Immobilization of Pb and Zn in Contaminated Soil Using Alumina–Silica Nano-Amendments Synthesized from Coal Fly Ash
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Synthesis Experiment of an ASNA Using Coal Fly Ash
2.3. The Immobilization Experiment of Heavy Metals in the Soil
2.4. Analytical Methods
2.5. Quality Control and Data Analysis
3. Results and Discussion
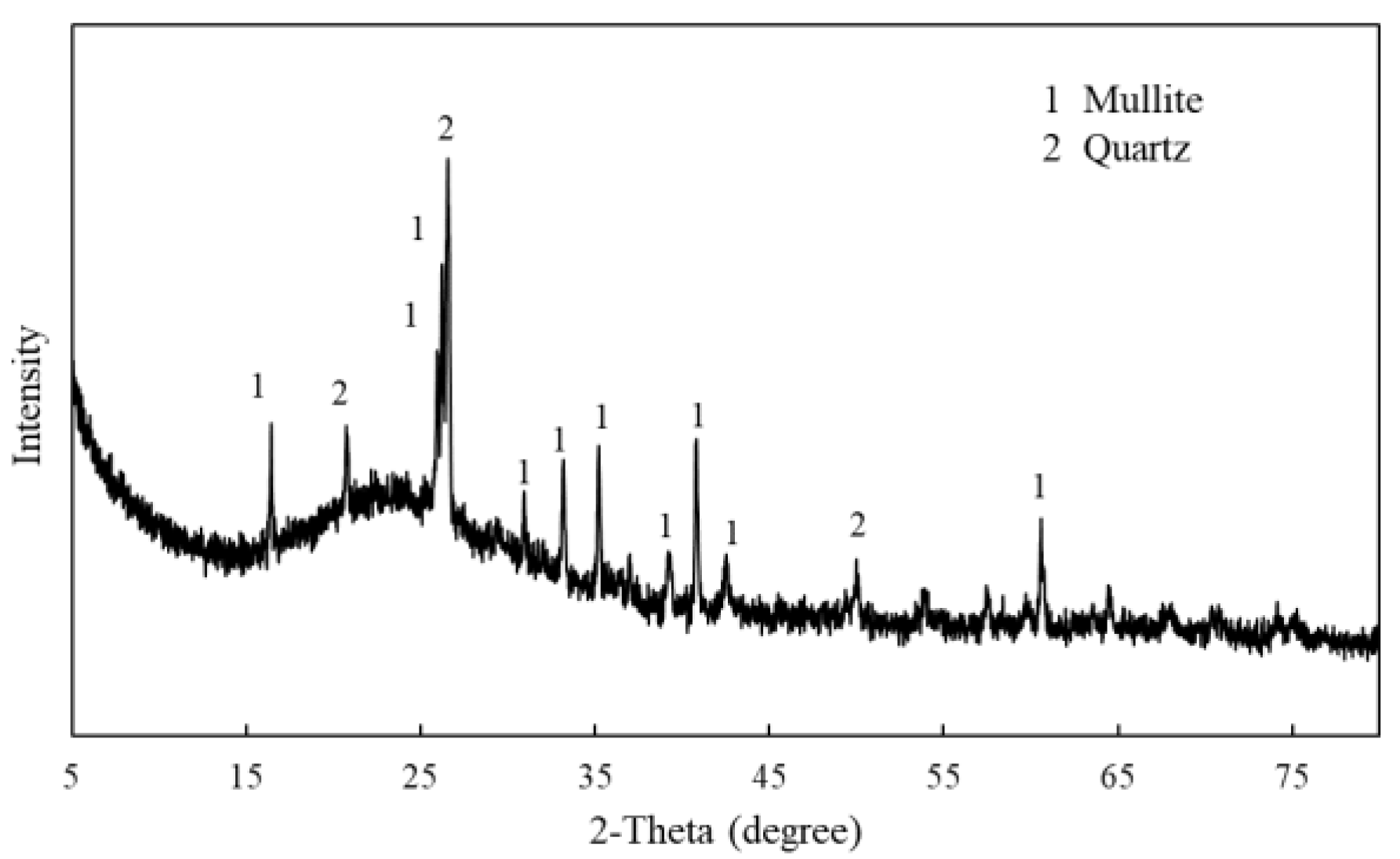
3.1. Characteristics of Coal Fly Ash
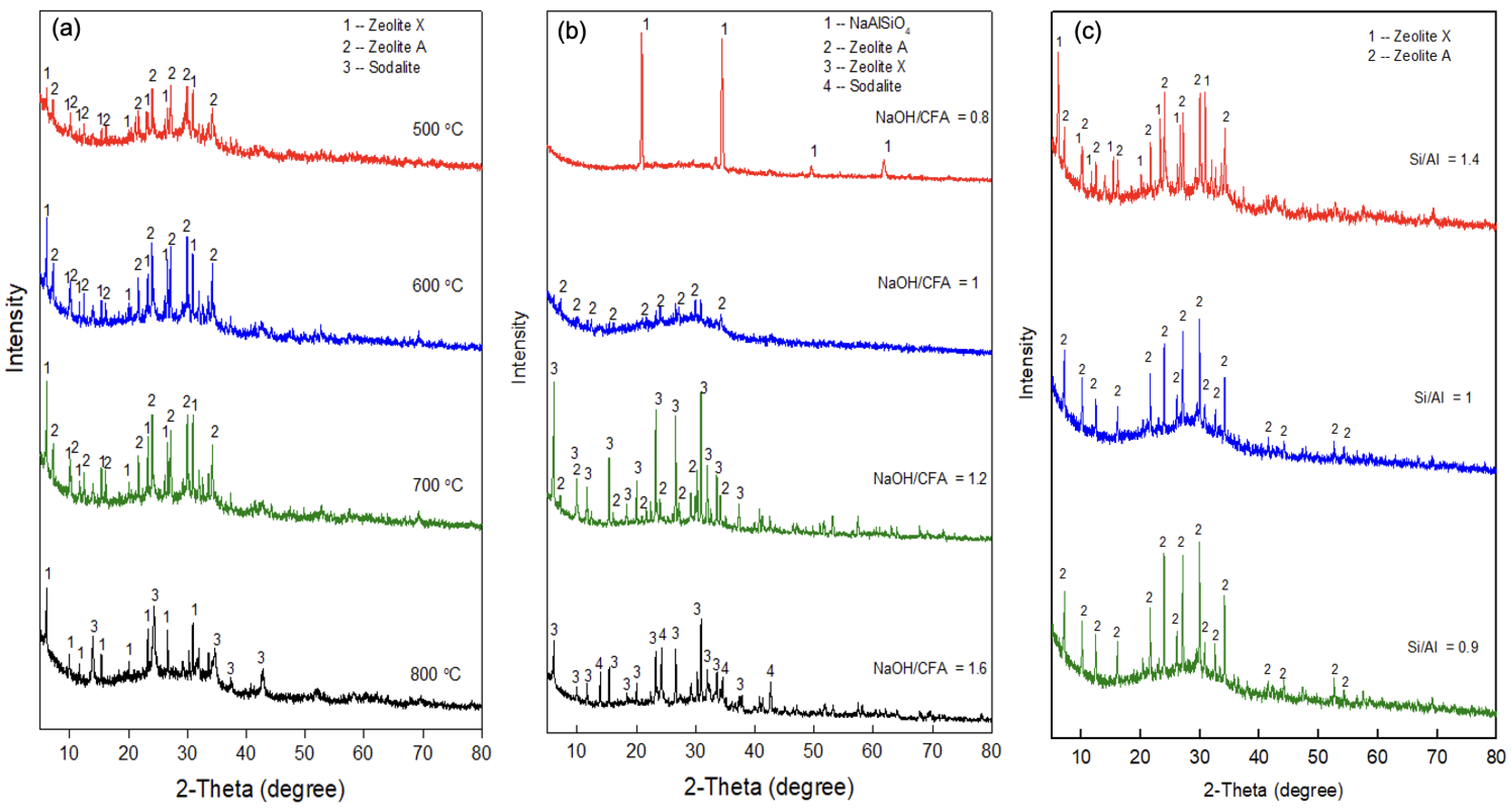
3.2. The Synthesis of the ASNA Using Coal Fly Ash
3.3. The Effect of the ASNA on the Immobilization of Pb and Zn in Contaminated Soil
3.3.1. The Changes in the Soil’s Physical and Chemical Properties
3.3.2. The Changes in the Bioavailability of Pb and Zn in the Soil
3.3.3. The Changes in the Chemical Speciation
3.4. Immobilization Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xu, D.M.; Yan, B.; Chen, T.; Lei, C.; Lin, H.Z.; Xiao, X.M. Contaminant Characteristics and Environmental Risk Assessment of Heavy Metals in the Paddy Soils from Lead (Pb)-Zinc (Zn) Mining Areas in Guangdong Province, South China. Environ. Sci. Pollut. Res. 2017, 24, 24387–24399. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Lei, C.; Yan, B.; Li, L.L.; Xu, D.M.; Ying, G.G. Spatial Distribution and Environmental Implications of Heavy Metals in Typical Lead (Pb)-Zinc (Zn) Mine Tailings Impoundments in Guangdong Province, South China. Environ. Sci. Pollut. Res. 2018, 25, 36702–36711. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Chen, T.; Zhang, Q.Y.; Long, L.S.; Chen, Z.; Fu, Z.P. Remediation of Lead Polluted Soil by Active Silicate Material Prepared from Coal Fly Ash. Ecotoxicol. Environ. Saf. 2020, 206, 111409. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Chen, L.; Ai, S. Speciation of Heavy Metals in Soils and Their Immobilization at Micro-Scale Interfaces among Diverse Soil Components. Sci. Total Environ. 2022, 825, 153862. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Q.; Du, W.; Lin, R.; Li, J.; Ai, F.; Yin, Y.; Ji, R.; Wang, X.; Guo, H. In-Situ Immobilization of Cadmium-Polluted Upland Soil: A Ten-Year Field Study. Ecotoxicol. Environ. Saf. 2021, 207, 111275. [Google Scholar] [CrossRef]
- Guo, F.; Ding, C.; Zhou, Z.; Huang, G.; Wang, X. Stability of Immobilization Remediation of Several Amendments on Cadmium Contaminated Soils as Affected by Simulated Soil Acidification. Ecotoxicol. Environ. Saf. 2018, 161, 164–172. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Li, R.; Zhang, Z. Immobilization of Lead and Cadmium in Contaminated Soil Using Amendments: A Review. Pedosphere 2015, 25, 555–568. [Google Scholar] [CrossRef]
- Du, Y.; Wang, X.; Ji, X.; Zhang, Z.; Saha, U.K.; Xie, W.; Xie, Y.; Wu, J.; Peng, B.; Tan, C. Effectiveness and Potential Risk of CaO Application in Cd-Contaminated Paddy Soil. Chemosphere 2018, 204, 130–139. [Google Scholar] [CrossRef]
- Cui, H.; Shen, L.; Yang, X.; Zhang, S.; Yi, Q.; Meng, L.; Zheng, X.; Wang, Q.; Zhou, J. Effects of Hematite on the Stabilization of Copper, Cadmium and Phosphorus in a Contaminated Red Soil Amended with Hydroxyapatite. Ecotoxicol. Environ. Saf. 2020, 201, 110830. [Google Scholar] [CrossRef]
- Kosolsaksakul, P.; Oliver, I.W.; Graham, M.C. Evaluating Cadmium Bioavailability in Contaminated Rice Paddy Soils and Assessing Potential for Contaminant Immobilisation with Biochar. J. Environ. Manag. 2018, 215, 49–56. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Liu, L.; Ulhassan, Z.; He, Z.; Yang, X. Sepiolite Clay: A Review of Its Applications to Immobilize Toxic Metals in Contaminated Soils and Its Implications in Soil–Plant System. Environ. Technol. Innov. 2021, 23, 101598. [Google Scholar] [CrossRef]
- Lv, G.; Yang, T.; Chen, Y.; Hou, H.; Liu, X.; Li, J.; Wei, L.; Li, J.-H. Biochar-Based Fertilizer Enhanced Cd Immobilization and Soil Quality in Soil-Rice System. Ecol. Eng. 2021, 171, 106396. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Lee, S.-E.; Lee, Y.H.; Tsang, D.C.W.; Rinklebe, J.; Kwon, E.E.; Ok, Y.S. Heavy Metal Immobilization and Microbial Community Abundance by Vegetable Waste and Pine Cone Biochar of Agricultural Soils. Chemosphere 2017, 174, 593–603. [Google Scholar] [CrossRef]
- Xu, D.M.; Fu, R.B.; Wang, J.X.; Shi, Y.X.; Guo, X.P. Chemical Stabilization Remediation for Heavy Metals in Contaminated Soils on the Latest Decade: Available Stabilizing Materials and Associated Evaluation Methods-A Critical Review. J. Clean. Prod. 2021, 321, 128730. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, L.; Li, Y.; Zha, J.; Tian, G.; Wang, F.; Zhang, H.; Liang, J. Synthesis of Zeolites by In-Situ Conversion of Geopolymers and Their Performance of Heavy Metal Ion Removal in Wastewater: A Review. J. Clean. Prod. 2022, 349, 131441. [Google Scholar] [CrossRef]
- Medykowska, M.; Wiśniewska, M.; Szewczuk-Karpisz, K.; Panek, R. Interaction Mechanism of Heavy Metal Ions with the Nanostructured Zeolites Surface—Adsorption, Electrokinetic and XPS Studies. J. Mol. Liq. 2022, 357, 119144. [Google Scholar] [CrossRef]
- Hudcová, B.; Osacký, M.; Vítková, M.; Mitzia, A.; Komárek, M. Investigation of Zinc Binding Properties onto Natural and Synthetic Zeolites: Implications for Soil Remediation. Microporous Mesoporous Mater. 2021, 317, 111022. [Google Scholar] [CrossRef]
- Wang, W.; Lu, T.; Liu, L.; Yang, X.; Sun, X.; Qiu, G.; Hua, D.; Zhou, D. Zeolite-Supported Manganese Oxides Decrease the Cd Uptake of Wheat Plants in Cd-Contaminated Weakly Alkaline Arable Soils. J. Hazard. Mater. 2021, 419, 126464. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cheng, L.; Zhang, D.; Zhang, F.; Zhou, S.; Ma, Y.; Guo, J.; Zhang, Y.; Xing, B. Stabilization of Pb, Cd, and Zn in Soil by Modified-Zeolite: Mechanisms and Evaluation of Effectiveness. Sci. Total Environ. 2022, 814, 152746. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, H.; Sun, Z.; Ma, E. New Technology and Application of Brick Making with Coal Fly Ash. J. Mater. Cycles Waste Manag. 2016, 18, 763–770. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Y.; Ma, S.; Zheng, S.; Zhang, Y.; Chu, P.K. Utilization of Coal Fly Ash in China: A Mini-Review on Challenges and Future Directions. Environ. Sci. Pollut. Res. 2021, 28, 18727–18740. [Google Scholar] [CrossRef] [PubMed]
- Kotova, O.B.; Shabalin, I.N.; Shushkov, D.A.; Kocheva, L.S. Hydrothermal Synthesis of Zeolites from Coal Fly Ash. Adv. Appl. Ceram. 2016, 115, 152–157. [Google Scholar] [CrossRef]
- Czuma, N.; Baran, P.; Franus, W.; Zabierowski, P.; Zarębska, K. Synthesis of Zeolites from Fly Ash with the Use of Modified Two-Step Hydrothermal Method and Preliminary SO2 Sorption Tests. Adsorpt. Sci. Technol. 2019, 37, 61–76. [Google Scholar] [CrossRef]
- Makgabutlane, B.; Nthunya, L.N.; Musyoka, N.; Dladla, B.S.; Nxumalo, E.N.; Mhlanga, S.D. Microwave-Assisted Synthesis of Coal Fly Ash-Based Zeolites for Removal of Ammonium from Urine. RSC Adv. 2020, 10, 2416–2427. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, C.; Zhang, H.; Zhang, X.; Wei, Y.; Jiang, K.; Zhang, B. Silicalite-1 Zeolite Membrane: Synthesis by Seed Method and Application in Organics Removal. Chemosphere 2019, 218, 984–991. [Google Scholar] [CrossRef]
- Shi, B.; Chang, Q. Green Synthesis of Fly Ash-based Zeolite Y by Mixed Alkali Fusion Method. Micro Nano Lett. 2021, 16, 540–545. [Google Scholar] [CrossRef]
- MEEPRC. Soil Environmental Quality–Risk Control Standard for Soil Contamination of Agricultural Land (GB15618-2018); Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018. [Google Scholar]
- Yu, J.; He, W.; Liu, B. Adsorption of Acid Orange Ⅱ with Two Step Modified Sepiolite: Optimization, Adsorption Performance, Kinetics, Thermodynamics and Regeneration. IJERPH 2020, 17, 1732. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Q.; Li, J.; Zhao, S.; Qie, M.; Wu, X.; Bai, Y.; Zhao, Y. Study on the Origin Traceability of Tibet Highland Barley (Hordeum Vulgare L.) Based on Its Nutrients and Mineral Elements. Food Chem. 2021, 346, 128928. [Google Scholar] [CrossRef]
- Nemati, K.; Bakar, N.K.A.; Abas, M.R.; Sobhanzadeh, E. Speciation of Heavy Metals by Modified BCR Sequential Extraction Procedure in Different Depths of Sediments from Sungai Buloh, Selangor, Malaysia. J. Hazard. Mater. 2011, 192, 402–410. [Google Scholar] [CrossRef]
- Pueyo, M.; López-Sánchez, J.F.; Rauret, G. Assessment of CaCl2, NaNO3 and NH4NO3 Extraction Procedures for the Study of Cd, Cu, Pb and Zn Extractability in Contaminated Soils. Anal. Chim. Acta 2004, 504, 217–226. [Google Scholar] [CrossRef]
- Feng, M.-H.; Shan, X.-Q.; Zhang, S.; Wen, B. A Comparison of the Rhizosphere-Based Method with DTPA, EDTA, CaCl2, and NaNO3 Extraction Methods for Prediction of Bioavailability of Metals in Soil to Barley. Environ. Pollut. 2005, 137, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Kede, M.L.F.M.; Correia, F.V.; Conceição, P.F.; Junior, S.F.S.; Marques, M.; Moreira, J.C.; Pérez, D.V. Evaluation of Mobility, Bioavailability and Toxicity of Pb and Cd in Contaminated Soil Using TCLP, BCR and Earthworms. Int. J. Environ. Res. Public Health 2014, 11, 11528–11540. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, H.; Naghdali, Z.; Ghaffari Kashani, T.; Farhadi, F. Conversion of High Silicon Fly Ash to Na-P1 Zeolite: Alkaline Fusion Followed by Hydrothermal Crystallization. Adv. Powder Technol. 2010, 21, 279–283. [Google Scholar] [CrossRef]
- Yaping, Y.; Xiaoqiang, Z.; Weilan, Q.; Mingwen, W. Synthesis of Pure Zeolites from Supersaturated Silicon and Aluminum Alkali Extracts from Fused Coal Fly Ash. Fuel 2008, 87, 1880–1886. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jiménez, A.; Palomo, A.; Sobrados, I.; Sanz, J. Effect of the SiO2/Na2O Ratio on the Alkali Activation of Fly Ash. Part II: 29Si MAS-NMR Survey. Microporous Mesoporous Mater. 2008, 109, 525–534. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A Review on Conventional and Novel Materials towards Heavy Metal Adsorption in Wastewater Treatment Application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Manrique, L.A.; Jones, C.A.; Dyke, P.T. Predicting Cation-Exchange Capacity from Soil Physical and Chemical Properties. Soil Sci. Soc. Am. J. 1991, 55, 787–794. [Google Scholar] [CrossRef]
- Cui, X.; Mao, P.; Sun, S.; Huang, R.; Fan, Y.; Li, Y.; Li, Y.; Zhuang, P.; Li, Z. Phytoremediation of Cadmium Contaminated Soils by Amaranthus Hypochondriacus L.: The Effects of Soil Properties Highlighting Cation Exchange Capacity. Chemosphere 2021, 283, 131067. [Google Scholar] [CrossRef]



| Components | SiO2 | Al2O3 | CaO | Fe2O3 | K2O | TiO2 | MgO | Na2O | SO3 | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| Wt% | 54.9 | 33.5 | 4.15 | 2.48 | 1.98 | 1.09 | 0.748 | 0.465 | 0.405 | 0.362 |
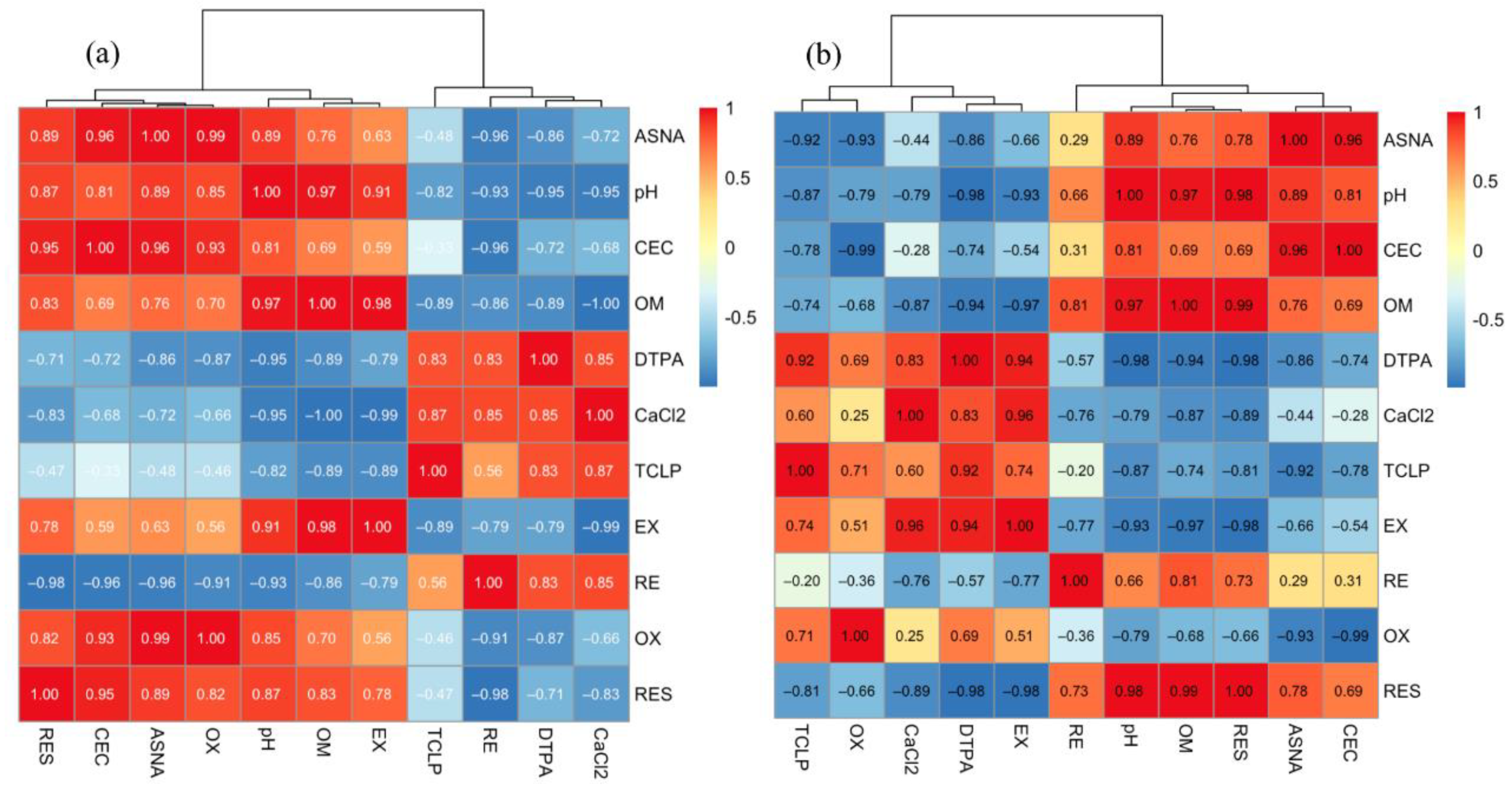
| ASNA | pH | CEC | OM | DTPA | CaCl2 | TCLP | EX | RE | OX | RES | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ASNA | 1 | ||||||||||
| pH | 0.885 | 1 | |||||||||
| CEC | 0.964 * | 0.814 | 1 | ||||||||
| OM | 0.755 | 0.971 * | 0.694 | 1 | |||||||
| DTPA | −0.863 | −0.950 * | −0.720 | −0.886 | 1 | ||||||
| CaCl2 | −0.724 | −0.954 * | −0.679 | −0.997 ** | 0.847 | 1 | |||||
| TCLP | −0.484 | −0.817 | −0.329 | −0.886 | 0.833 | 0.872 | 1 | ||||
| EX | 0.629 | 0.908 | 0.588 | 0.981 * | −0.788 | −0.992 ** | −0.886 | 1 | |||
| RE | −0.956 * | −0.934 | −0.961 * | −0.865 | 0.831 | 0.854 | 0.562 | −0.787 | 1 | ||
| OX | 0.992 ** | 0.851 | 0.934 | 0.702 | −0.866 | −0.661 | −0.459 | 0.558 | −0.912 | 1 | |
| RES | 0.889 | 0.871 | 0.946 | 0.826 | −0.710 | −0.831 | −0.473 | 0.775 | −0.979 * | 0.824 | 1 |
| ASNA | pH | CEC | OM | DTPA | CaCl2 | TCLP | EX | RE | OX | RES | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ASNA | 1 | ||||||||||
| pH | 0.885 | 1 | |||||||||
| CEC | 0.964 * | 0.814 | 1 | ||||||||
| OM | 0.755 | 0.971 * | 0.694 | 1 | |||||||
| DTPA | −0.859 | −0.980 * | −0.738 | −0.938 | 1 | ||||||
| CaCl2 | −0.438 | −0.789 | −0.284 | −0.869 | 0.834 | 1 | |||||
| TCLP | −0.916 | −0.865 | −0.777 | −0.736 | 0.919 | 0.602 | 1 | ||||
| EX | −0.660 | −0.929 | −0.540 | −0.969 * | 0.942 | 0.960 * | 0.741 | 1 | |||
| RE | 0.291 | 0.657 | 0.314 | 0.813 | −0.569 | −0.755 | −0.204 | −0.772 | 1 | ||
| OX | −0.930 | −0.788 | −0.993 ** | −0.684 | 0.691 | 0.250 | 0.705 | 0.511 | −0.359 | 1 | |
| RES | 0.782 | 0.981 * | 0.686 | 0.989 * | −0.976 * | −0.892 | −0.812 | −0.983 * | 0.734 | −0.659 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, C.; Huang, H.; Ye, H.; Fu, Z.; Peng, P.; Zhang, S.; Long, L. Immobilization of Pb and Zn in Contaminated Soil Using Alumina–Silica Nano-Amendments Synthesized from Coal Fly Ash. Int. J. Environ. Res. Public Health 2022, 19, 16204. https://doi.org/10.3390/ijerph192316204
Lei C, Huang H, Ye H, Fu Z, Peng P, Zhang S, Long L. Immobilization of Pb and Zn in Contaminated Soil Using Alumina–Silica Nano-Amendments Synthesized from Coal Fly Ash. International Journal of Environmental Research and Public Health. 2022; 19(23):16204. https://doi.org/10.3390/ijerph192316204
Chicago/Turabian StyleLei, Chang, Hao Huang, Haoxin Ye, Zhiping Fu, Peipei Peng, Shaoqing Zhang, and Laishou Long. 2022. "Immobilization of Pb and Zn in Contaminated Soil Using Alumina–Silica Nano-Amendments Synthesized from Coal Fly Ash" International Journal of Environmental Research and Public Health 19, no. 23: 16204. https://doi.org/10.3390/ijerph192316204
APA StyleLei, C., Huang, H., Ye, H., Fu, Z., Peng, P., Zhang, S., & Long, L. (2022). Immobilization of Pb and Zn in Contaminated Soil Using Alumina–Silica Nano-Amendments Synthesized from Coal Fly Ash. International Journal of Environmental Research and Public Health, 19(23), 16204. https://doi.org/10.3390/ijerph192316204







