Development and Validation of a Nomogram Based on Metabolic Risk Score for Assessing Lymphovascular Space Invasion in Patients with Endometrial Cancer
Abstract
1. Introduction
2. Materials and Methods
- Patient selection
- Cohort definition and variable recode
- (1)
- basic demographics, including age at diagnosis, menopause status, BMI;
- (2)
- comorbidities, including diabetes, hypertension;
- (3)
- vital signs, including systolic blood pressure (SBP), diastolic blood pressure (DBP), PP;
- (4)
- laboratory tests, including FPG, total cholesterol (TC), TG, HDL-C, MRS, cancer antigen 125 (CA125), cancer antigen 199 (CA199);
- (5)
- pathological features including LVSI, ascites cytology, histology, grade, LNM, myometrial invasion (MI), cervical stromal invasion (CI).
- Statistical analysis
3. Results
3.1. Patient Characteristics
3.2. Risk Factors of LVSI
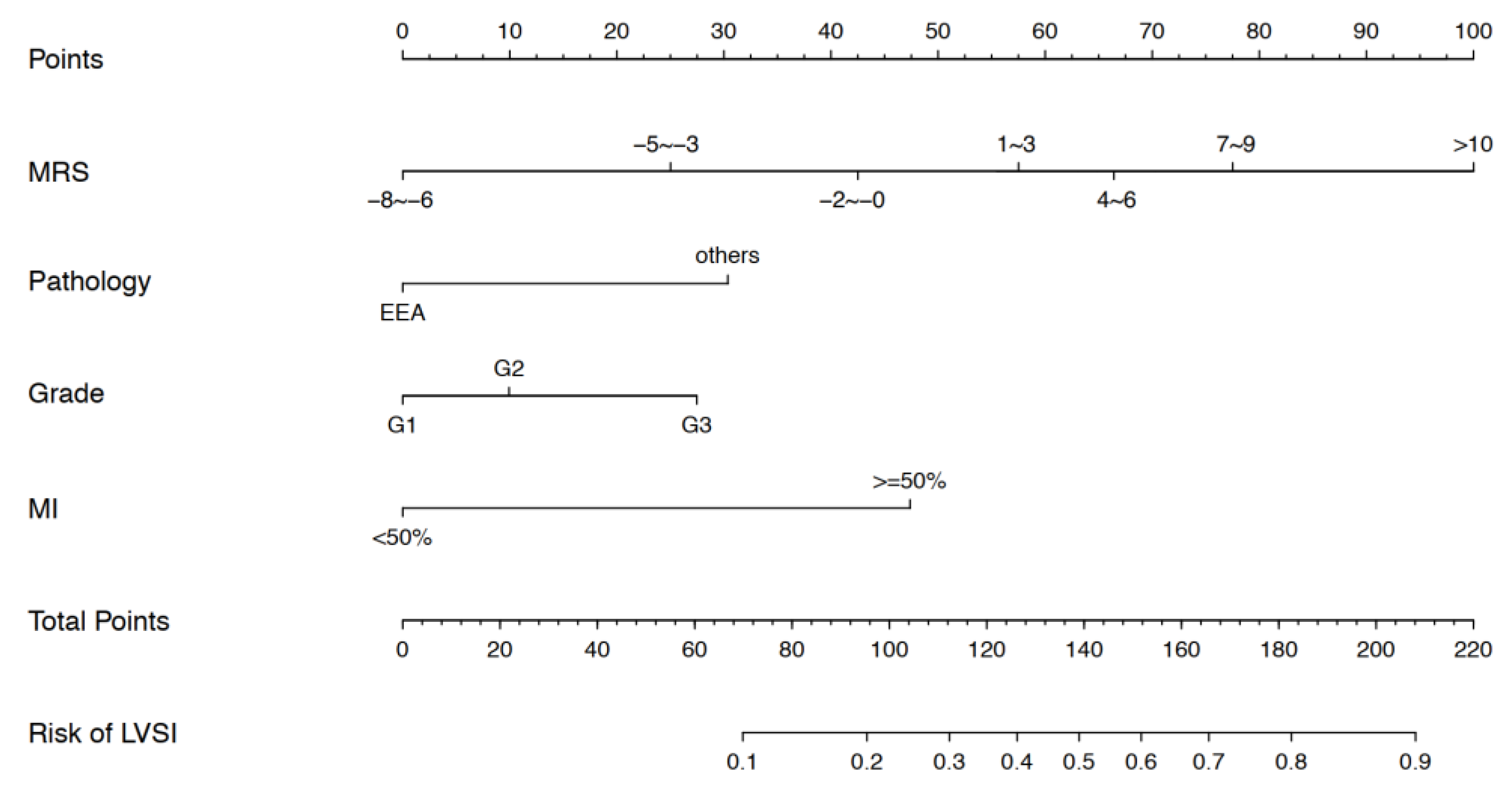
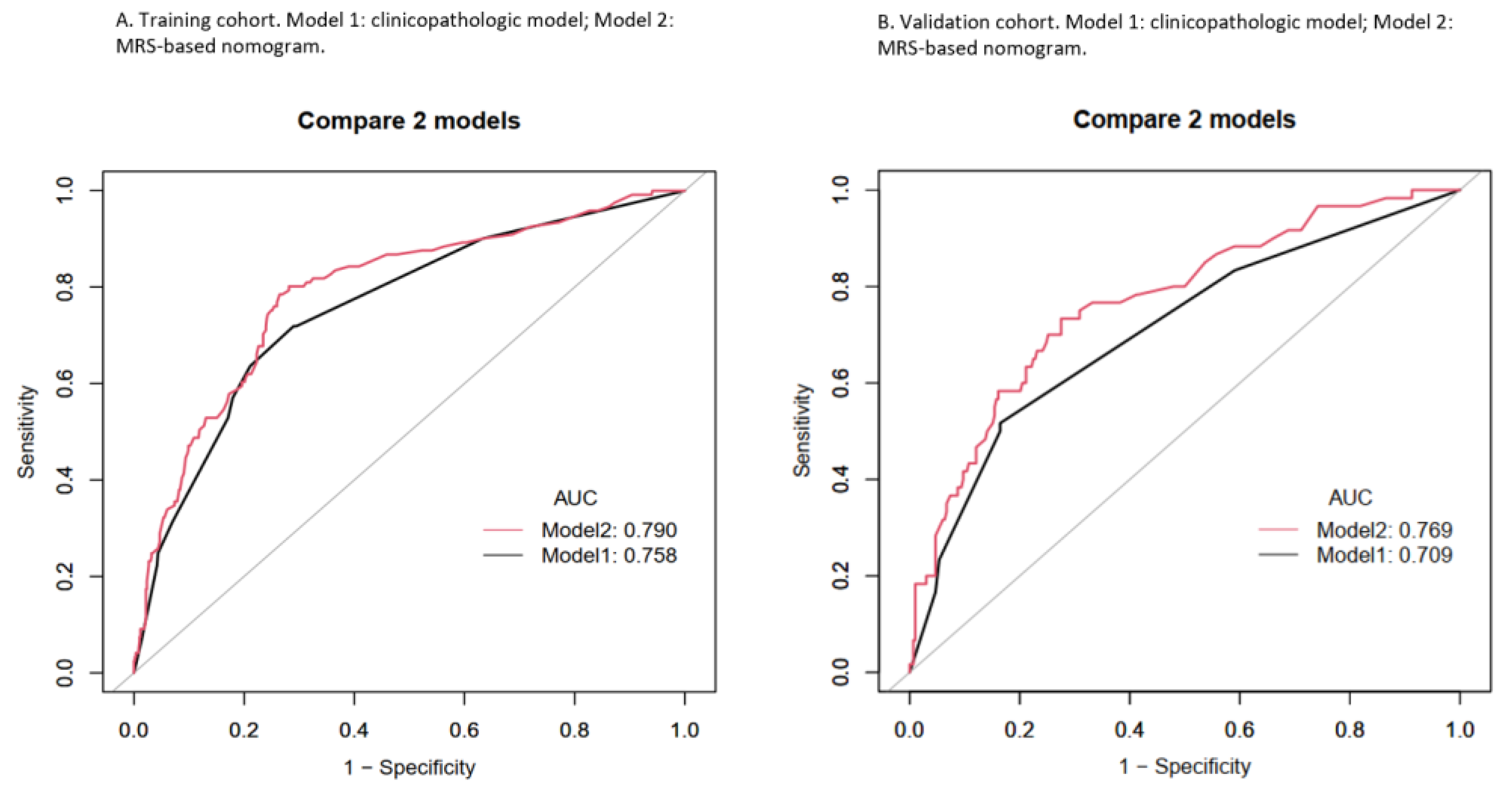
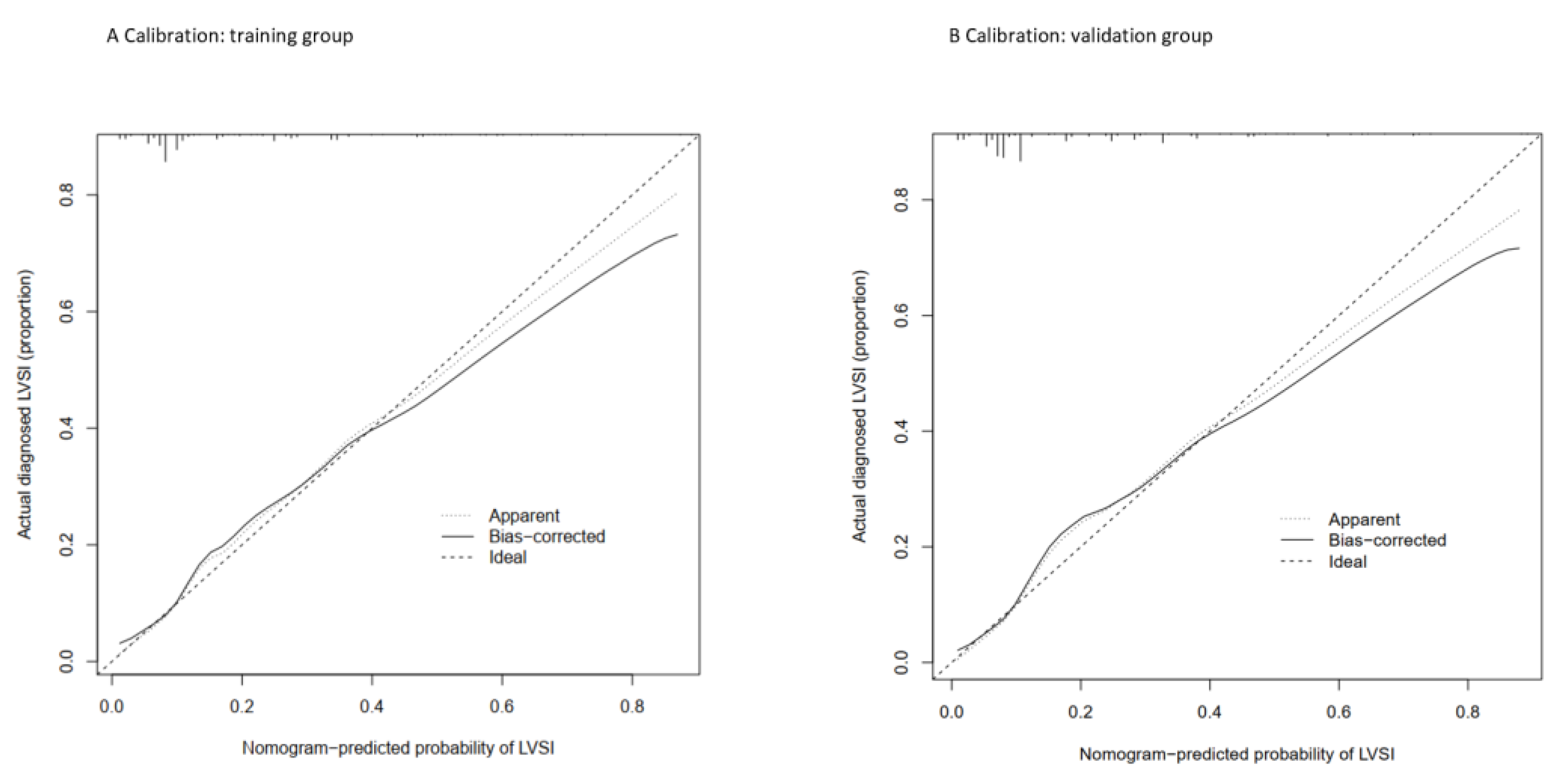
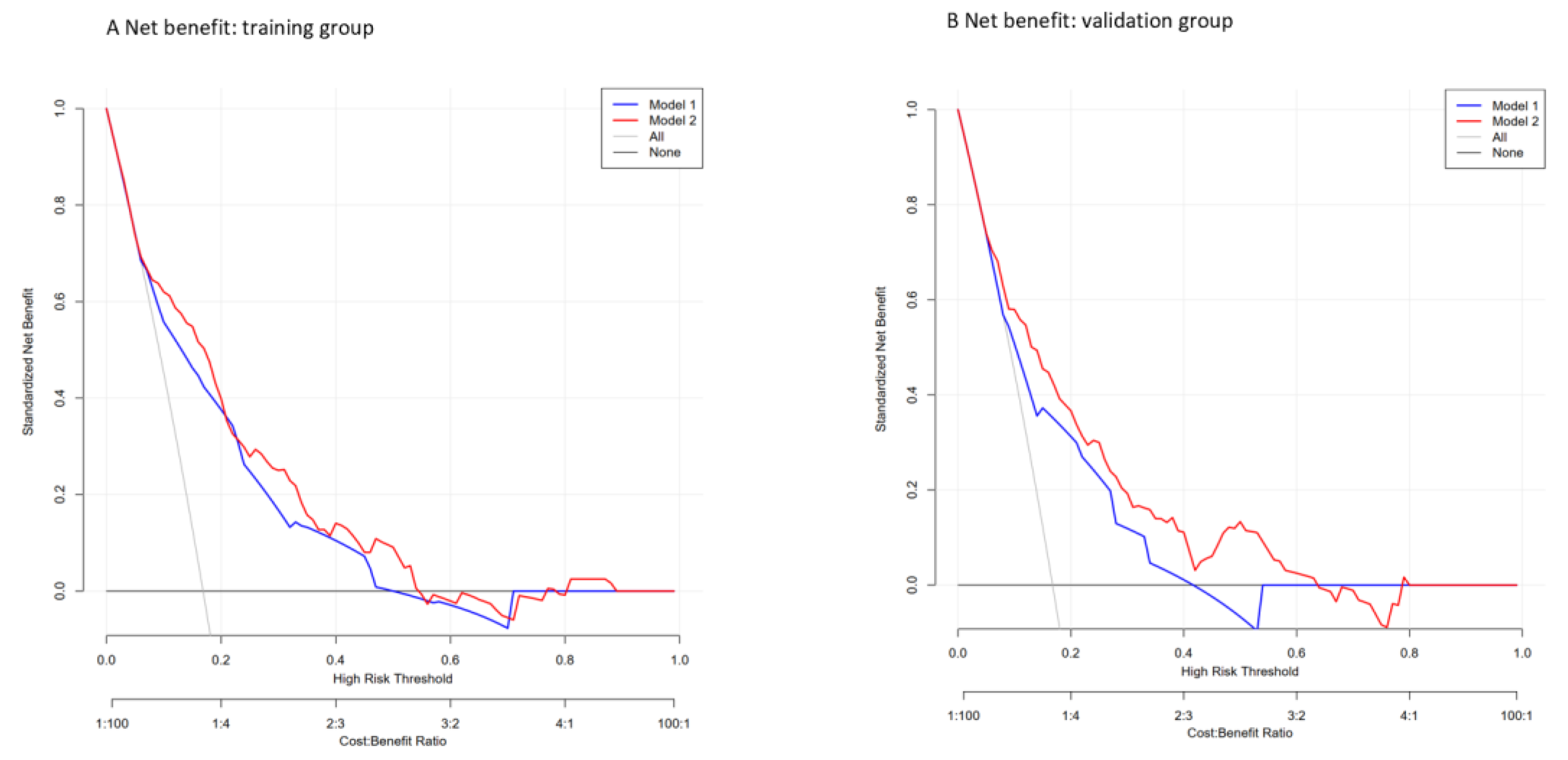
3.3. Nomogram Development and Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henley, S.J.; Ward, E.M.; Scott, S.; Ma, J.; Anderson, R.N.; Firth, A.U.; Thomas, C.C.; Islami, F.; Weir, H.K.; Lewis, D.R.; et al. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer 2020, 126, 2225–2249. [Google Scholar] [CrossRef]
- Banas, T.; Juszczyk, G.; Pitynski, K.; Nieweglowska, D.; Ludwin, A.; Czerw, A. Incidence and mortality rates in breast, corpus uteri, and ovarian cancers in Poland (1980–2013): An analysis of population-based data in relation to socioeconomic changes. OncoTargets Ther. 2016, 9, 5521–5530. [Google Scholar] [CrossRef]
- Piechocki, M.; Koziołek, W.; Sroka, D.; Matrejek, A.; Miziołek, P.; Saiuk, N.; Sledzik, M.; Jaworska, A.; Bereza, K.; Pluta, E.; et al. Trends in Incidence and Mortality of Gynecological and Breast Cancers in Poland (1980–2018). Clin. Epidemiol. 2022, 14, 95–114. [Google Scholar] [CrossRef]
- Creasman, W.T.; Odicino, F.; Maisonneuve, P.; Quinn, M.A.; Beller, U.; Benedet, J.L.; Heintz, A.P.; Ngan, H.Y.; Pecorelli, S. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2006, 95 (Suppl. S1), S105–S143. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-up. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2015, 117, 559–581. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, E.; Margulis, V.; Karakiewicz, P.I.; Roscigno, M.; Mikami, S.; Lotan, Y.; Remzi, M.; Bolenz, C.; Langner, C.; Weizer, A.; et al. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Simpkins, F.; Papadia, A.; Kunos, C.; Michener, C.; Frasure, H.; AbuShahin, F.; Mariani, A.; Bakkum-Gamez, J.N.; Landrum, L.; Moore, K.; et al. Patterns of recurrence in stage I endometrioid endometrial adenocarcinoma with lymphovascular space invasion. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2013, 23, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Pollom, E.L.; Conklin, C.M.; von Eyben, R.; Folkins, A.K.; Kidd, E.A. Nomogram to Predict Risk of Lymph Node Metastases in Patients With Endometrioid Endometrial Cancer. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2016, 35, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Jorge, S.; Hou, J.Y.; Tergas, A.I.; Burke, W.M.; Huang, Y.; Hu, J.C.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L.; Wright, J.D. Magnitude of risk for nodal metastasis associated with lymphvascular space invasion for endometrial cancer. Gynecol. Oncol. 2016, 140, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Bosse, T.; Peters, E.E.; Creutzberg, C.L.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Mens, J.W.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Smit, V.T.; Nout, R.A. Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer--A pooled analysis of PORTEC 1 and 2 trials. Eur. J. Cancer 2015, 51, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Guntupalli, S.R.; Zighelboim, I.; Kizer, N.T.; Zhang, Q.; Powell, M.A.; Thaker, P.H.; Goodfellow, P.J.; Mutch, D.G. Lymphovascular space invasion is an independent risk factor for nodal disease and poor outcomes in endometrioid endometrial cancer. Gynecol. Oncol. 2012, 124, 31–35. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Li, L.; Hu, M.; Chen, L.; Xu, B.; Song, Q. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: A population-based analysis. Cancer Commun. 2020, 40, 301–312. [Google Scholar] [CrossRef]
- Jin, R.; Zheng, Y.; Gao, T.; Zhang, Y.; Wang, B.; Hang, J.; Li, H. A nomogram for preoperative prediction of prolonged air leak after pulmonary malignancy resection. Transl. Lung Cancer Res. 2021, 10, 3616–3626. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, S.; Ma, Y.; Li, W.; Tian, J.; Liu, T. A nomogram prediction model for lymph node metastasis in endometrial cancer patients. BMC Cancer 2021, 21, 748. [Google Scholar] [CrossRef]
- Li, X.; Fan, Y.; Dong, Y.; Cheng, Y.; Zhou, J.; Wang, Z.; Li, X.; Wang, J. Development and Validation of Nomograms Predicting the Overall and the Cancer-Specific Survival in Endometrial Cancer Patients. Front. Med. 2020, 7, 614629. [Google Scholar] [CrossRef]
- Shen, L.; Xie, L.; Li, R.; Shan, B.; Liang, S.; Tian, W.; Wang, H.; Ren, Y. A preoperative prediction model for predicting coexisting adnexa malignancy of patients with G1/G2 endometrioid endometrial cancer. Gynecol. Oncol. 2020, 159, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Y.; Liu, P.; Zhang, X. Developing a validated nomogram for predicting ovarian metastasis in endometrial cancer patients: A retrospective research. Arch. Gynecol. Obstet. 2022, 305, 719–729. [Google Scholar] [CrossRef]
- Hammarsten, J.; Damber, J.E.; Haghsheno, M.A.; Mellström, D.; Peeker, R. A stage-dependent link between metabolic syndrome components and incident prostate cancer. Nat. Rev. Urol. 2018, 15, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Dibaba, D.T.; Ogunsina, K.; Braithwaite, D.; Akinyemiju, T. Metabolic syndrome and risk of breast cancer mortality by menopause, obesity, and subtype. Breast Cancer Res. Treat. 2019, 174, 209–218. [Google Scholar] [CrossRef]
- Shou, H.; Yan, K.; Song, J.; Zhao, L.; Zhang, Y.; Ni, J. Metabolic syndrome affects the long-term survival of patients with non-endometrioid carcinoma of the uterine corpus. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2020, 148, 96–101. [Google Scholar] [CrossRef]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Sha, H.; Hu, D.; Wu, S.; Peng, F.; Xu, G.; Fan, G.; Lin, X.; Chen, G.; Liang, B.; Chen, Y.; et al. Baseline Metabolic Risk Score and Postsurgical Esophageal Cancer-Specific Mortality: The Fujian Prospective Investigation of Cancer (FIESTA) Study. J. Cancer 2018, 9, 1173–1181. [Google Scholar] [CrossRef]
- Watanabe, T.; Honma, R.; Kojima, M.; Nomura, S.; Furukawa, S.; Soeda, S.; Watanabe, S.; Fujimori, K. Prediction of lymphovascular space invasion in endometrial cancer using the 55-gene signature selected by DNA microarray analysis. PLoS ONE 2019, 14, e0223178. [Google Scholar] [CrossRef]
- Mariani, A.; Webb, M.J.; Keeney, G.L.; Aletti, G.; Podratz, K.C. Predictors of lymphatic failure in endometrial cancer. Gynecol. Oncol. 2002, 84, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Koskas, M.; Bassot, K.; Graesslin, O.; Aristizabal, P.; Barranger, E.; Clavel-Chapelon, F.; Haddad, B.; Luton, D.; Darai, E.; Rouzier, R. Impact of lymphovascular space invasion on a nomogram for predicting lymph node metastasis in endometrial cancer. Gynecol. Oncol. 2013, 129, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, S.C.; Borah, B.J.; Bakkum-Gamez, J.N.; Weaver, A.L.; McGree, M.E.; Haas, L.R.; Keeney, G.L.; Mariani, A.; Podratz, K.C. Prospective assessment of survival, morbidity, and cost associated with lymphadenectomy in low-risk endometrial cancer. Gynecol. Oncol. 2012, 127, 5–10. [Google Scholar] [CrossRef]
- Benedetti Panici, P.; Basile, S.; Maneschi, F.; Alberto Lissoni, A.; Signorelli, M.; Scambia, G.; Angioli, R.; Tateo, S.; Mangili, G.; Katsaros, D.; et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. J. Natl. Cancer Inst. 2008, 100, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, R.; Burzawa, J.K.; Tsunoda, A.T.; Hosaka, M.; Frumovitz, M.; Westin, S.N.; Munsell, M.F.; Ramirez, P.T. Lymphovascular Space Invasion Portends Poor Prognosis in Low-Risk Endometrial Cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2015, 25, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Cohn, D.E.; Horowitz, N.S.; Mutch, D.G.; Kim, S.M.; Manolitsas, T.; Fowler, J.M. Should the presence of lymphvascular space involvement be used to assign patients to adjuvant therapy following hysterectomy for unstaged endometrial cancer? Gynecol. Oncol. 2002, 87, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Sari, M.E.; Yalcin, İ.; Sahin, H.; Meydanli, M.M.; Gungor, T. Risk factors for paraaortic lymph node metastasis in endometrial cancer. Int. J. Clin. Oncol. 2017, 22, 937–944. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Capozzi, V.A.; Rosati, A.; Rumolo, V.; Ferrari, F.; Gullo, G.; Karaman, E.; Karaaslan, O.; HacioĞlu, L. Novelties of ultrasound imaging for endometrial cancer preoperative workup. Minerva Med. 2021, 112, 3–11. [Google Scholar] [CrossRef]
- Luo, Y.; Mei, D.; Gong, J.; Zuo, M.; Guo, X. Multiparametric MRI-Based Radiomics Nomogram for Predicting Lymphovascular Space Invasion in Endometrial Carcinoma. J. Magn. Reson. Imaging JMRI 2020, 52, 1257–1262. [Google Scholar] [CrossRef]
- Long, L.; Sun, J.; Jiang, L.; Hu, Y.; Li, L.; Tan, Y.; Cao, M.; Lan, X.; Zhang, J. MRI-based traditional radiomics and computer-vision nomogram for predicting lymphovascular space invasion in endometrial carcinoma. Diagn. Interv. Imaging 2021, 102, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Yoon, J.H.; Lee, S.J.; Song, M.J.; Kim, J.H.; Lee, H.N.; Jung, G.; Yoo, J.G. Prediction of lymphovascular space invasion in patients with endometrial cancer. Int. J. Med. Sci. 2021, 18, 2828–2834. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.A.; Sozzi, G.; Uccella, S.; Ceni, V.; Cianciolo, A.; Gambino, G.; Armano, G.; Pugliese, M.; Scambia, G.; Chiantera, V.; et al. Novel preoperative predictive score to evaluate lymphovascular space involvement in endometrial cancer: An aid to the sentinel lymph node algorithm. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2020, 30, 806–812. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, H.; Wang, X. Preoperative CA125 and fibrinogen in patients with endometrial cancer: A risk model for predicting lymphovascular space invasion. J. Gynecol. Oncol. 2017, 28, e11. [Google Scholar] [CrossRef]
- Mendonça, F.M.; de Sousa, F.R.; Barbosa, A.L.; Martins, S.C.; Araújo, R.L.; Soares, R.; Abreu, C. Metabolic syndrome and risk of cancer: Which link? Metab. Clin. Exp. 2015, 64, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Pourhoseingholi, M.A.; Ashtari, S.; Hajizadeh, N.; Zali, M.R. Metabolic Syndrome, Gastric Cancer Mortality and Competing Risk Survival Analysis. EBioMedicine 2017, 15, 4–5. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Liu, W.Y.; Zhu, G.Q.; Wang, O.C.; Ma, R.M.; Huang, G.Q.; Shi, K.Q.; Guo, G.L.; Braddock, M.; Zheng, M.H. Metabolic syndrome contributes to an increased recurrence risk of non-metastatic colorectal cancer. Oncotarget 2015, 6, 19880–19890. [Google Scholar] [CrossRef] [PubMed]
- Hursting, S.D.; Hursting, M.J. Growth signals, inflammation, and vascular perturbations: Mechanistic links between obesity, metabolic syndrome, and cancer. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Kokts-Porietis, R.L.; McNeil, J.; Nelson, G.; Courneya, K.S.; Cook, L.S.; Friedenreich, C.M. Prospective cohort study of metabolic syndrome and endometrial cancer survival. Gynecol. Oncol. 2020, 158, 727–733. [Google Scholar] [CrossRef] [PubMed]
| Metabolic Risk Factors | Quintile: Range | Score |
|---|---|---|
| BMI | Q1: 17.15–19.72 | −1 |
| Q2: 19.72–21.22 | 0 | |
| Q3: 21.22–22.60 | 0 | |
| Q4: 22.60–24.77 | 1 | |
| Q5: 24.77–29.05 | 1 | |
| PP | Q1: 25.00–35.00 | −2 |
| Q2: 35.00–40.00 | −1 | |
| Q3: 40.00–50.00 | 0 | |
| Q4: 50.00–55.00 | 1 | |
| Q5: 55.00–80.00 | 2 | |
| FBG | Q1: 3.76–4.51 | −2 |
| Q2: 4.51–4.93 | −1 | |
| Q3: 4.93–5.52 | 0 | |
| Q4: 5.52–7.47 | 2 | |
| Q5: 7.47–13.69 | 9 | |
| TG | Q1: 0.41–0.67 | 0 |
| Q2: 0.67–0.87 | 0 | |
| Q3: 0.87–1.08 | 0 | |
| Q4: 1.08–1.51 | 0 | |
| Q5: 1.51–3.56 | 1 | |
| HDL-C | Q1: 0.47–0.78 | 5 |
| Q2: 0.78–0.96 | 3 | |
| Q3: 0.96–1.18 | 2 | |
| Q4: 1.18–1.45 | 0 | |
| Q5: 1.45–2.04 | −3 |
| Training Cohort (n = 718) | Validation Cohort (n = 358) | |
|---|---|---|
| Age, mean ± SD | 55.99 ± 9.54 | 56.42 ± 9.23 |
| BMI, mean ± SD | 26.20 ± 4.52 | 26.31 ± 4.46 |
| SBP, mean ± SD | 128.05 ± 16.04 | 128.48 ± 15.99 |
| DBP, mean ± SD | 78.75 ± 9.59 | 78.70 ± 9.80 |
| PP, mean ± SD | 49.30 ± 13.23 | 49.78 ± 12.68 |
| FPG, mean ± SD | 5.95 ± 1.66 | 5.94 ± 1.75 |
| TC, mean ± SD | 4.98 ± 1.05 | 4.94 ± 1.15 |
| TG, mean ± SD | 1.63 ± 0.86 | 1.56 ± 0.99 |
| HDL-C, mean ± SD | 1.24 ± 0.29 | 1.22 ± 0.34 |
| MRS, mean ± SD | 2.36 ± 4.31 | 2.58 ± 4.31 |
| CA125, mean ± SD | 52.41 ± 173.43 | 79.29 ± 395.40 |
| CA199, mean ± SD | 35.07 ± 85.80 | 44.73 ± 108.02 |
| Menopause status, n (%) | ||
| No | 252 (35.10%) | 119 (33.24%) |
| Yes | 466 (64.90%) | 239 (66.76%) |
| Diabetes, n (%) | ||
| No | 555 (77.3%) | 287 (80.17%) |
| Yes | 163 (22.7%) | 71 (19.83%) |
| Hypertension, n (%) | ||
| No | 433 (60.31%) | 202 (56.42%) |
| Yes | 285 (39.69%) | 156 (43.58%) |
| Ascites cytology | ||
| Negative | 665 (94.33%) | 326 (92.88%) |
| Positive | 40 (5.67%) | 25 (7.12%) |
| LNM, n (%) | ||
| Negative | 661 (92.06%) | 324 (90.50%) |
| Positive | 57 (7.94%) | 34 (9.50%) |
| Histology, n (%) | ||
| Adenocarcinoma | 659 (91.78%) | 327 (91.34%) |
| others | 59 (8.22%) | 31 (8.66%) |
| Grade, n (%) | ||
| G1 | 265 (36.91%) | 133 (37.15%) |
| G2 | 324 (45.13%) | 152 (42.46%) |
| G3 | 129 (17.97%) | 73 (20.39%) |
| MI, n (%) | ||
| <1/2 | 549 (76.46%) | 278 (77.65%) |
| ≥1/2 | 169 (23.54%) | 80 (22.35%) |
| CI, n (%) | ||
| Negative | 649 (90.39%) | 315 (87.99%) |
| Positive | 69 (9.61%) | 43 (12.01%) |
| LVSI | ||
| Negative | 597 (83.15%) | 298 (83.24%) |
| Positive | 121 (16.85%) | 60 (16.76%) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age | 1.04 (1.02, 1.06) | 0.0002 | 0.650 (0.379–1.116) | 0.118 |
| BMI | 0.96 (0.91, 1.00) | 0.0582 | ||
| MRS | 1.15 (1.09, 1.20) | <0.0001 | 1.124 (1.067–1.185) | <0.001 |
| SBP | 1.00 (0.99, 1.01) | 0.8016 | ||
| DBP | 0.98 (0.96, 1.00) | 0.1221 | ||
| PP | 1.006 (0.99, 1.02) | 0.414 | ||
| FPG | 1.07 (0.96, 1.19) | 0.2298 | ||
| TC | 1.17 (0.97, 1.41) | 0.1007 | ||
| TG | 0.86 (0.67, 1.10) | 0.2213 | ||
| HDL-C | 0.59 (0.29, 1.18) | 0.1340 | ||
| CA125 | 1.00 (1.00, 1.00) | 0.0192 | 1.001 (1.000–1.002) | 0.188 |
| CA199 | 1.00 (1.00, 1.00) | 0.5715 | ||
| Diabetes | ||||
| No | 1.0 | |||
| Yes | 1.42 (0.91, 2.21) | 0.1214 | ||
| Hypertension | ||||
| No | 1.0 | |||
| Yes | 0.88 (0.59, 1.32) | 0.5372 | ||
| Menopause status | ||||
| No | 1.0 | |||
| Yes | 2.11 (1.33, 3.33) | 0.0015 | 1.617 (0.908–2.879) | 0.103 |
| Histology | ||||
| Adenocarcinoma | 1.0 | |||
| Others | 4.31 (2.46, 7.55) | <0.0001 | 2.723 (1.370–5.415) | 0.004 |
| Grade | ||||
| G1 | 1.0 | |||
| G2 | 2.15 (1.28, 3.61) | 0.0037 | 1.800 (1.050–3.07.) | 0.032 |
| G3 | 5.26 (3.00, 9.24) | <0.0001 | 3.490 (1.870–6.520) | <0.001 |
| MI | ||||
| <1/2 | 1.0 | |||
| ≥1/2 | 5.26 (3.48, 7.96) | <0.0001 | 4.286 (2.663–6.896) | <0.001 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age | 1.06 (1.02, 1.09) | 0.0009 | 0.781 (0.505–1.208) | 0.267 |
| BMI | 0.96 (0.90, 1.03) | 0.2835 | ||
| MRS | 1.09 (1.02, 1.16) | 0.0083 | 1.092 (1.047–1.139) | <0.001 |
| SBP | 1.00 (0.98, 1.02) | 0.8885 | ||
| DBP | 0.99 (0.96, 1.02) | 0.4505 | ||
| PP | 1.00 (0.98, 1.03) | 0.684 | ||
| FPG | 1.11 (0.96, 1.27) | 0.1564 | ||
| TC | 1.06 (0.83, 1.35) | 0.6330 | ||
| TG | 0.83 (0.56, 1.22) | 0.3372 | ||
| HDL-C | 0.74 (0.31, 1.74) | 0.4844 | ||
| CA125 | 1.00 (1.00, 1.00) | 0.0990 | 1.000 (1.000–1.001) | 0.156 |
| CA199 | 1.00 (1.00, 1.00) | 0.4396 | ||
| Diabetes | ||||
| No | 1.0 | |||
| Yes | 1.79 (0.95, 3.37) | 0.0730 | ||
| Hypertension | ||||
| No | 1.0 | |||
| Yes | 0.99 (0.56, 1.73) | 0.9669 | ||
| Menopause status | ||||
| No | 1.0 | |||
| Yes | 2.24 (1.14, 4.40) | 0.0192 | 1.433 (0.885–2.322) | 0.144 |
| Histology | ||||
| Adenocarcinoma | 1.0 | |||
| Others | 5.87 (2.72, 12.71) | <0.0001 | 3.080 (1.778–5.338) | <0.001 |
| Grade | ||||
| G1 | 1.0 | |||
| G2 | 1.98 (0.92, 4.23) | 0.0787 | 1.880 (0.870–4.070) | 0.110 |
| G3 | 6.14 (2.81, 13.40) | <0.0001 | 3.650 (1.540–8.630) | 0.003 |
| MI | ||||
| <1/2 | 1.0 | |||
| ≥1/2 | 4.96 (2.75, 8.95) | <0.0001 | 4.013 (2.720–5.920) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, X.; Yang, X.; Wang, J. Development and Validation of a Nomogram Based on Metabolic Risk Score for Assessing Lymphovascular Space Invasion in Patients with Endometrial Cancer. Int. J. Environ. Res. Public Health 2022, 19, 15654. https://doi.org/10.3390/ijerph192315654
Wang J, Li X, Yang X, Wang J. Development and Validation of a Nomogram Based on Metabolic Risk Score for Assessing Lymphovascular Space Invasion in Patients with Endometrial Cancer. International Journal of Environmental Research and Public Health. 2022; 19(23):15654. https://doi.org/10.3390/ijerph192315654
Chicago/Turabian StyleWang, Jingyuan, Xingchen Li, Xiao Yang, and Jianliu Wang. 2022. "Development and Validation of a Nomogram Based on Metabolic Risk Score for Assessing Lymphovascular Space Invasion in Patients with Endometrial Cancer" International Journal of Environmental Research and Public Health 19, no. 23: 15654. https://doi.org/10.3390/ijerph192315654
APA StyleWang, J., Li, X., Yang, X., & Wang, J. (2022). Development and Validation of a Nomogram Based on Metabolic Risk Score for Assessing Lymphovascular Space Invasion in Patients with Endometrial Cancer. International Journal of Environmental Research and Public Health, 19(23), 15654. https://doi.org/10.3390/ijerph192315654





