1. Introduction
We live in an ageing society. According to the fourth national oral survey in China, 4.5% of 65–74-year-old people present an edentulous jaw, with 22.5 teeth remaining, and 63.2% of the dentition defects have been restored with different restoration plans. With economic growth and an increased awareness of oral health issues, more and more elderly people with tooth loss are receiving dental implants in response to the 2030 Healthy China initiative.
Unfortunately, dental implant failures are also on the rise due to various reasons, including patient-related factors (systemic diseases, poor oral hygiene, etc.) and operator-related factors (poor clinical experience, aggressive surgical or prosthetic plan, etc.), which plague patients and implantologists. When an implant exhibits pathological loosening, spontaneous pain, pyorrhea, and irreversible surrounding-bone resorption, it must be removed [
1]. Chranovic et al. discovered 642 cases of implant failure (6.36%) out of 10,096 implants, with 176 implants (1.74%) failing before a secondary surgery [
2]. A one-year retrospective study also revealed a 4.2% (362/8540) early failure rate of implant restorations within one year of implantation [
3].
It was recommended nearly 40 years ago to reinstall implants in sites where failed implants had been removed and healed well [
4]. Grossman and Levin revealed the survival rate of 31 reimplanted implants and suggested that it was significantly lower than that of common implantation (70.97% vs. 93.08%), indicating a higher risk of reimplantation treatment [
5]. According to Mardinger et al., the survival rate of 144 implant failures followed by reimplantation was 92.36%, with only one out of seven cases of the secondary reimplantation failing [
6]. In Wang et al.’s study, a success rate of 90.6% and a cumulative survival rate of 94.6% were discovered in 67 cases of early reimplantation failure, with an average follow-up of 67 months [
7].
In such situations, the implantologist’s clinical competence, experience, and personal habits (implant brand preference, subtle differences in surgery, etc.) can have an impact on the prognosis. It is challenging to rule out the interference of a single implantologist’s subjective factors in a retrospective study with a large sample of patients from multiple implantologists. In this study, the clinical cases from a certain implantologist during his first five years of clinical practice (1 January 2017 to 31 December 2021) were screened, and all the failed cases were thoroughly evaluated, including those who received the reimplantation. Based on the outcome of the implants placed in the previously failed sites, we preliminarily examined the possible influence of risk factors in reimplantation, including patient systemic and local factors, as well as flaws in treatment design and execution. In these complex and challenging clinical situations, which give the patients nonnegligible pain and trauma, additional attention was paid to the patients’ psychological status. We analyzed and evaluated the patients’ satisfaction with the co-located reimplantation and its changes throughout the treatment process. The purpose of this study was to evaluate the clinical outcome of dental implants placed in previously failed sites and discuss risk factors that matter in reimplantation. We hypothesized that reimplantation treatment could yield an acceptable clinical outcome based on a thorough evaluation, consideration of the risk factors, and effective communication with the patients. We hope that this study can inform the development of a young implantologist in dealing with consecutive emerging implant failures in the early career stage, which could provide a useful reference for their peers.
4. Discussion
The causes of implant failures are usually a combination of factors, with a relatively low incidence (2.4% in this study) and serious consequences when they do occur. The reasons for early implant failure include medical factors (mainly related to the operators), such as an immediate/early loading with inadequate primary stability, clinician inexperience, and osteonecrosis due to intraoperative heat production, and patient-related factors such as systemic diseases, poor oral hygiene, and severe periodontitis. While a late implant failure can be attributed to an occlusal overload, adhesive residue, poor restoration design, etc. (operator-related factors), excessive occlusal forces, poor oral hygiene, an inflammation of adjacent teeth, etc. (patient-related factors) can also be responsible [
15,
16]. There are a number of behavioral patterns patients exhibit when implants fail, including but not limited to: (1) opting for a reimplantation attempt, (2) choosing other restoration means, (3) opting for other treatment modalities (fixed partial denture, removable denture, etc.), (4) abandoning reimplantation due to high risks or objective circumstances that do not meet the requirements for reimplantation, (5) losing confidence and terminating the treatment. Regardless of the patient’s behavior, the goal of improving the prognosis for the patient’s subsequent treatment and increasing patient satisfaction should always be at the heart of the implantologist’s decision.
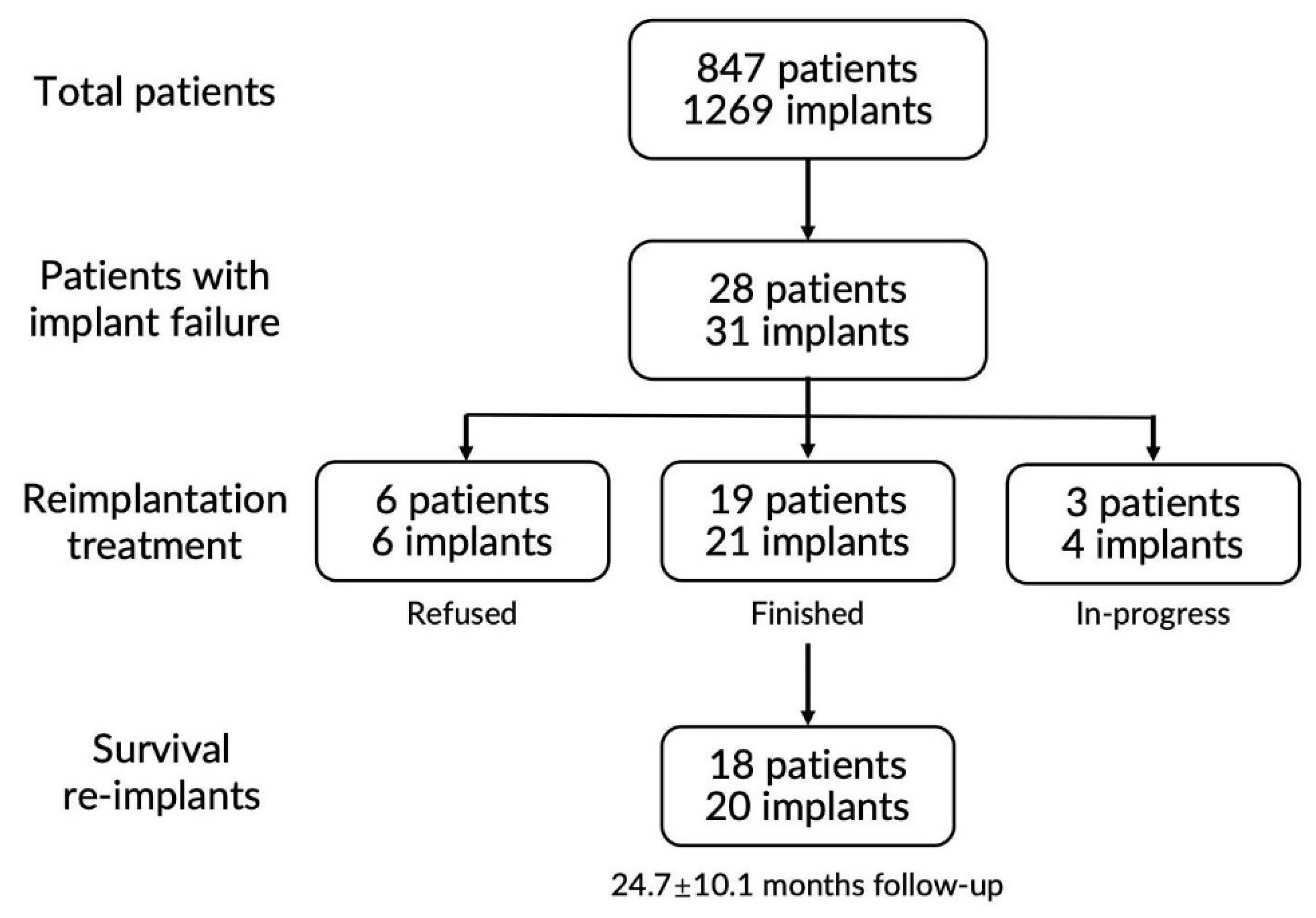
In this study, 28 patients experienced failure after the primary implantation. The reimplantation was abandoned by six (21.4%) patients, indicating that reimplantation was relatively well-received by the majority of patients. The main reasons the patients chose to forgo reimplantation varied (
Appendix C). Here, 19 reimplanted patients with a total of 21 implants revealed an overall survival rate of 95.2% and an overall success rate of 90.4%, with a mean follow-up of 24.7 ± 9.8 months, which was relatively acceptable based on the large-scale systematic reviews [
17,
18]. No unanimous opinion has been reached regarding the prognosis of reimplantation. Gomes et al. systematically reviewed and meta-analyzed the clinical literature related to reimplantation and showed that the implant reimplantation survival rates (88.7%) and survival rates for second-attempt reimplantations (67.1%) were lower than those for conventional non-reimplantable implants [
19]. On the other hand, a study by Wang et al. showed a high success rate (90.6%) and cumulative survival rate (94.6%) in 67 reinserted implants at an average of 67 months after surgery, which might be associated with the early failures of primary implants [
7]. In this study, a total of 20 reinserted implants completed the restoration and kept on functioning for 16.5 ± 8.7 months postoperatively, showing no pain, tenderness, or pathological exudate. The mean peri-implant PD was 2.7 ± 1.0 mm; the mean mSBI was 0.7 ± 0.7; and the mean MBL was 0.5 ± 0.6 mm, with only one implant showing MBL greater than 2 mm but less than 4 mm (in #6). All the results indicated an overall healthy state of the peri-implant soft and hard tissues of the re-inserted implants. According to Wang et al., 67 early failed sites were reimplanted and exhibited a mean 1.7 ± 1.3 mm MBL, with 1 implant’s MBL being 4 mm and 3 implants’ MBL being 2–4 mm at a mean 69.4 month follow-up [
5]. Nevertheless, significant changes in MBL were observed between 12 months and 24 months postoperatively, and at 24 months compared to 36 months postoperatively, suggesting some active remodeling of the peri-implant bone tissue after reimplantation [
20]. Besides the above-mentioned results, in further studies, more objective evaluation criteria are recommended to assess the prosthodontic outcome of reimplantation comprehensively, such as the functional implant prosthodontic score (FIPS) [
21].
According to this study, there was no statistical association between the prognosis of reimplantation and a number of conventional risk factors, including age, gender, smoking habits, history of periodontitis, edentulism, and oral hygiene. In clinical practice, most implant failures cannot be attributed to a single factor or even if the cause was ambiguous, which would emerge in the reimplantation phase. In terms of systemic diseases, only three patients reported mild hypertension in our study. As a growing number of old patients with systemic diseases, including type 1 diabetes [
22], receive dental implant treatment, the influence of systemic diseases on the prognosis of dental reimplantation needs further large-sample clinical studies. Interestingly, bruxism was a factor that attracted attention in the reimplantation treatment. Bruxism is a relative explicit risk factor in dental implant treatment and can be managed by prosthodontics [
23]. Here, we recommend a protective polyethylene terephthalate occlusal guard as an appropriate and cost-effective intervention.
Although dental implant failure prolonged the time of edentulism and loss of occlusal support, which could deteriorate the health of patient’s masticatory system [
24], we still recommend a conventional or late timing for the reimplantation, allowing the hard and soft tissues to heal uneventfully (at least 12 weeks post-removal of the failed implant [
25], and removing as many of the detrimental physiological influences from the primary failure as possible. However, in a few cases, such as in the aesthetic zone, immediate or early reimplantation could be chosen to shorten the treatment period, to better preserve the bone volume, and reduce the atrophy of the alveolar bone caused by edentulism [
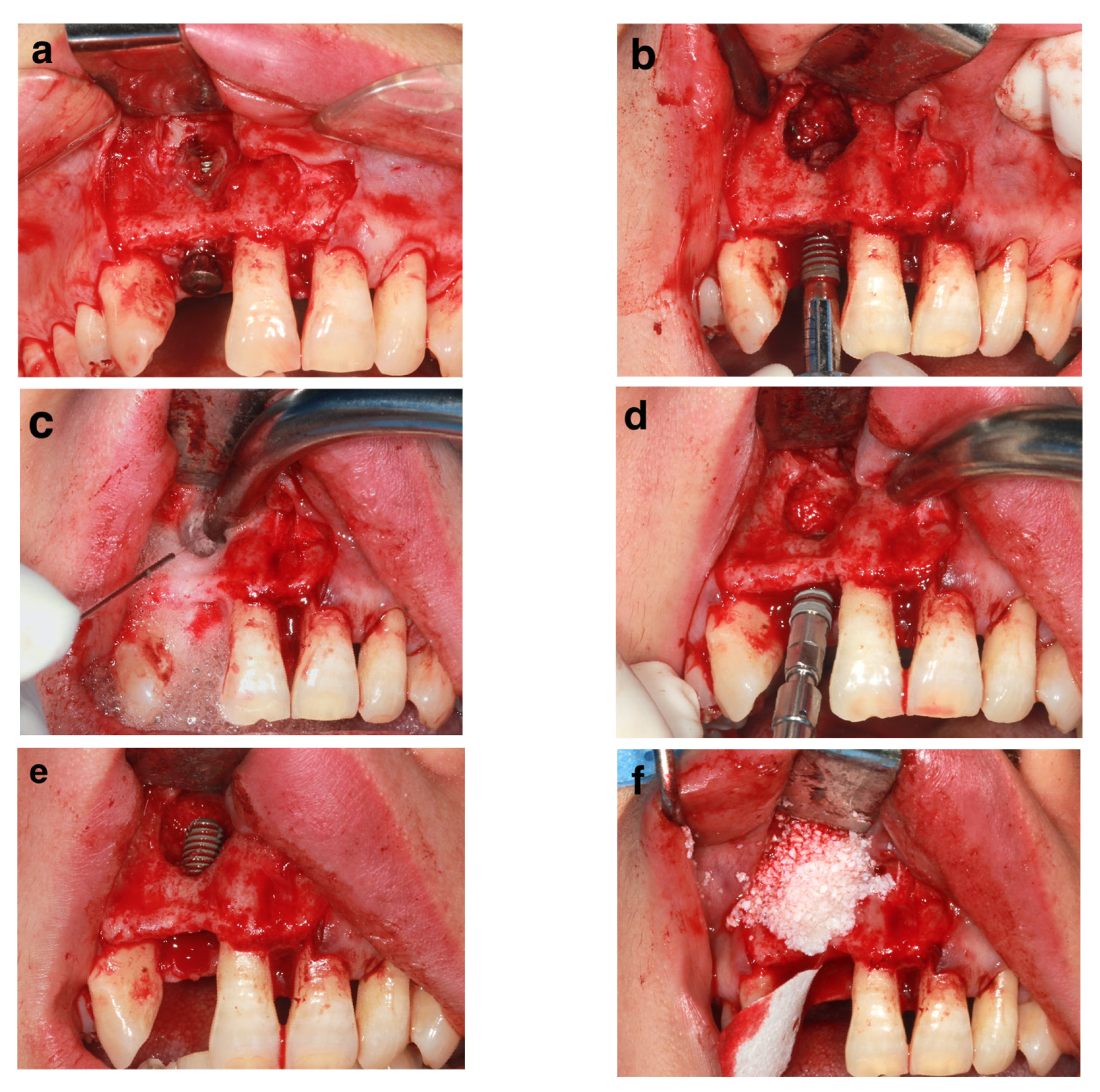
26]. The average interval between the removal of the failed implant and reimplantation was 6.7 ± 3.3 months, with only one patient receiving immediate reimplantation (patient #17). With relatively good conditions (>2 mm intact cervical buccal bone and thorough debridement), the immediately re-inserted implant yielded a successful clinical outcome 46 months later (
Figure 3).
No statistical differences were found when comparing the lengths and diameters of the reinserted implants with the primary implants in this study, and most of the reinserted implants were the same length and diameter as the primary implants. The length and diameter of the implant had an important impact on its osseointegration and initial osseointegration area [
27], as well as its mechanical strength [
28]. In the study by He et al., all 15 reinserted implants were significantly larger in diameter than the primary implants, which suggested that wider implants might be beneficial to the prognosis of reimplantation [
29]. Choosing a wider implant to be reinserted was also recommended by some authors [
5,
30]. However, the decision on the implant’s physical parameters should be based on the local site situation itself, the volume of available bone, and the anticipated biting force of the patient. A longer or wider implant could be chosen in some cases to provide better primary stability, such as in immediate reimplantation. When the primary implant failed because of a fracture, a wider implant was recommended to resist possible abnormal loading, especially for some middle-aged men with robust biting muscles.
Despite no statistical difference, in this study, the re-inserted implants were more in embedded healing than the primary implants, which implies the implantologist’s conservative decision-making in terms of the implant healing. Generally, when the primary stability of an implant is relatively adequate (torque ≥ 35 Ncm), a healing abutment can be delivered to accelerate the shaping of the soft tissues. Healing abutments can accelerate the shaping of soft tissues, but when eating and chewing, it is inevitable that the healing abutment will be compressed and the load will be transferred rigidly to the implant–bone interface. Animal studies have found that a 10 N static immediate load can increase the proportion of poorly structured new bone during the initial healing phase of a dental implant, which is detrimental to the functional loading of the implant [
31]. We observed that placing implants in the mandibular anterior region puts them at a higher risk of pathological micromotion resulting from tongue movement. Two patients (#7 and #18) definitely reported that they could not help licking the healing abutments after the primary implantation. A retrospective study by Zhang et al. on early failure found that simultaneous application of healing abutments and bone grafting resulted in a significantly higher risk of early failure [
32]. We recommended embedded healing as a way to provide an uneventful osteointegration for re-inserted implants.
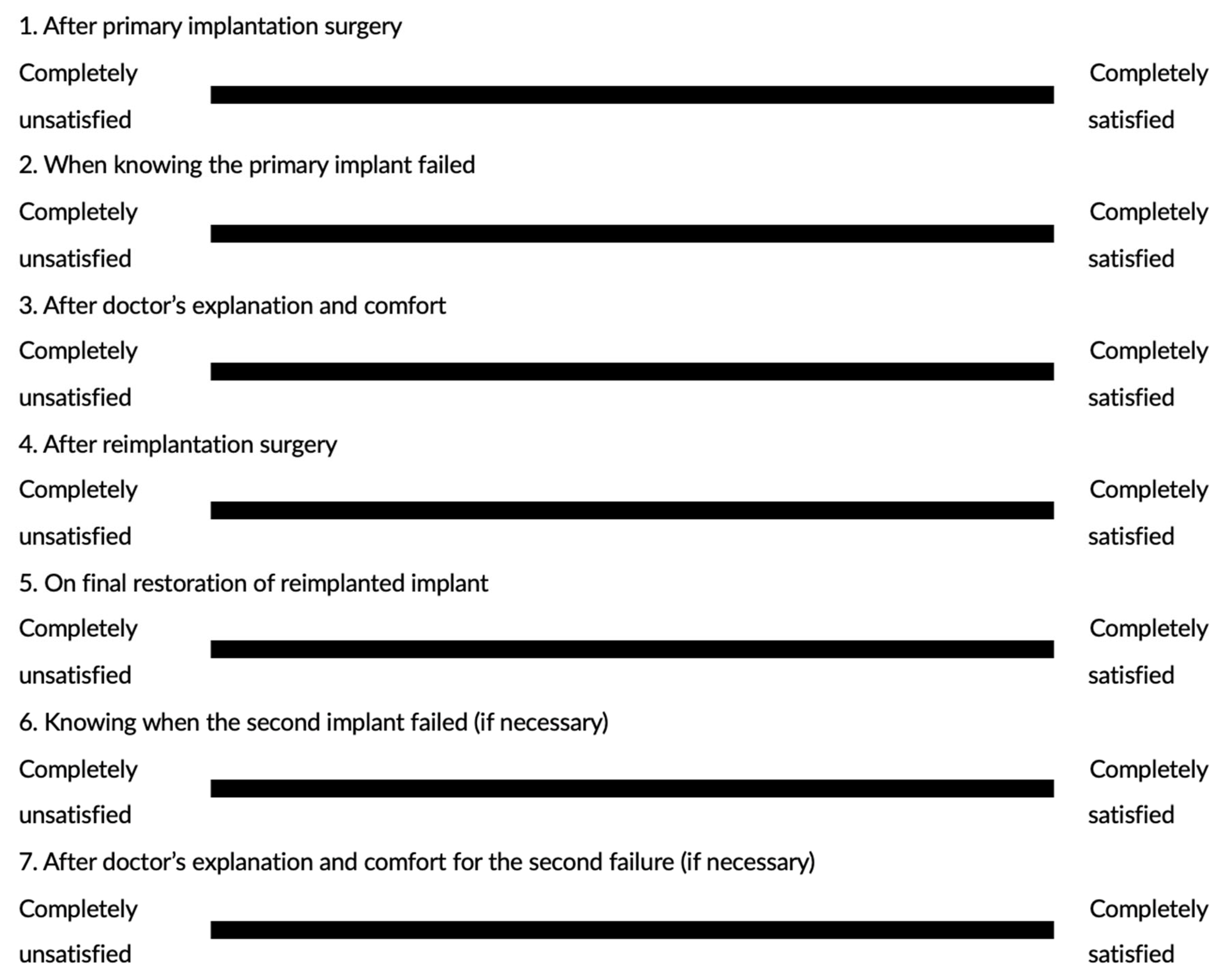
Without a complex questionnaire design, the VAS method allowed patients to recall their overall satisfaction at key time points from the primary implantation without deep consideration, and they could write on the VAS scales to present the level of satisfaction with the least amount of recall bias. Interestingly, the mean satisfaction of the 18 reimplanted patients dropped from 85.1 ± 17.3 post-primary implantation to 48.3 ± 23.6 when they knew the implant failure had occurred, but the mean score soared back to 81.2 ± 15.0 after the implantologist’s meticulous explanation and comfort, with both changes showing statistical significance. Then, the satisfaction level remained over 80 with slight increases after the reimplantation and successful restoration. This dramatic development indicated that implant failure did largely influence the patient’s satisfaction with the implant treatment, but it can be reversed by the implantologist’s explanation, and most essentially, successful reimplantation. Since the failure of surgical procedures can have a significant psychological impact on patients [
33], when re-treating, medical decisions and communication strategies must be more sensitive to the patient’s psychological state. Therefore, it is crucial to actively reassure the patient and create a psychological development process. Therefore, we recommend some tips for implantologists when implant failure occurs: (1) Acknowledge that implant failure does happen and try to accept all the patients’ complaints; at first, be helpful in calming the patient’s possible agitation and anger. (2) Meticulously explain all the possible reasons impartially, but do NOT purely attribute them to either the patient or the implantologist. Blaming the patient can exacerbate the conflict, while blaming the implantologist can undermine the patient’s trust in the implantologist. (3) Cooperate with the patient in the further treatment planning and respect the patient’s final decision (no matter what the outcome). A collaborative model of patient–clinician communication promotes better clinical outcomes.
This study included all the reimplantation treatment cases in the first 5 years of the implantologist’s career in implant dentistry, which was informative to some extent. The majority of patients came from Hangzhou or other cities and counties in the province with relatively high economic development and a favorable attitude toward dental implant treatment. Prior to taking up autonomous clinical practice, the implantologist, with a PhD degree, had finished a 3-year nationwide standardized pre-clinical training course and a 1-year specialized internship, directly supervised by a senior implantologist. This provided him with a relatively solid theoretical foundation and basic clinical experience, which is currently the mainstay of doctor training in China’s AAA stomatology hospital. The clinical outcomes of all the patients who had received reimplantation by such a single implantologist could provide some guidance for young implantologists in dealing with implant failure cases.
The popularity of dental implant treatment stems from the high economic level and the unavoidable commercialization of the promotion. However, the relatively high cost of implant restorative treatment invariably affects patients’ expectations of implant treatment outcomes. The failure of implantation can seriously undermine a patient’s trust in the treatment and the implantologist, which will put enormous pressure on implantologists to try a second implantation attempt, making the cases more complex. The question of how to properly deal with dental implant failure and improve the prognosis of reimplantation treatment still requires more investigation with large-scale samples and longer follow-up.