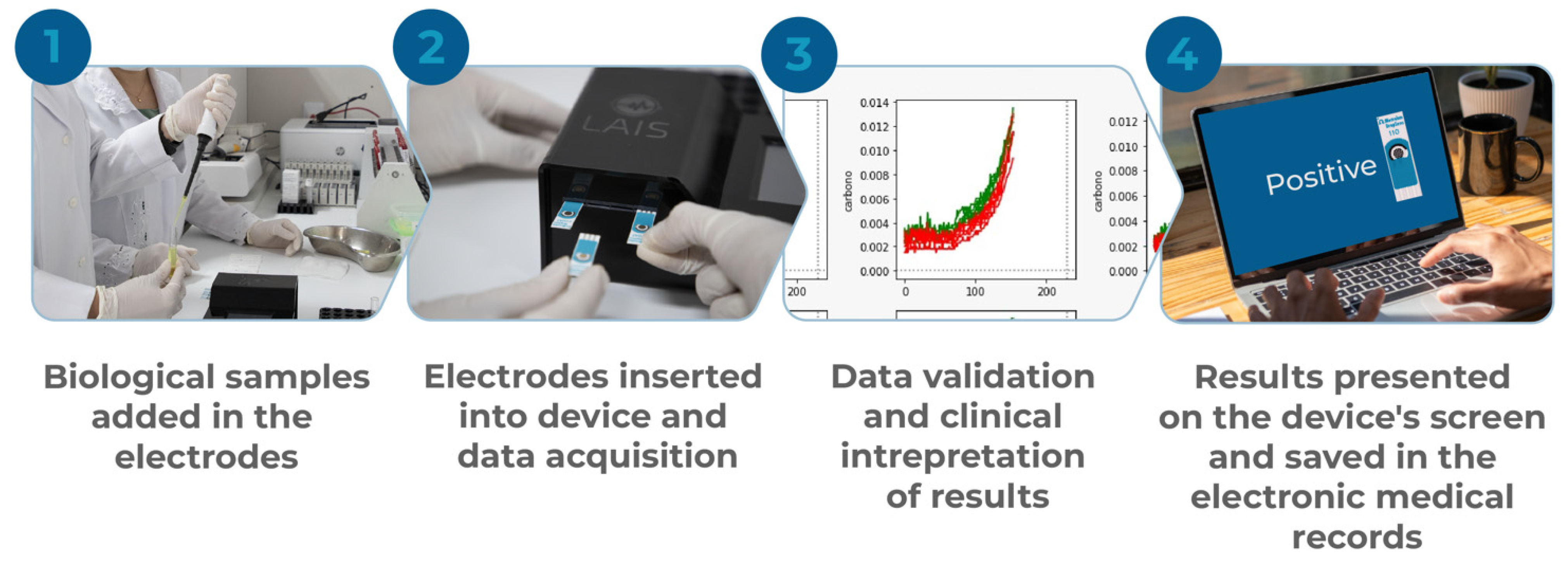
2.1. General Properties of the Developed Device
2.2. Biological Sample Processing and Quantification of Voltammetric Signal
2.3. Electrode Preparation and Immobilization of Biological Baits
2.4. Data Processing, Batch Normalization, and Interpretation of Results
2.5. Statistical Analysis
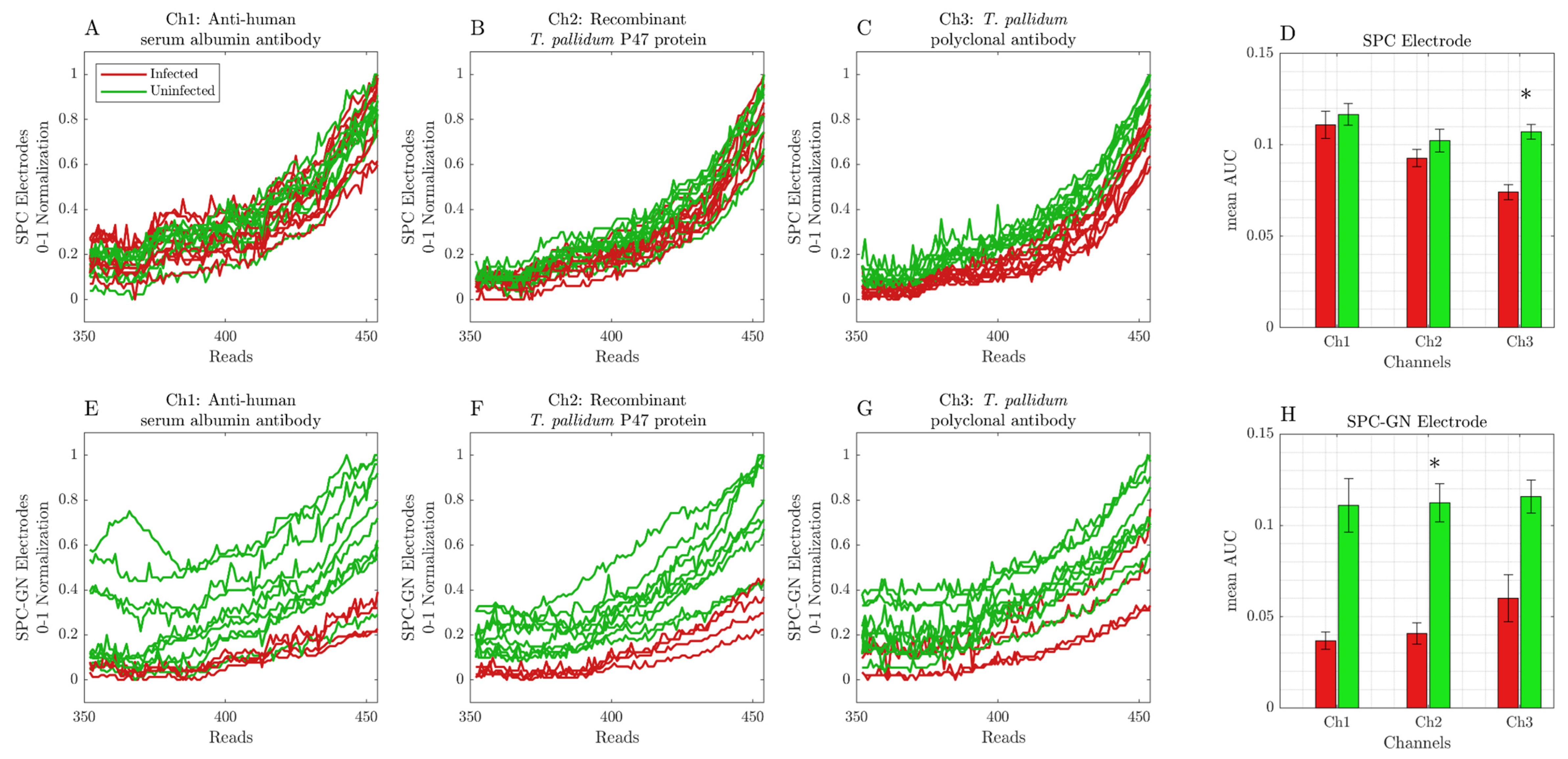
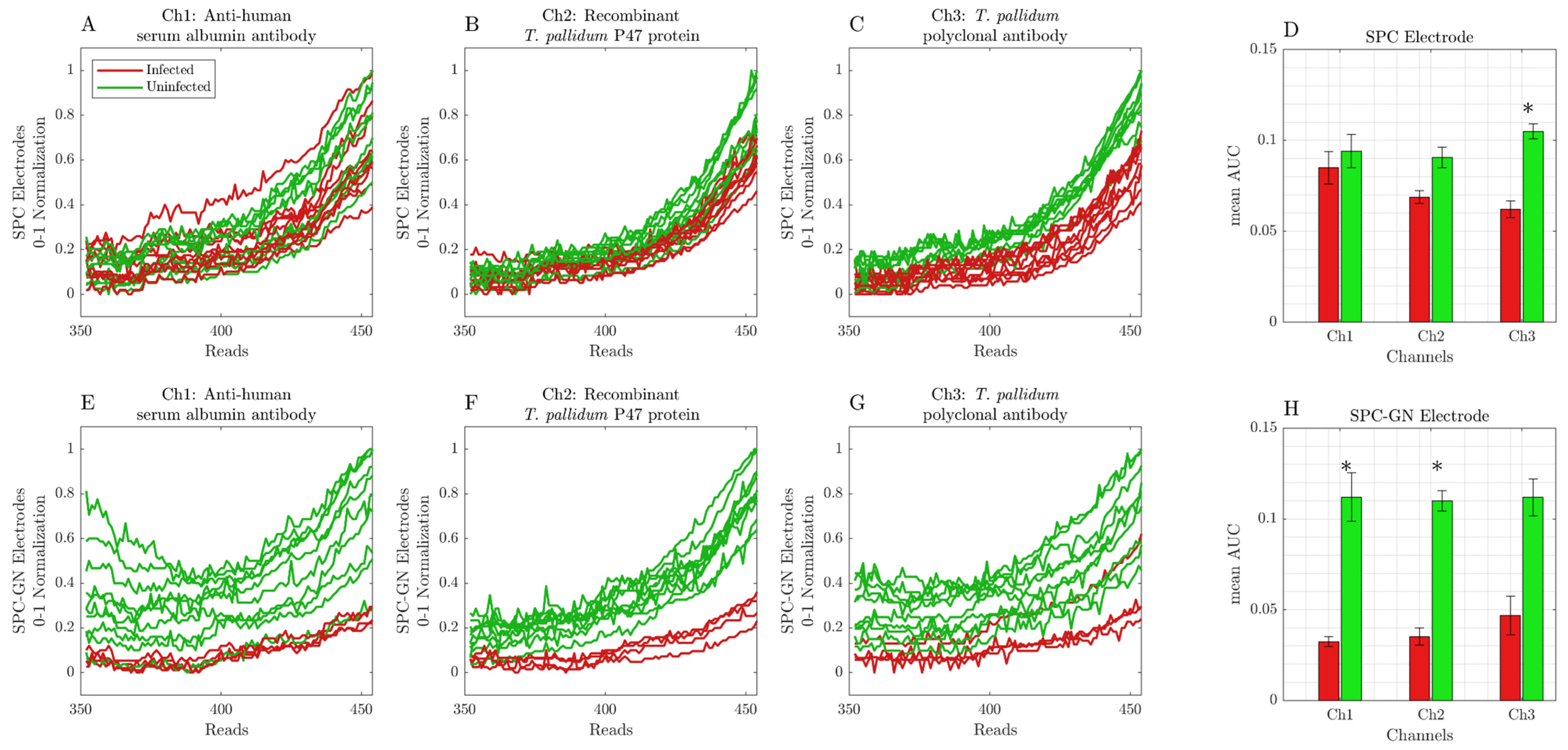
3. Results
Screen-Printed Carbon Modified with Gold Nanoparticle Electrodes Can Be Used as a Platform to Identify Antigens or Antibodies in Infected Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC Preliminary 2021 Data: Syphilis. Available online: https://www.cdc.gov/std/statistics/2020/preliminary2021.htm (accessed on 22 August 2022).
- Tanne, J.H. COVID-19: Sexually Transmitted Diseases Surged in US during Pandemic. BMJ 2022, 377, o1275. [Google Scholar] [CrossRef] [PubMed]
- WHO Data on Syphilis. Available online: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/data-on-syphilis (accessed on 22 August 2022).
- Eisinger, R.W.; Erbelding, E.; Fauci, A.S. Refocusing Research on Sexually Transmitted Infections. J. Infect. Dis. 2020, 222, 1432–1434. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention Sexually Transmitted Infections Treatment Guidelines, 2021—Syphilis. Available online: https://www.cdc.gov/std/treatment-guidelines/syphilis.htm (accessed on 17 October 2022).
- Ministério da Saúde Protocolo Clínico e Diretrizes Terapêuticas Para Atenção Integral Às Pessoas Com Infecções Sexualmente Transmissíveis. Available online: https://www.gov.br/aids/pt-br/centrais-de-conteudo/pcdts/2022/ist/pcdt-ist-2022_isbn-1.pdf/view (accessed on 27 November 2022).
- Ceccarelli, G.; Borrazzo, C.; Lazzaro, A.; Innocenti, G.P.; Celani, L.; Cavallari, E.N.; Pinacchio, C.; Santinelli, L.; Mastroianni, C.M.; d’Ettorre, G. Diagnostic Issues of Asymptomatic Neurosyphilis in HIV-Positive Patients: A Retrospective Study. Brain Sci. 2019, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, S.; Sánchez-Tirado, E.; Agüí, L.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Simultaneous Determination of CXCL7 Chemokine and MMP3 Metalloproteinase as Biomarkers for Rheumatoid Arthritis. Talanta 2021, 234, 122705. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Pan, G.; Guan, S.; Rong, S.; Wang, D.; Gao, Z.; Tian, P.; Li, Q. A Broad-Range Disposable Electrochemical Biosensor Based on Screen-Printed Carbon Electrodes for Detection of Human Noroviruses. Front. Bioeng. Biotechnol. 2022, 10, 845660. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Bezaatpour, A.; Jafari, H.; Boukherroub, R.; Szunerits, S. Electrochemical Methodologies for the Detection of Pathogens. ACS Sens. 2018, 3, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.; Guo, X.; Musavi, L.; Lin, C.-S.; Chen, S.-H.; Wu, V.C.H. Gold Nanoparticle-Modified Carbon Electrode Biosensor for the Detection of Listeria Monocytogenes. Ind. Biotechnol. 2013, 9, 31–36. [Google Scholar] [CrossRef]
- Baradoke, A.; Jose, B.; Pauliukaite, R.; Forster, R.J. Properties of Anti-CA125 Antibody Layers on Screen-Printed Carbon Electrodes Modified by Gold and Platinum Nanostructures. Electrochim. Acta 2019, 306, 299–306. [Google Scholar] [CrossRef]
- Sharafeldin, M.; McCaffrey, K.; Rusling, J.F. Influence of Antibody Immobilization Strategy on Carbon Electrode Immunoarrays. Analyst 2019, 144, 5108–5116. [Google Scholar] [CrossRef] [PubMed]
- Patro, S.G.K.; Sahu, K.K. Normalization: A Preprocessing Stage. IARJSET 2015, 2, 20–22. [Google Scholar] [CrossRef]
- Teng, C.M. Data, Data, Everywhere. In Philosophy of Statistics; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1099–1117. [Google Scholar]
- Ministério da Saúde Epidemiological Report—Syphilis. 2021. Available online: http://www.aids.gov.br/pt-br/pub/2021/boletim-epidemiologico-de-sifilis-2021 (accessed on 10 February 2022).
- European Centre for Disease Prevention and Control Syphilis—Annual Epidemiological Report for 2018; ECDC: Stockholm, Sweden, 2020.
- Spicknall, I.H.; Kreisel, K.M.; Weinstock, H.S. Estimates of the Prevalence and Incidence of Syphilis in the United States, 2018. Sex. Transm. Dis. 2021, 48, 247–252. [Google Scholar] [CrossRef] [PubMed]

| Sample ID | Serological Status | Incubation Time | Reading Results | |||||
|---|---|---|---|---|---|---|---|---|
| SPC Electrodes | SPC-GN Electrodes | |||||||
| Ch1 | Ch2 | Ch3 | Ch1 | Ch2 | Ch3 | |||
| #1 | Reagent (1:16) | T0′ | 0.0073023 | 0.0048307 | 0.0046663 | 0.0085546 | 0.0058676 | 0.0050748 |
| T15′ | 0.007248 | 0.0044141 | 0.0046389 | 0.0102268 | 0.0075225 | 0.0078614 | ||
| #2 | Reagent (1:8) | T0′ | 0.0066464 | 0.0048928 | 0.0046928 | 0.0066712 | 0.0064598 | 0.0058732 |
| T15′ | 0.0070614 | 0.0055667 | 0.0049399 | 0.0082176 | 0.0067742 | 0.0072676 | ||
| #3 | Reagent (1:8) | T0′ | 0.0062958 | 0.0045392 | 0.0042912 | 0.0102056 | 0.0062748 | 0.0080925 |
| T15′ | 0.0075369 | 0.0054023 | 0.0050748 | 0.0111369 | 0.0069585 | 0.009382 | ||
| #4 | Reagent (1:8) | T0′ | 0.0064392 | 0.0054418 | 0.0049408 | 0.0064092 | 0.0055343 | 0.0042261 |
| T15′ | 0.0065085 | 0.0058232 | 0.0048578 | 0.0079186 | 0.0073196 | 0.0051402 | ||
| #5 | Reagent (1:32) | T0′ | 0.0066752 | 0.0050833 | 0.0050637 | 0.0054958 | 0.0049605 | 0.0063 |
| T15′ | 0.0065742 | 0.0048722 | 0.0047098 | 0.0062386 | 0.0069281 | 0.0084974 | ||
| #6 | Not reagent | T0′ | 0.0052951 | 0.0038428 | 0.0033974 | 0.0045085 | 0.0036376 | 0.0030425 |
| T15′ | 0.0052376 | 0.0050915 | 0.0033176 | 0.0044732 | 0.0041791 | 0.0026961 | ||
| #7 | Not reagent | T0′ | 0.0059196 | 0.0045167 | 0.0036095 | 0.0047245 | 0.0034605 | 0.0031814 |
| T15′ | 0.0056761 | 0.0044562 | 0.0034755 | 0.0051882 | 0.0044399 | 0.0030239 | ||
| #8 | Not reagent | T0′ | 0.0063853 | 0.0032065 | 0.0037765 | - | - | - |
| T15′ | 0.0065418 | 0.0030444 | 0.0034376 | - | - | - | ||
| #9 | Not reagent | T0′ | 0.0042196 | 0.0041471 | 0.0039121 | - | - | - |
| T15′ | 0.0042706 | 0.0039232 | 0.0037206 | - | - | - | ||
| #10 | Not reagent | T0′ | 0.0050824 | 0.0043886 | 0.0037699 | - | - | - |
| T15′ | 0.0048477 | 0.0041954 | 0.0035725 | - | - | - | ||
| Sample ID | Serological Status | Incubation Time | Reading Results | |||||
|---|---|---|---|---|---|---|---|---|
| SPC Electrodes | SPC-GN Electrodes | |||||||
| Ch1 | Ch2 | Ch3 | Ch1 | Ch2 | Ch3 | |||
| #1 | Reagent (1:16) | T0′ | 0.0061614 | 0.0050206 | 0.0054392 | 0.0053752 | 0.0115748 | 0.0060915 |
| T15′ | 0.006133 | 0.0048654 | 0.0058974 | 0.0051804 | 0.0081693 | 0.0063118 | ||
| #2 | Reagent (1:8) | T0′ | 0.0071621 | 0.0045608 | 0.0055788 | 0.0179023 | 0.0073248 | 0.0043092 |
| T15′ | 0.0067964 | 0.0051062 | 0.0057716 | 0.0109739 | 0.0072356 | 0.0042765 | ||
| #3 | Reagent (1:8) | T0′ | 0.0062062 | 0.0042696 | 0.0054667 | 0.0073343 | 0.0064624 | 0.0062797 |
| T15′ | 0.0058938 | 0.004919 | 0.0060235 | 0.0074324 | 0.0066493 | 0.0089889 | ||
| #4 | Reagent (1:8) | T0′ | 0.0051379 | 0.0038879 | 0.0050614 | 0.012567 | 0.0063592 | 0.0050092 |
| T15′ | 0.0050918 | 0.0038203 | 0.0062732 | 0.0142846 | 0.0078542 | 0.0063647 | ||
| #5 | Reagent (1:32) | T0′ | 0.0067141 | 0.0051461 | 0.0058124 | 0.0084686 | 0.0091882 | 0.0046758 |
| T15′ | 0.006716 | 0.0051173 | 0.0047245 | 0.0113376 | 0.0095065 | 0.0049608 | ||
| #6 | Not reagent | T0′ | 0.0076271 | 0.0055431 | 0.0038944 | 0.0054523 | 0.0037611 | 0.0037 |
| T15′ | 0.0073258 | 0.0037948 | 0.0035039 | 0.0052284 | 0.0038824 | 0.0034193 | ||
| #7 | Not reagent | T0′ | 0.0084239 | 0.0046605 | 0.0046275 | 0.0046131 | 0.0042807 | 0.0040147 |
| T15′ | 0.0108144 | 0.0045082 | 0.0045366 | 0.0048908 | 0.0048435 | 0.0049637 | ||
| #8 | Not reagent | T0′ | 0.0081389 | 0.0048268 | 0.0045644 | - | - | - |
| T15′ | 0.0087944 | 0.0046984 | 0.0041039 | - | - | - | ||
| #9 | Not reagent | T0′ | 0.0063222 | 0.0043167 | 0.0042317 | - | - | - |
| T15′ | 0.0057637 | 0.0044886 | 0.0040026 | - | - | - | ||
| #10 | Not reagent | T0′ | 0.0077984 | 0.004785 | 0.0040369 | - | - | - |
| T15′ | 0.0083092 | 0.0047706 | 0.0041108 | - | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, G.M.C.; Carvalho, D.D.A.; Cruz, A.S.; Morais, E.K.L.; Sales-Moioli, A.I.L.; Pinto, T.K.B.; Almeida, M.C.D.; Sanchez-Gendriz, I.; Fernandes, F.; Barbalho, I.M.P.; et al. Development of a Cyclic Voltammetry-Based Method for the Detection of Antigens and Antibodies as a Novel Strategy for Syphilis Diagnosis. Int. J. Environ. Res. Public Health 2022, 19, 16206. https://doi.org/10.3390/ijerph192316206
Barros GMC, Carvalho DDA, Cruz AS, Morais EKL, Sales-Moioli AIL, Pinto TKB, Almeida MCD, Sanchez-Gendriz I, Fernandes F, Barbalho IMP, et al. Development of a Cyclic Voltammetry-Based Method for the Detection of Antigens and Antibodies as a Novel Strategy for Syphilis Diagnosis. International Journal of Environmental Research and Public Health. 2022; 19(23):16206. https://doi.org/10.3390/ijerph192316206
Chicago/Turabian StyleBarros, Gabriel M. C., Dionísio D. A. Carvalho, Agnaldo S. Cruz, Ellen K. L. Morais, Ana Isabela L. Sales-Moioli, Talita K. B. Pinto, Melise C. D. Almeida, Ignacio Sanchez-Gendriz, Felipe Fernandes, Ingridy M. P. Barbalho, and et al. 2022. "Development of a Cyclic Voltammetry-Based Method for the Detection of Antigens and Antibodies as a Novel Strategy for Syphilis Diagnosis" International Journal of Environmental Research and Public Health 19, no. 23: 16206. https://doi.org/10.3390/ijerph192316206
APA StyleBarros, G. M. C., Carvalho, D. D. A., Cruz, A. S., Morais, E. K. L., Sales-Moioli, A. I. L., Pinto, T. K. B., Almeida, M. C. D., Sanchez-Gendriz, I., Fernandes, F., Barbalho, I. M. P., Santos, J. P. Q., Henriques, J. M. O., Teixeira, C. A. D., Gil, P., Gama, L., Miranda, A. E., Coutinho, K. D., Galvão-Lima, L. J., & Valentim, R. A. M. (2022). Development of a Cyclic Voltammetry-Based Method for the Detection of Antigens and Antibodies as a Novel Strategy for Syphilis Diagnosis. International Journal of Environmental Research and Public Health, 19(23), 16206. https://doi.org/10.3390/ijerph192316206
















