Open Questions on the Electromagnetic Field Contribution to the Risk of Neurodegenerative Diseases
Abstract
1. Introduction
2. Neurodegenerative Diseases (NDD)
| Disease | Main Neuropathology | Symptoms |
|---|---|---|
| Alzheimer’s disease (AD) | Beta-amyloid deposits and neurofibrillary tangles in the cerebral cortex and subcortical grey matter |
|
| Parkinson’s disease (PD) | Loss of neurons that produce dopamine–a chemical messenger in the brain |
|
| Amyotrophic lateral sclerosis (ALS) | Loss of neurons in the motor cortex (upper motor neurons) and motor neurons in the brain stem and central spinal cord (lower motor neurons) |
|
| Multiple sclerosis (MS) | Inflammatory demyelinating processes in the brain and spinal cord (CNS) |
|
3. Electromagnetic Field and Neurodegenerative Diseases
3.1. Earlier Epidemiological Studies
3.2. Laboratory Experiments
4. Evidence for Accelerating Neurological Mortality (NM) 2000–2015
5. Discussion
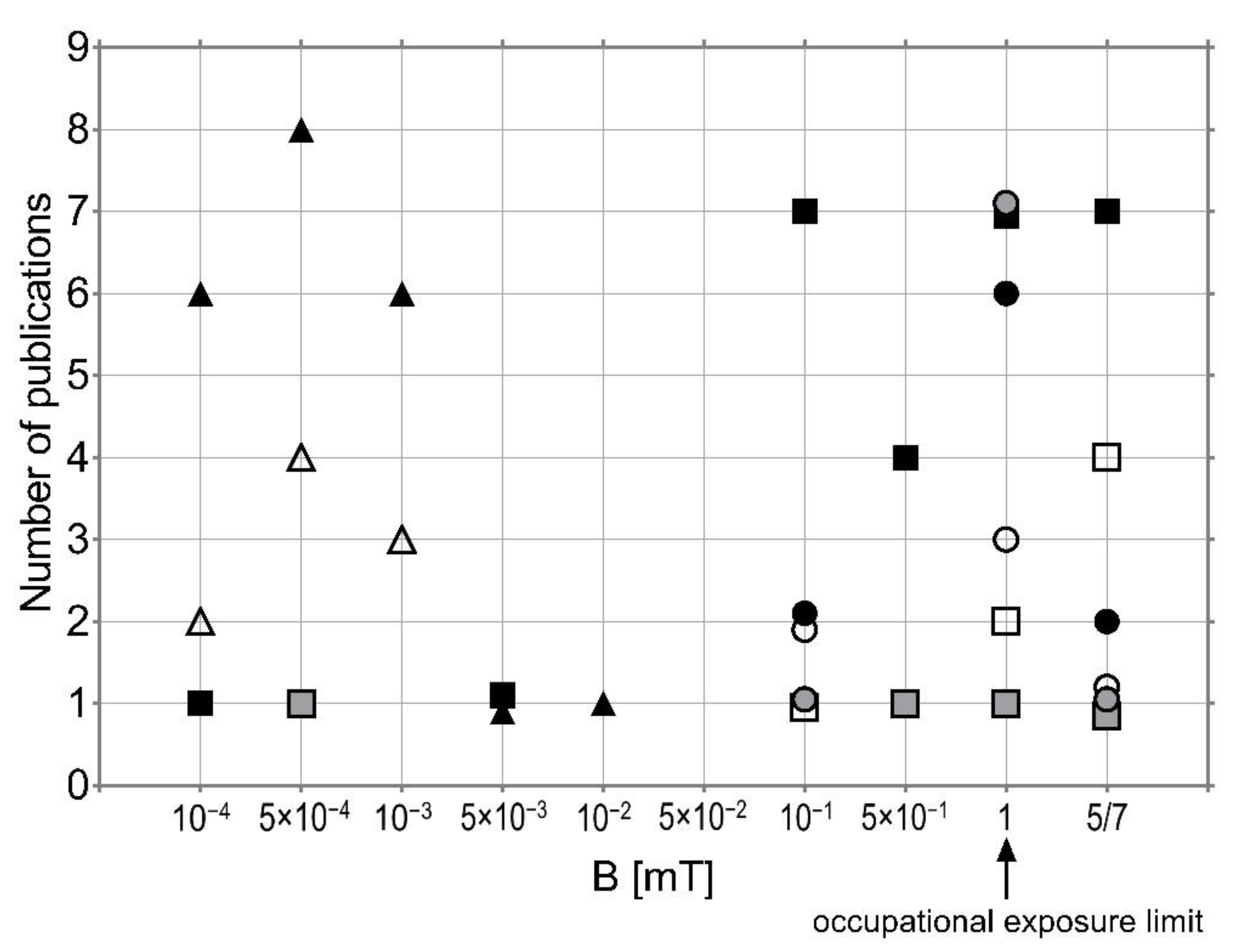
6. Conclusions
- Studies investigating the possible effects of EMF exposure on NDD are too diverse with regard to applied EMF, the duration of exposure, and statistical methods to draw any reasonable and satisfactory conclusion.
- The difficulties with the identification and experimental validation of the EMF influence mechanism are due to the variability of biological responses and a lack of consistency in the findings.
- There are a number of significant factors besides EMF influencing NDD, such as age, a low level or lack of education, or serious or repeated minor head injuries, and various toxic environmental and occupational agents (including such things as solvents, pesticides, and toxic metals).
- EMF may interact with other multiple environmental pollutants and/or occupational factors.
- EMF may have a beneficial impact as a mild stress factor inducing protection against various stressors or, on the contrary, may disturb the stress response of cells, leading to oxidative damage and functional impairment
- There is no concrete evidence of the positive or negative effects of EMF, however, research should still be carried out in this field, so as not to overlook such a risk factor.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Terzi, M.; Ozberk, B.; Deniz, O.G.; Kaplan, S. The Role of Electromagnetic Fields in Neurological Disorders. J. Chem. Neuroanat. 2016, 75, 77–84. [Google Scholar] [CrossRef]
- Mattsson, M.-O.; Simkó, M. Is There a Relation between Extremely Low Frequency Magnetic Field Exposure, Inflammation and Neurodegenerative Diseases? A Review of in Vivo and in Vitro Experimental Evidence. Toxicology 2012, 301, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lai, H. Neurological Effects of Non-Ionizing Electromagnetic Fields. The Bioinitiative Report 2012. 2012. Available online: http://www.bioinitiative.org (accessed on 3 October 2022).
- Saliev, T.; Begimbetova, D.; Masoud, A.-R.; Matkarimov, B. Biological Effects of Non-Ionizing Electromagnetic Fields: Two Sides of a Coin. Prog. Biophys. Mol. Biol. 2019, 141, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowska, J.; Jankowska, M.; Gas, P. Electromagnetic Fields and Neurodegenerative Diseases. Przegląd Elektrotechniczny 2019, 1, 129–133. [Google Scholar] [CrossRef]
- Hosseinabadi, M.B.; Khanjani, N.; Ebrahimi, M.H.; Haji, B.; Abdolahfard, M. The Effect of Chronic Exposure to Extremely Low-Frequency Electromagnetic Fields on Sleep Quality, Stress, Depression and Anxiety. Electromagn. Biol. Med. 2019, 38, 96–101. [Google Scholar] [CrossRef]
- Touitou, Y.; Selmaoui, B.; Lambrozo, J. Assessment of Cortisol Secretory Pattern in Workers Chronically Exposed to ELF-EMF Generated by High Voltage Transmission Lines and Substations. Environ. Int. 2022, 161, 107103. [Google Scholar] [CrossRef] [PubMed]
- Touitou, Y.; Lambrozo, J.; Mauvieux, B.; Riedel, M. Evaluation in Humans of ELF-EMF Exposure on Chromogranin A, a Marker of Neuroendocrine Tumors and Stress. Chronobiol. Int. 2020, 37, 60–67. [Google Scholar] [CrossRef]
- Reilly, J.P. Peripheral Nerve Stimulation by Induced Electric Currents: Exposure to Time-Varying Magnetic Fields. Med. Biol. Eng. Comput. 1989, 27, 101. [Google Scholar] [CrossRef]
- Madkan, A.; Blank, M.; Elson, E.; Chou, K.-C.; Geddis, M.S.; Goodman, R. Steps to the Clinic with ELF EMF. Nat. Sci. 2009, 1, 157–165. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Non-Ionizing Radiation, Part 2: Radiofrequency Electromagnetic Fields. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer (IARC): Lyon, France, 2013; Volume 102. Available online: http://monographs.iarc.fr/ENG/Monographs/vol102/ (accessed on 3 October 2022).
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Non-Ionizing Radiation, Part 1: Static and Extremely Low-Frequency (ELF) Electric and Magnetic Fields. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer (IARC): Lyon, France, 2002; Volume 80, Available online: http://monographs.iarc.fr/ENG/Monographs/vol80/ (accessed on 3 October 2022).
- Hazlewood, C.F.; Markov, M. Trigger Points and Systemic Effect for EMF Therapy. Environmentalist 2009, 29, 232–239. [Google Scholar] [CrossRef]
- Jankowska, M.; Pawlowska-Mainville, A.; Stankiewicz, M.; Rogalska, J.; Wyszkowska, J. Exposure to 50 Hz Electromagnetic Field Changes the Efficiency of the Scorpion Alpha Toxin. J. Venom. Anim. Toxins Trop. Dis. 2015, 21, 38. [Google Scholar] [CrossRef][Green Version]
- Wyszkowska, J.; Jędrzejewski, T.; Piotrowski, J.; Wojciechowska, A.; Stankiewicz, M.; Kozak, W. Evaluation of the Influence of in Vivo Exposure to Extremely Low-Frequency Magnetic Fields on the Plasma Levels of pro-Inflammatory Cytokines in Rats. Int. J. Radiat. Biol. 2018, 94, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Klimek, A.; Nowakowska, A.; Kletkiewicz, H.; Wyszkowska, J.; Maliszewska, J.; Jankowska, M.; Peplowski, L.; Rogalska, J. Bidirectional Effect of Repeated Exposure to Extremely Low-Frequency Electromagnetic Field (50 Hz) of 1 and 7 MT on Oxidative/Antioxidative Status in Rat’s Brain: The Prediction for the Vulnerability to Diseases. Oxidative Med. Cell. Longev. 2022, 2022, e1031211. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, C.; Costantini, E.; Kamal, M.A.; Reale, M. Experimental Model for ELF-EMF Exposure: Concern for Human Health. Saudi J. Biol. Sci. 2015, 22, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) Potential Health Effects of Exposure to Electromagnetic Fields (EMF). Available online: https://health.ec.europa.eu/publications/potential-health-effects-exposure-electromagnetic-fields-emf_en (accessed on 26 September 2022).
- Ahlbom, A. Neurodegenerative Diseases, Suicide and Depressive Symptoms in Relation to EMF. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2001, 22, S132–S143. [Google Scholar] [CrossRef]
- Riancho, J.; Sanchez de la Torre, J.R.; Paz-Fajardo, L.; Limia, C.; Santurtun, A.; Cifra, M.; Kourtidis, K.; Fdez-Arroyabe, P. The Role of Magnetic Fields in Neurodegenerative Diseases. Int. J. Biometeorol. 2021, 65, 107–117. [Google Scholar] [CrossRef]
- Filippini, T.; Hatch, E.E.; Vinceti, M. Residential Exposure to Electromagnetic Fields and Risk of Amyotrophic Lateral Sclerosis: A Dose–Response Meta-Analysis. Sci. Rep. 2021, 11, 11939. [Google Scholar] [CrossRef]
- Migliore, L.; Coppedè, F. Environmental-Induced Oxidative Stress in Neurodegenerative Disorders and Aging. Mutat. Res. Toxicol. Environ. Mutagen. 2009, 674, 73–84. [Google Scholar] [CrossRef]
- Reale, M.; Kamal, M.A.; Patruno, A.; Costantini, E.; D’Angelo, C.; Pesce, M.; Greig, N.H. Neuronal Cellular Responses to Extremely Low Frequency Electromagnetic Field Exposure: Implications Regarding Oxidative Stress and Neurodegeneration. PLoS ONE 2014, 9, e104973. [Google Scholar] [CrossRef]
- Pritchard, C.; Silk, A.; Hansen, L. Are Rises in Electro-Magnetic Field in the Human Environment, Interacting with Multiple Environmental Pollutions, the Tripping Point for Increases in Neurological Deaths in the Western World? Med. Hypotheses 2019, 127, 76–83. [Google Scholar] [CrossRef]
- Easton, D.M. Gompertzian Growth and Decay: A Powerful Descriptive Tool for Neuroscience. Physiol. Behav. 2005, 86, 407–414. [Google Scholar] [CrossRef] [PubMed]
- WHO. Mortality Database—WHO. Available online: https://www.who.int/data/data-collection-tools/who-mortality-database (accessed on 26 September 2022).
- Villarini, M.; Gambelunghe, A.; Giustarini, D.; Ambrosini, M.V.; Fatigoni, C.; Rossi, R.; Dominici, L.; Levorato, S.; Muzi, G.; Piobbico, D. No Evidence of DNA Damage by Co-Exposure to Extremely Low Frequency Magnetic Fields and Aluminum on Neuroblastoma Cell Lines. Mutat. Res. Toxicol. Environ. Mutagen. 2017, 823, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Zubko, O.; Gould, R.L.; Gay, H.C.; Cox, H.J.; Coulson, M.C.; Howard, R.J. Effects of Electromagnetic Fields Emitted by GSM Phones on Working Memory: A Meta-analysis. Int. J. Geriatr. Psychiatry 2017, 32, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, F.; Botarelli, E.; Mele, G.; Polo, L.; Zoncu, D.; Renati, P.; Sgarlata, C.; Rollone, M.; Ricevuti, G.; Maurizi, N.; et al. An Innovative Intervention for the Treatment of Cognitive Impairment–Emisymmetric Bilateral Stimulation Improves Cognitive Functions in Alzheimer’s Disease and Mild Cognitive Impairment: An Open-Label Study. Neuropsychiatr. Dis. Treat. 2015, 11, 2391–2404. [Google Scholar] [CrossRef]
- Cichoń, N.; Rzeźnicka, P.; Bijak, M.; Miller, E.; Miller, S.; Saluk, J. Extremely Low Frequency Electromagnetic Field Reduces Oxidative Stress during the Rehabilitation of Post-Acute Stroke Patients. Adv. Clin. Exp. Med. 2018, 27, 1285–1293. [Google Scholar] [CrossRef]
- WHO. Extremely Low Frequency Fields. Environmental Health Criteria Monograph No. 238. Available online: https://www.who.int/publications-detail-redirect/9789241572385 (accessed on 26 September 2022).
- Cannon, J.R.; Greenamyre, J.T. The Role of Environmental Exposures in Neurodegeneration and Neurodegenerative Diseases. Toxicol. Sci. 2011, 124, 225–250. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Alzheimer’s Disease Fact Sheet. Available online: https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet (accessed on 3 October 2018).
- Neuropathology. Available online: https://neuropathology-web.org/ (accessed on 27 September 2022).
- García, A.M.; Sisternas, A.; Hoyos, S.P. Occupational Exposure to Extremely Low Frequency Electric and Magnetic Fields and Alzheimer Disease: A Meta-Analysis. Int. J. Epidemiol. 2008, 37, 329–340. [Google Scholar] [CrossRef]
- Hug, K.; Röösli, M.; Rapp, R. Magnetic Field Exposure and Neurodegenerative Diseases–Recent Epidemiological Studies. Soz.-Präventivmedizin 2006, 51, 210–220. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, G.; Chen, C.; Yu, Y.; Xu, Z. Association between Extremely Low-Frequency Electromagnetic Fields Occupations and Amyotrophic Lateral Sclerosis: A Meta-Analysis. PLoS ONE 2012, 7, e48354. [Google Scholar] [CrossRef]
- Savitz, D.A.; Checkoway, H.; Loomis, D.P. Magnetic Field Exposure and Neurodegenerative Disease Mortality among Electric Utility Workers. Epidemiol. Camb. Mass. 1998, 9, 398–404. [Google Scholar] [CrossRef]
- Haakansson, N.; Gustavsson, P.; Johansen, C.; Floderus, B. Neurodegenerative Diseases in Welders and Other Workers Exposed to High Levels of Magnetic Fields. Epidemiology 2003, 14, 420–426. [Google Scholar] [CrossRef]
- Feychting, M.; Jonsson, F.; Pedersen, N.L.; Ahlbom, A. Occupational Magnetic Field Exposure and Neurodegenerative Disease. Epidemiology 2003, 14, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Huss, A.; Spoerri, A.; Egger, M.; Röösli, M.; Study, S.N.C. Residence near Power Lines and Mortality from Neurodegenerative Diseases: Longitudinal Study of the Swiss Population. Am. J. Epidemiol. 2009, 169, 167–175. [Google Scholar] [CrossRef]
- Andel, R.; Crowe, M.; Feychting, M.; Pedersen, N.L.; Fratiglioni, L.; Johansson, B.; Gatz, M. Work-Related Exposure to Extremely Low-Frequency Magnetic Fields and Dementia: Results from the Population-Based Study of Dementia in Swedish Twins. J. Gerontol. Ser. Biomed. Sci. Med. Sci. 2010, 65, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Davanipour, Z.; Tseng, C.-C.; Lee, P.-J.; Markides, K.S.; Sobel, E. Severe Cognitive Dysfunction and Occupational Extremely Low Frequency Magnetic Field Exposure among Elderly Mexican Americans. Br. J. Med. Med. Res. 2014, 4, 1641–1662. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.; Poulsen, A.H.; Rod, N.H.; Frei, P.; Hansen, J.; Grell, K.; Raaschou-Nielsen, O.; Schüz, J.; Johansen, C. Occupational Exposure to Extremely Low-Frequency Magnetic Fields and Risk for Central Nervous System Disease: An Update of a Danish Cohort Study among Utility Workers. Int. Arch. Occup. Environ. Health 2017, 90, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Johansen, C. Exposure to Electromagnetic Fields and Risk of Central Nervous System Disease in Utility Workers. Epidemiology 2000, 11, 539–543. [Google Scholar] [CrossRef]
- Parlett, L.E.; Bowman, J.D.; van Wijngaarden, E. Evaluation of Occupational Exposure to Magnetic Fields and Motor Neuron Disease Mortality in a Population-Based Cohort. J. Occup. Environ. Med. Coll. Occup. Environ. Med. 2011, 53, 1447. [Google Scholar] [CrossRef]
- Gunnarsson, L.-G.; Bodin, L. Occupational Exposures and Neurodegenerative Diseases—A Systematic Literature Review and Meta-Analyses. Int. J. Environ. Res. Public. Health 2019, 16, 337. [Google Scholar] [CrossRef]
- Frei, P.; Poulsen, A.H.; Mezei, G.; Pedersen, C.; Cronberg Salem, L.; Johansen, C.; Roosli, M.; Schuz, J. Residential Distance to High-Voltage Power Lines and Risk of Neurodegenerative Diseases: A Danish Population-Based Case-Control Study. Am. J. Epidemiol. 2013, 177, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, A.H.; Stenager, E.; Johansen, C.; Bentzen, J.; Friis, S.; Schüz, J. Mobile Phones and Multiple Sclerosis—A Nationwide Cohort Study in Denmark. PLoS ONE 2012, 7, e34453. [Google Scholar]
- Rollwitz, J.; Lupke, M.; Simkó, M. Fifty-Hertz Magnetic Fields Induce Free Radical Formation in Mouse Bone Marrow-Derived Promonocytes and Macrophages. Biochim. Biophys. Acta BBA-Gen. Subj. 2004, 1674, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Kıvrak, E.G.; Yurt, K.K.; Kaplan, A.A.; Alkan, I.; Altun, G. Effects of Electromagnetic Fields Exposure on the Antioxidant Defense System. J. Microsc. Ultrastruct. 2017, 5, 167–176. [Google Scholar] [CrossRef]
- Consales, C.; Merla, C.; Marino, C.; Benassi, B. Electromagnetic Fields, Oxidative Stress, and Neurodegeneration. Int. J. Cell Biol. 2012, 2012, 683897. [Google Scholar] [CrossRef]
- Mahaki, H.; Jabarivasal, N.; Sardarian, K.; Zamani, A. Effects of Various Densities of 50 Hz Electromagnetic Field on Serum IL-9, IL-10, and TNF-α Levels. Int. J. Occup. Environ. Med. 2020, 11, 24–32. [Google Scholar] [CrossRef]
- Falone, S.; Mirabilio, A.; Carbone, M.C.; Zimmitti, V.; Di Loreto, S.; Mariggiò, M.A.; Mancinelli, R.; Di Ilio, C.; Amicarelli, F. Chronic Exposure to 50 Hz Magnetic Fields Causes a Significant Weakening of Antioxidant Defence Systems in Aged Rat Brain. Int. J. Biochem. Cell Biol. 2008, 40, 2762–2770. [Google Scholar] [CrossRef]
- Ivancsits, S.; Diem, E.; Jahn, O.; Rüdiger, H.W. Age-Related Effects on Induction of DNA Strand Breaks by Intermittent Exposure to Electromagnetic Fields. Mech. Ageing Dev. 2003, 124, 847–850. [Google Scholar] [CrossRef]
- Arendash, G.W.; Mori, T.; Dorsey, M.; Gonzalez, R.; Tajiri, N.; Borlongan, C. Electromagnetic Treatment to Old Alzheimer’s Mice Reverses β-Amyloid Deposition, Modifies Cerebral Blood Flow, and Provides Selected Cognitive Benefit. PLoS ONE 2012, 7, e35751. [Google Scholar] [CrossRef]
- Maaroufi, K.; Had-Aissouni, L.; Melon, C.; Sakly, M.; Abdelmelek, H.; Poucet, B.; Save, E. Spatial Learning, Monoamines and Oxidative Stress in Rats Exposed to 900MHz Electromagnetic Field in Combination with Iron Overload. Behav. Brain Res. 2014, 258, 80–89. [Google Scholar] [CrossRef]
- Teimori, F.; Khaki, A.A.; Hemmati, R.; Rajabzadeh, A. Probably Role of Antioxidants Against EMFs-Induced Effects on Central Nervous System Structures: A Mini Review. Crescent J. Med. Biol. Sci. 2017, 4, 92–98. [Google Scholar]
- Deng, Y.; Zhang, Y.; Jia, S.; Liu, J.; Liu, Y.; Xu, W.; Liu, L. Effects of Aluminum and Extremely Low Frequency Electromagnetic Radiation on Oxidative Stress and Memory in Brain of Mice. Biol. Trace Elem. Res. 2013, 156, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Wang, C.; Lv, R.; Song, T. Extremely Low-Frequency Magnetic Exposure Appears to Have No Effect on Pathogenesis of Alzheimer’s Disease in Aluminum-Overloaded Rat. PLoS ONE 2013, 8, e71087. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zuo, H.; Wang, D.; Peng, R.; Song, T.; Wang, S.; Xu, X.; Gao, Y.; Li, Y.; Wang, S. Improvement of Spatial Memory Disorder and Hippocampal Damage by Exposure to Electromagnetic Fields in an Alzheimer’s Disease Rat Model. PLoS ONE 2015, 10, e0126963. [Google Scholar] [CrossRef] [PubMed]
- Mariucci, G.; Villarini, M.; Moretti, M.; Taha, E.; Conte, C.; Minelli, A.; Aristei, C.; Ambrosini, M.V. Brain DNA Damage and 70-KDa Heat Shock Protein Expression in CD1 Mice Exposed to Extremely Low Frequency Magnetic Fields. Int. J. Radiat. Biol. 2010, 86, 701–710. [Google Scholar] [CrossRef]
- Bouwens, M.; de Kleijn, S.; Ferwerda, G.; Cuppen, J.J.; Savelkoul, H.F.J.; Kemenade, B.M.L.V. Low-Frequency Electromagnetic Fields Do Not Alter Responses of Inflammatory Genes and Proteins in Human Monocytes and Immune Cell Lines. Bioelectromagnetics 2012, 33, 226–237. [Google Scholar] [CrossRef]
- Rothstein, M.A.; Cai, Y.; Marchant, G.E. The Ghost in Our Genes: Legal and Ethical Implications of Epigenetics. Health Matrix 2009, 19, 1–62. [Google Scholar]
- Pritchard, C.; Rosenorn-Lanng, E.; Silk, A.; Hansen, L. Controlled Population-Based Comparative Study of USA and International Adult [55–74] Neurological Deaths 1989–2014. Acta Neurol. Scand. 2017, 136, 698–707. [Google Scholar] [CrossRef]
- demografia.stat.gov.pl Baza Demografia—Główny Urząd Statystyczny. Available online: https://demografia.stat.gov.pl/BazaDemografia/StartIntro.aspx (accessed on 26 September 2022).
- Bonvicini, C.; Scassellati, C.; Benussi, L.; Di Maria, E.; Maj, C.; Ciani, M.; Fostinelli, S.; Mega, A.; Bocchetta, M.; Lanzi, G. Next Generation Sequencing Analysis in Early Onset Dementia Patients. J. Alzheimers Dis. 2019, 67, 243–256. [Google Scholar] [CrossRef]
- Strand, B.H.; Knapskog, A.-B.; Persson, K.; Edwin, T.H.; Bjertness, E.; Engedal, K.; Selbaek, G. The Loss in Expectation of Life Due to Early-Onset Mild Cognitive Impairment and Early-Onset Dementia in Norway. Dement. Geriatr. Cogn. Disord. 2019, 47, 355–365. [Google Scholar] [CrossRef]
- Meet the Team. Available online: https://www.youngdementiauk.org/meet-team (accessed on 27 December 2019).
- Pritchard, C.; Silk, A.; Hansen, L. Accelerating Rises of Neurological Deaths in the UK and the Other Western Countries 2000-2015: Urgent Need for Policy Response 2019. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3494401 (accessed on 3 October 2022).
- Zeni, O.; Simkó, M.; Scarfi, M.R.; Mattsson, M.-O. Cellular Response to ELF-MF and Heat: Evidence for a Common Involvement of Heat Shock Proteins? Front. Public Health 2017, 5, 280. [Google Scholar] [CrossRef] [PubMed]
- Blank, M.; Goodman, R. Electromagnetic Fields Stress Living Cells. Pathophysiology 2009, 16, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, F.; Ricevuti, G. Extremely Low Frequency Electromagnetic Fields Stimulation Modulates Autoimmunity and Immune Responses: A Possible Immuno-Modulatory Therapeutic Effect in Neurodegenerative Diseases. Neural Regen. Res. 2016, 11, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Carmody, S.; Wu, X.L.; Lin, H.; Blank, M.; Skopicki, H.; Goodman, R. Cytoprotection by Electromagnetic Field-Induced Hsp70: A Model for Clinical Application. J. Cell. Biochem. 2000, 79, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Falone, S.; Grossi, M.R.; Cinque, B.; D’Angelo, B.; Tettamanti, E.; Cimini, A.; Di Ilio, C.; Amicarelli, F. Fifty Hertz Extremely Low-Frequency Electromagnetic Field Causes Changes in Redox and Differentiative Status in Neuroblastoma Cells. Int. J. Biochem. Cell Biol. 2007, 39, 2093–2106. [Google Scholar] [CrossRef]
- International Commission on Non-Ionizing Radiation Protection. Guidelines for Limiting Exposure to Time-Varying Electric and Magnetic Fields (1 Hz to 100 KHz). Health Phys. 2010, 99, 818–836. [Google Scholar] [CrossRef]
| Country | Total Male NM ASDR | Total Female NM ASDR | |||
|---|---|---|---|---|---|
| NM/pm | Change | NM/pm | Change | ||
| [%] | [%] | ||||
| 1. | Australia 2000 | 246 | 231 | ||
| 2015 | 383 | 56 | 380 | 65 | |
| 2. | Austria 2000 | 129 | 91 | ||
| 2015 | 226 | 75 | 202 | 122 | |
| 3. | Belgium 2000 | 238 | 274 | ||
| 2015 | 405 | 70 | 367 | 34 | |
| 4. | Canada 2000 | 358 | 345 | ||
| 2015 | 393 | 10 | 399 | 16 | |
| 5. | Denmark 2000 | 246 | 206 | ||
| 2015 | 405 | 65 | 419 | 103 | |
| 6. | France 2000 | 331 | 280 | ||
| 2014 | 334 | 1 | 322 | 15 | |
| 7. | Finland 2000 | 481 | 462 | ||
| 2015 | 999 | 108 | 938 | 103 | |
| 8. | Germany 2000 | 169 | 117 | ||
| 2015 | 302 | 79 | 262 | 124 | |
| 9. | Greece2000 | 151 | 77 | ||
| 2015 | 228 | 51 | 101 | 31 | |
| 10. | Ireland 2000 | 217 | 194 | ||
| 2014 | 408 | 88 | 405 | 109 | |
| 11. | Italy 2000 | 231 | 200 | ||
| 2015 | 288 | 25 | 270 | 35 | |
| 12. | Japan 2000 | 71 | 49 | ||
| 2015 | 125 | 76 | 100 | 104 | |
| 13. | Netherland 2000 | 260 | 272 | ||
| 2015 | 477 | 83 | 482 | 92 | |
| 14. | New Zealand 2000 | 291 | 238 | ||
| 2013 | 344 | 18 | 342 | 44 | |
| 15. | Norway 2000 | 262 | 204 | ||
| 2015 | 368 | 40 | 309 | 51 | |
| 16. | Portugal 2000 | 162 | 121 | ||
| 2014 | 292 | 80 | 228 | 88 | |
| 17. | Spain 2000 | 298 | 291 | ||
| 2015 | 394 | 32 | 401 | 38 | |
| 18. | Sweden 2000 | 260 | 251 | ||
| 2015 | 398 | 53 | 436 | 74 | |
| 19. | Switzerland 2000 | 312 | 274 | ||
| 2015 | 346 | 11 | 400 | 46 | |
| 20. | UK 2000 | 217 | 192 | ||
| 2015 | 531 | 145 | 558 | 191 | |
| 21. | USA 2000 | 330 | 325 | ||
| 2015 | 557 | 69 | 606 | 86 | |
| Population of 55–74 Years Old | Total Population | Total NM | |||
|---|---|---|---|---|---|
| Country | 2000–2015 | Change | 2000–2015 | Change | Odds Ratio |
| [%] | [%] | ||||
| Canada | |||||
| Neurological mortality: | 2649–3652 | +38 | 19,293–35,091 | +82 | 59% |
| Population (in millions): | 0.496–0.761 | +53 | 30.791–35.255 | +14 | |
| France | |||||
| Neurological mortality: | 6236–5997 | −4 | 40,594–71,543 | +76 | 62% |
| Population (in millions): | 10.628–13.956 | +31 | 58.898–64.129 | +9 | |
| Germany | |||||
| Neurological mortality: | 5790–9332 | +61 | 22,543–73,310 | +225 | 227% |
| Population (in millions:) | 18.424–19.491 | +6 | 82.188–81.687 | −1 | |
| Italy | |||||
| Neurological mortality: | 5693–6542 | +68 | 27,554–61,678 | +124 | 110% |
| Population (in millions): | 12.598–14.231 | +13 | 56.924–60.731 | +7 | |
| Japan | |||||
| Neurological mortality: | 4438–8099 | +82 | 14,023–56,027 | +299 | 300% |
| Population (in millions): | 29.392–33.471 | +14 | 125.612–125.319 | −1 | |
| Spain | |||||
| Neurological mortality: | 3892–5007 | +29 | 26,679–62,871 | +135 | 104% |
| Population (in millions): | 7.888–9.876 | +25 | 40.174–46.410 | +16 | |
| UK | |||||
| Neurological mortality: | 4650–9019 | +94 | 24,601–103,550 | +321 | 286% |
| Population (in millions): | 11.065–13.792 | +25 | 59.704–65.110 | +9 | |
| USA | |||||
| Neurological mortality: | 21,818–48,047 | +120 | 174,708–436,438 | +150 | 120% |
| Population (in millions:) | 42.666–67.380 | +58 | 281.421–319.929 | +14 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyszkowska, J.; Pritchard, C. Open Questions on the Electromagnetic Field Contribution to the Risk of Neurodegenerative Diseases. Int. J. Environ. Res. Public Health 2022, 19, 16150. https://doi.org/10.3390/ijerph192316150
Wyszkowska J, Pritchard C. Open Questions on the Electromagnetic Field Contribution to the Risk of Neurodegenerative Diseases. International Journal of Environmental Research and Public Health. 2022; 19(23):16150. https://doi.org/10.3390/ijerph192316150
Chicago/Turabian StyleWyszkowska, Joanna, and Colin Pritchard. 2022. "Open Questions on the Electromagnetic Field Contribution to the Risk of Neurodegenerative Diseases" International Journal of Environmental Research and Public Health 19, no. 23: 16150. https://doi.org/10.3390/ijerph192316150
APA StyleWyszkowska, J., & Pritchard, C. (2022). Open Questions on the Electromagnetic Field Contribution to the Risk of Neurodegenerative Diseases. International Journal of Environmental Research and Public Health, 19(23), 16150. https://doi.org/10.3390/ijerph192316150








