Ti/PbO2 Electrode Efficiency in Catalytic Chloramphenicol Degradation and Its Effect on Antibiotic Resistance Genes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Apparatus
2.3. Experimental Methods
2.3.1. Ti/PbO2 Electrode Preparation
2.3.2. CAP Test Method
2.3.3. High Throughput Sequencing and qPCR
2.4. Data Analysis
3. Results and Discussion
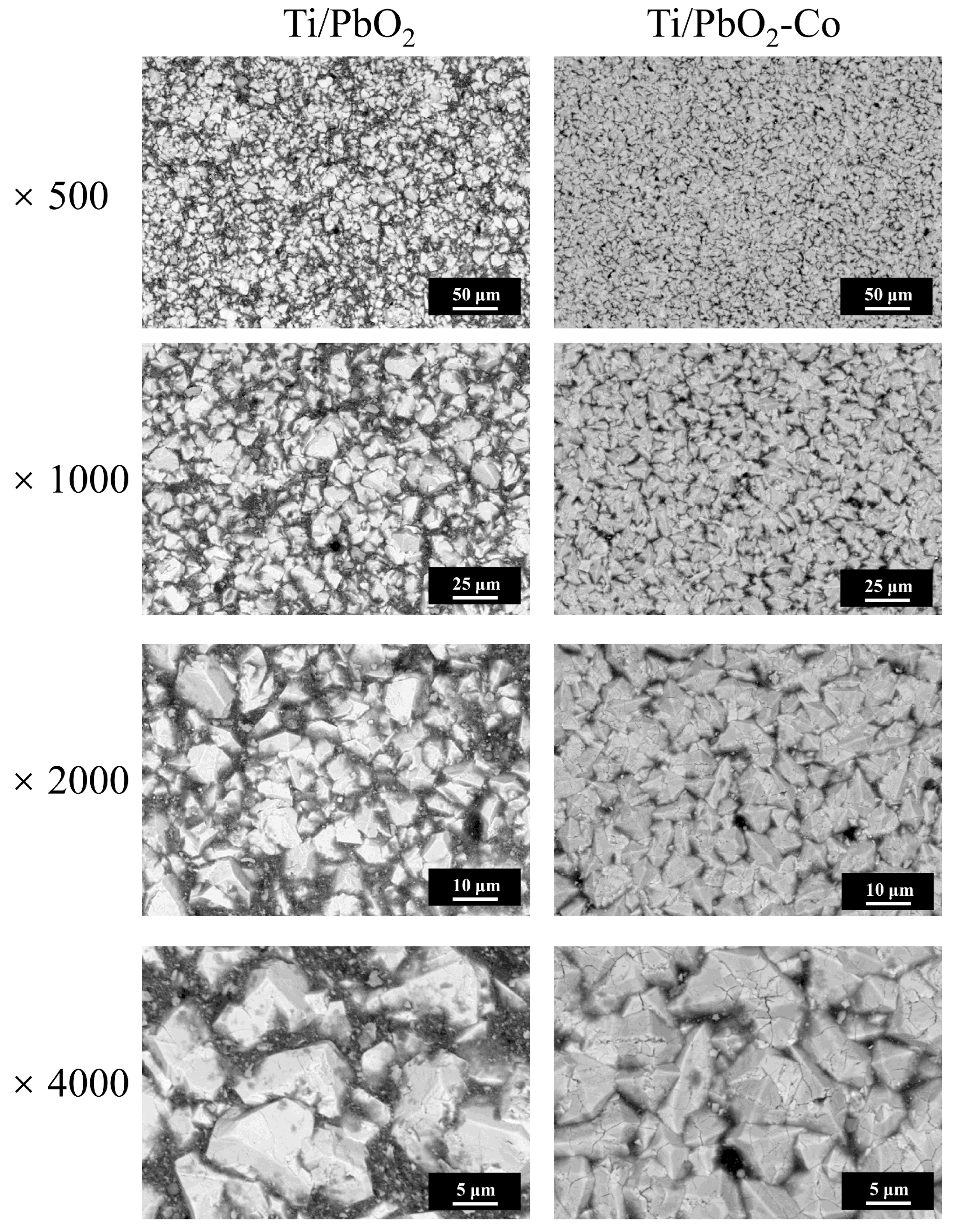
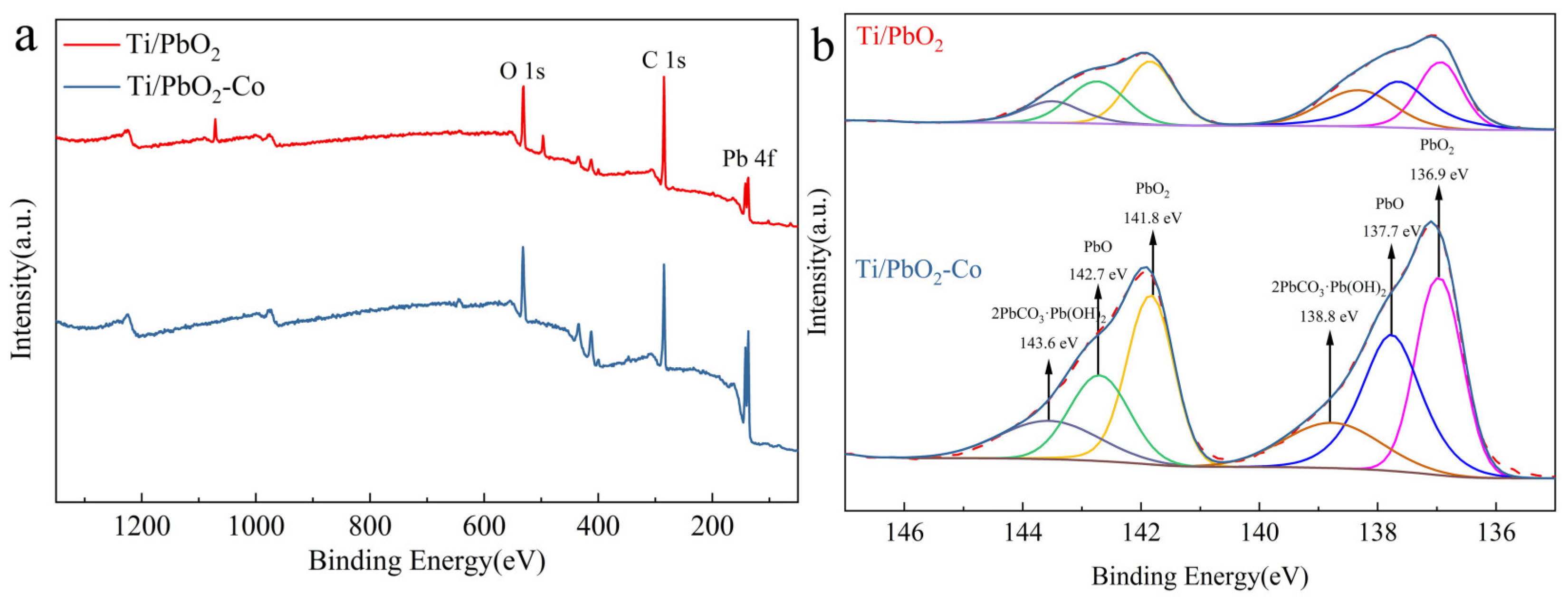
3.1. Morphological and Structural Characterization
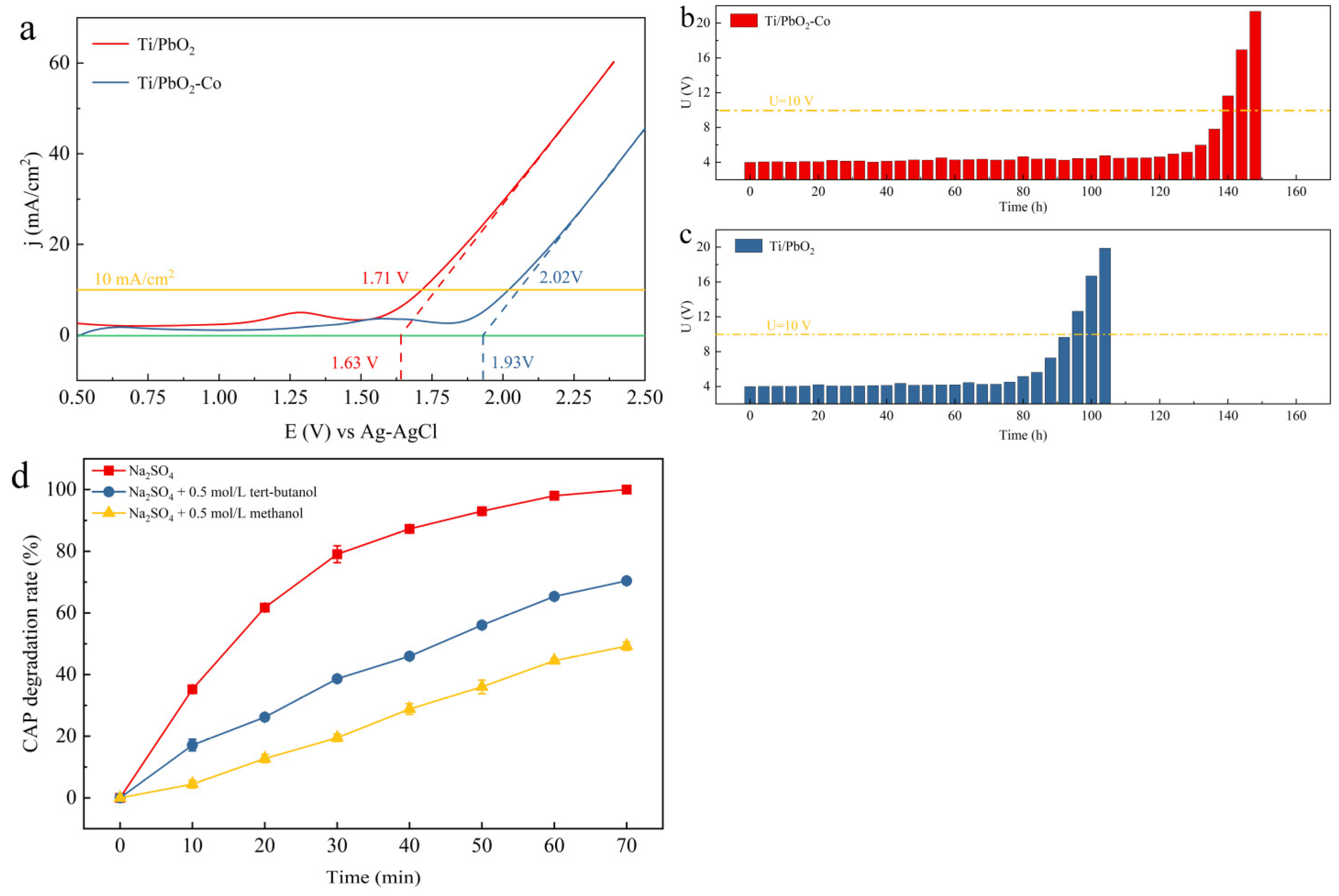
3.2. Electrochemical Property Characterization
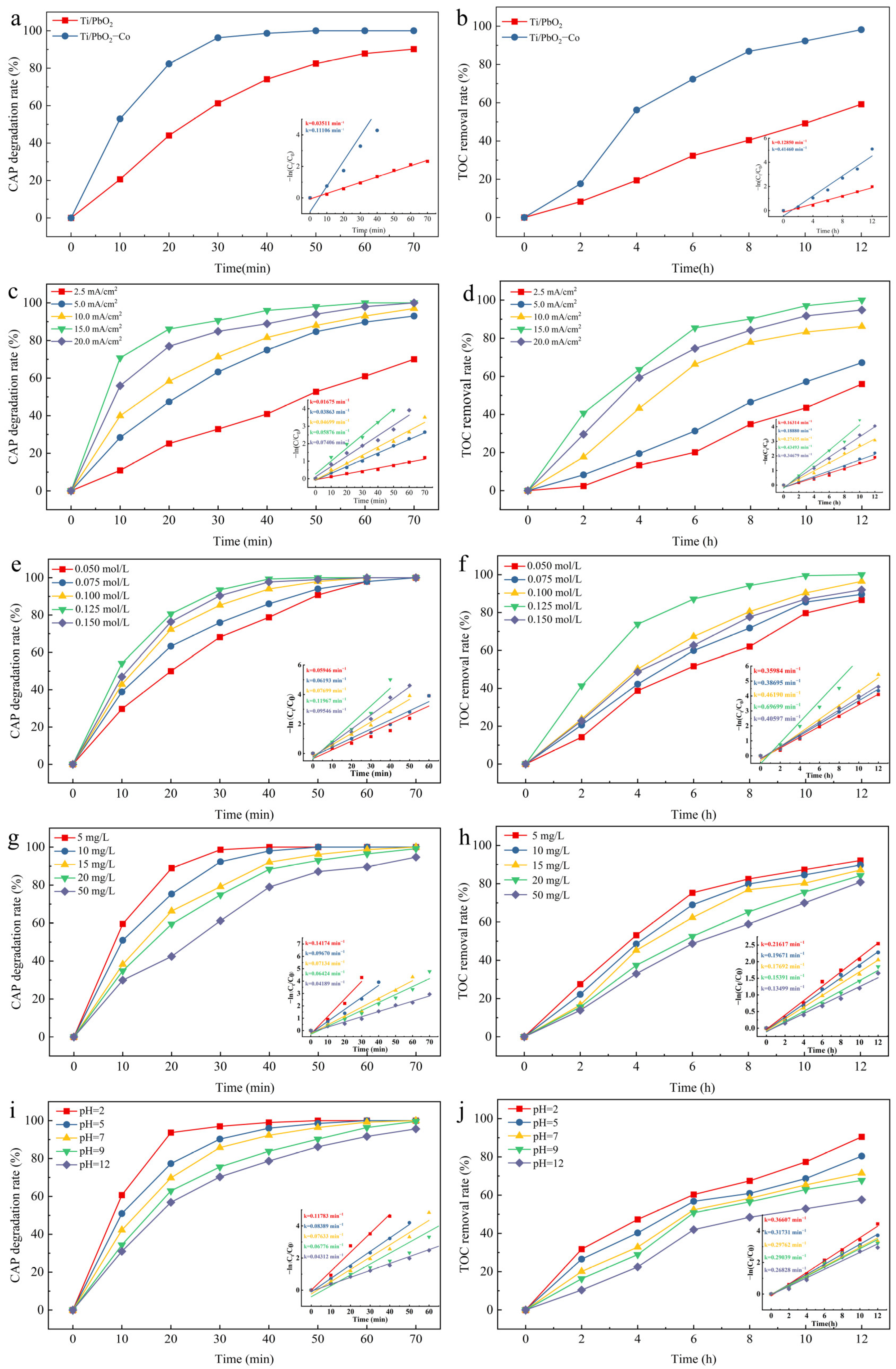
3.3. Studies Affecting the CAP Degradation Effect
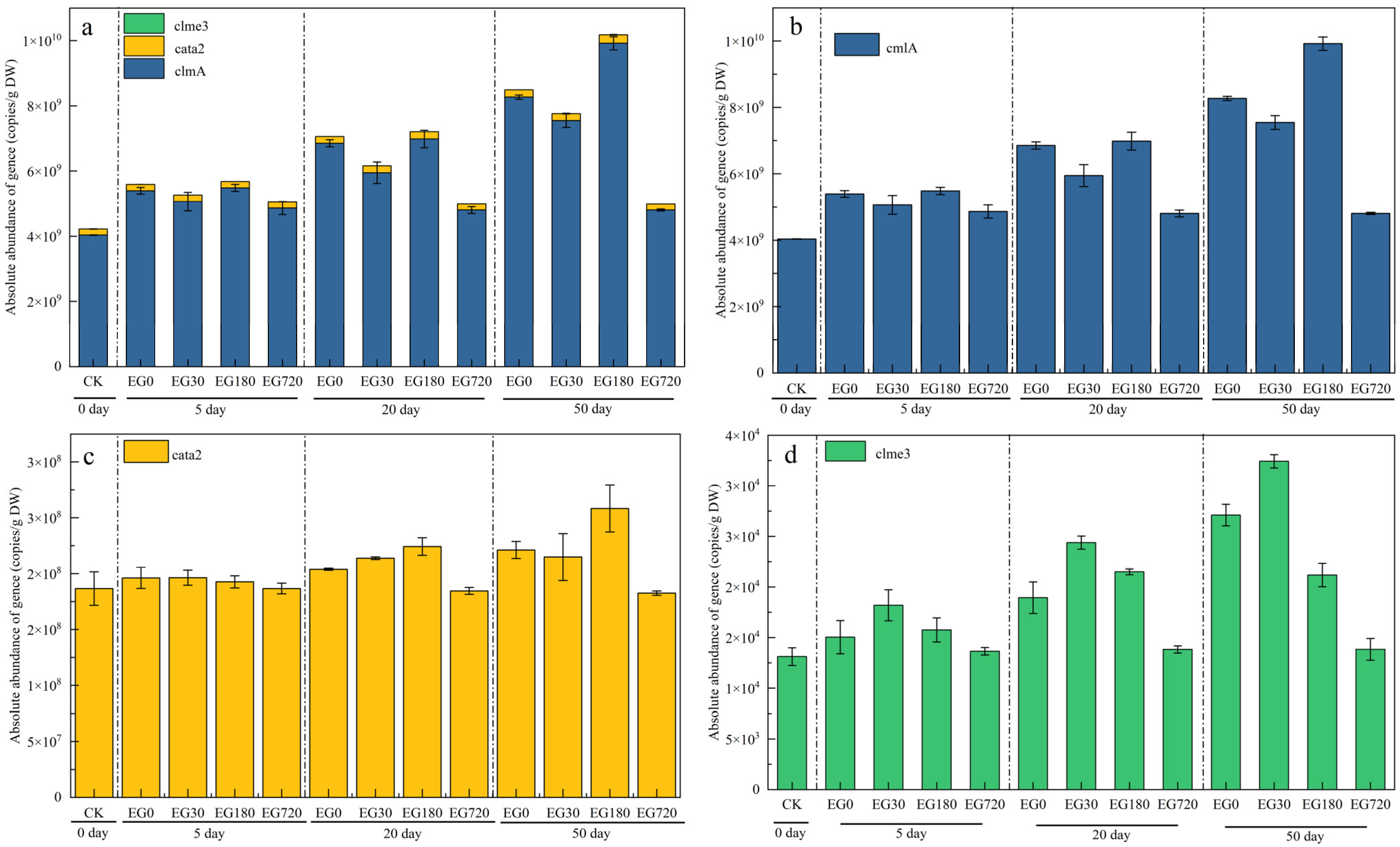
3.4. Effect of CAP Degradation Intermediates on Resistance Genes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Jin, X.; Feng, C.; Wang, Z.; Wu, F.; Johnson, A.C.; Xiao, H.; Hollert, H.; Giesy, J.P. Ecological risk assessment of fifty pharmaceuticals and personal care products (PPCPs) in Chinese surface waters: A proposed multiple-level system. Environ. Int. 2020, 136, 105454. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Steele, J.C.; Meng, X.Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Tekle-Roettering, A.; von Sonntag, C.; Reisz, E.; vom Eyser, C.; Lutze, H.V.; Tuerk, J.; Naumov, S.; Schmidt, W.; Schmidt, T.C. Ozonation of anilines: Kinetics, stoichiometry, product identification and elucidation of pathways. Water Res. 2016, 98, 147–159. [Google Scholar] [CrossRef]
- Onal, A. Overview on liquid chromatographic analysis of tetracycline residues in food matrices. Food Chem. 2011, 127, 197–203. [Google Scholar] [CrossRef]
- Carrales-Alvarado, D.H.; Rodriguez-Ramos, I.; Leyva-Ramos, R.; Mendoza-Mendoza, E.; Villela-Martinez, D.E. Effect of surface area and physical-chemical properties of graphite and graphene-based materials on their adsorption capacity towards metronidazole and trimethoprim antibiotics in aqueous solution. Chem. Eng. J. 2020, 402, 126155. [Google Scholar] [CrossRef]
- Mansurov, R.R.; Zverev, V.S.; Safronov, A.P. Dynamics of diffusion-limited photocatalytic degradation of dye by polymeric hydrogel with embedded TiO2 nanoparticles. J. Catal. 2022, 406, 9–18. [Google Scholar] [CrossRef]
- Morais, E.; O’Modhrain, C.; Thampi, K.R.; Sullivan, J.A. RuO2/TiO2 photocatalysts prepared via a hydrothermal route: Influence of the presence of TiO2 on the reactivity of RuO2 in the artificial photosynthesis reaction. J. Catal. 2021, 401, 288–296. [Google Scholar] [CrossRef]
- Ioannidou, E.; Frontistis, Z.; Antonopoulou, M.; Venieri, D.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Solar photocatalytic degradation of sulfamethoxazole over tungsten—Modified TiO2. Chem. Eng. J. 2017, 318, 143–152. [Google Scholar] [CrossRef]
- Liu, N.; Fei, F.H.; Dai, W.X.; Lei, J.Q.; Bi, F.K.; Wang, B.T.; Quan, G.X.; Zhang, X.D.; Tang, L. Visible-light-assisted persulfate activation by SnS2/MIL-88B(Fe) Z-scheme heterojunction for enhanced degradation of ibuprofen. J. Colloid Interf. Sci. 2022, 625, 965–977. [Google Scholar] [CrossRef]
- Pruden, A.; Arabi, M.; Storteboom, H.N. Correlation Between Upstream Human Activities and Riverine Antibiotic Resistance Genes. Environ. Sci. Technol. 2012, 46, 11541–11549. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Mao, D.Q.; Rysz, M.; Zhou, D.X.; Zhang, H.J.; Xu, L.; Alvarez, P.J.J. Trends in Antibiotic Resistance Genes Occurrence in the Haihe River, China. Environ. Sci. Technol. 2010, 44, 7220–7225. [Google Scholar] [CrossRef]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planet Health 2018, 2, E398–E405. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikov, M.; Mueller, M.; McBeth, C.; Lee, J.C.; Campbell, J.; Sharon, A.; Sauer-Budge, A.F. Rapid phenotypic stress-based microfluidic antibiotic susceptibility testing of Gram-negative clinical isolates. Sci. Rep. 2017, 7, 10. [Google Scholar] [CrossRef]
- Pei, R.; Kim, S.C.; Carlson, K.H.; Pruden, A. Effect of River Landscape on the sediment concentrations of antibiotics and corresponding antibiotic resistance genes (ARG). Water Res. 2006, 40, 2427–2435. [Google Scholar] [CrossRef]
- Comninellis, C. Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochem. Acta 1994, 39, 1857–1862. [Google Scholar] [CrossRef]
- Ali, T.; Muhammad, N.; Qian, Y.J.; Liu, S.S.; Wang, S.; Wang, M.F.; Qian, T.; Yan, C.L. Recent advances in material design and reactor engineering for electrocatalytic ambient nitrogen fixation. Mater. Chem. Front. 2022, 6, 843–879. [Google Scholar] [CrossRef]
- Brinzila, C.I.; Pacheco, M.J.; Ciriaco, L.; Ciobanu, R.C.; Lopes, A. Electrodegradation of tetracycline on BDD anode. Chem. Eng. J. 2012, 209, 54–61. [Google Scholar] [CrossRef]
- Sun, Y.; Li, P.; Zheng, H.; Zhao, C.; Xiao, X.; Xu, Y.; Sun, W.; Wu, H.; Ren, M. Electrochemical treatment of chloramphenicol using Ti-Sn/gamma-Al2O3 particle electrodes with a three-dimensional reactor. Chem. Eng. J. 2017, 308, 1233–1242. [Google Scholar] [CrossRef]
- Wang, J.B.; Zhi, D.; Zhou, H.; He, X.W.; Zhang, D.Y. Evaluating tetracycline degradation pathway and intermediate toxicity during the electrochemical oxidation over a Ti/Ti4O7 anode. Water Res. 2018, 137, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, C.; Wu, J.; Niu, J. Insights into the electrochemical degradation of sulfamethoxazole and its metabolite by Ti/SnO2-Sb/Er-PbO2 anode. Chin. Chem. Lett. 2020, 31, 2673–2677. [Google Scholar] [CrossRef]
- Liang, B.; Cheng, H.Y.; Kong, D.Y.; Gao, S.H.; Sun, F.; Cui, D.; Kong, F.Y.; Zhou, A.J.; Liu, W.Z.; Ren, N.Q.; et al. Accelerated reduction of chlorinated nitroaromatic antibiotic chloramphenicol by biocathode. Environ. Sci. Technol. 2013, 47, 5353–5361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, J.; Chen, B. Effect of Ce(NO3)4 on the electrochemical properties of Ti/PbO2–TiO2–Ce(NO3)4 electrode for zinc electrowinning. Appl. Phys. A 2019, 125, 150. [Google Scholar] [CrossRef]
- Watson, P.R.; Somorjai, G.A. The formation of oxygen-containing organic molecules by the hydrogenation of carbon monoxide using a lanthanum rhodate catalyst. J. Catal. 1982, 74, 282–295. [Google Scholar] [CrossRef]
- An, H.; Li, Q.; Tao, D.J.; Cui, H.; Xu, X.T.; Ding, L.; Sun, L.; Zhai, J.P. The synthesis and characterization of Ti/SnO2-Sb2O3/PbO2 electrodes: The influence of morphology caused by different electrochemical deposition time. Appl. Surf. Sci. 2011, 258, 218–224. [Google Scholar] [CrossRef]
- Velichenko, A.B.; Devilliers, D. Electrodeposition of fluorine-Doped lead dioxide. Cheminform 2007, 38, 269–376. [Google Scholar] [CrossRef]
- Ansari, A.; Nematollahi, D. A comprehensive study on the electrocatalytic degradation, electrochemical behavior and degradation mechanism of malachite green using electrodeposited nanostructured beta-PbO2 electrodes. Water Res. 2018, 144, 462–473. [Google Scholar] [CrossRef]
- Duan, X.Y.; Xu, F.; Wang, Y.N.; Chen, Y.W.; Chang, L.M. Fabrication of a hydrophobic SDBS-PbO2 anode for electrochemical degradation of nitrobenzene in aqueous solution. Electrochim. Acta 2018, 282, 662–671. [Google Scholar] [CrossRef]
- Pei, K.S.; Xiao, L.W. Morphologic study of electrochemically formed lead dioxide. Electrochim. Acta 2003, 48, 1743–1747. [Google Scholar] [CrossRef]
- Wu, H.; Yang, T.; Du, Y.; Shen, L.; Ho, G.W. Identification of facet-governing reactivity in hematite for oxygen evolution. Adv. Mater. 2018, 30, 1804341. [Google Scholar] [CrossRef]
- Gui, L.; Chen, Z.; Chen, B.; Song, Y.; Yeasmin, F. Preparation and characterization of ZnO/PEG-Co(II)-PbO2 nanocomposite electrode and an investigation of the electrocatalytic degradation of phenol. J. Hazard. Mater. 2020, 399, 123018. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.F.; Zhang, X.; Wang, P.Q.; Ren, Y.; Meng, X.X.; Ding, Y.; Zhang, T.; Jiang, W.Q. Electrochemical degradation of doxycycline hydrochloride on Bi/Ce co-doped Ti/PbO2 anodes: Efficiency and mechanism. J. Environ. Chem. Eng. 2022, 10, 108430. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Jiang, W.Q.; Dong, H.; Hu, X.Y.; Fang, B.H.; Gao, G.F.; Zhao, R. Study on the electrochemical removal mechanism of oxytetracycline by a Ti/IrO2-Ta2O5 plate. Int. J. Environ. Res. Public Health 2021, 18, 1708. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.S.; Huang, W.M.; Lin, H.B.; Lu, H.Y.; Zhang, W.L. Effect of SnO2-Sb2O5 interlayer on electrochemical performances of a Ti-substrate lead dioxide electrode. Chin. J. Chem. 2012, 30, 2059–2065. [Google Scholar] [CrossRef]
- Li, J.; Yan, J.F.; Yao, G.; Zhang, Y.H.; Li, X.; Lai, B. Improving the degradation of atrazine in the three-dimensional (3D) electrochemical process using CuFe2O4 as both particle electrode and catalyst for persulfate activation. Chem. Eng. J. 2019, 361, 1317–1332. [Google Scholar] [CrossRef]
- Song, S.; Fan, J.Q.; He, Z.Q.; Zhan, L.Y.; Liu, Z.W.; Chen, J.M.; Xu, X.H. Electrochemical degradation of azo dye CI Reactive Red 195 by anodic oxidation on Ti/SnO2-Sb/PbO2 electrodes. Electrochim. Acta 2010, 55, 3606–3613. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Z.Y.; Chen, X.C. Effects of experimental parameters on 2,4-dichlorphenol degradation over Er-chitosan-PbO2 electrode. J. Hazard. Mater. 2010, 178, 867–874. [Google Scholar] [CrossRef]
- Dong, H.; Fu, Y.L.; Wang, P.Q.; Jiang, W.Q.; Gao, G.F.; Zhang, X. Degradation of chloramphenicol by Ti/PbO2-La anodes and alteration in bacterial community and antibiotics resistance genes. Environ. Pollut. 2022, 301, 119031. [Google Scholar] [CrossRef]
- Marshall, A.T.; Haverkamp, R.G. Electrocatalytic activity of IrO2-RuO2 supported on Sb-doped SnO2 nanoparticles. Electrochim. Acta 2010, 55, 1978–1984. [Google Scholar] [CrossRef]
- Qu, X.A.; Tian, M.; Liao, B.Q.; Chen, A.C. Enhanced electrochemical treatment of phenolic pollutants by an effective adsorption and release process. Electrochim. Acta 2010, 55, 5367–5374. [Google Scholar] [CrossRef]
- Shaikh, J.S.; Pawar, R.C.; Tarwal, N.L.; Patil, D.S.; Patil, P.S. Supercapacitor behavior of CuO-PAA hybrid films: Effect of PAA concentration. J. Alloy. Compd. 2011, 509, 7168–7174. [Google Scholar] [CrossRef]
- Oturan, N.; Wu, J.; Zhang, H.; Sharma, V.K.; Oturan, M.A. Electrocatalytic destruction of the antibiotic tetracycline in aqueous medium by electrochemical advanced oxidation processes: Effect of electrode materials. Appl. Catal. B Environ. 2013, 140, 92–97. [Google Scholar] [CrossRef]
- Samet, Y.; Agengui, L.; Abdelhedi, R. Anodic oxidation of chlorpyrifos in aqueous solution at lead dioxide electrodes. J. Electroanal. Chem. 2010, 650, 152–158. [Google Scholar] [CrossRef]
- Ammar, H.B.; Ben Brahim, M.; Abdelhedi, R.; Samet, Y. Green electrochemical process for metronidazole degradation at BDD anode in aqueous solutions via direct and indirect oxidation. Sep. Purif. Technol. 2016, 157, 9–16. [Google Scholar] [CrossRef]
- Salazar, C.; Contreras, N.; Mansilla, H.D.; Yanez, J.; Salazar, R. Electrochemical degradation of the antihypertensive losartan in aqueous medium by electro-oxidation with boron-doped diamond electrode. J. Hazard. Mater. 2016, 319, 84–92. [Google Scholar] [CrossRef]
- Devilliers, D.; Thi, M.T.D.; Mahé, E.; Dauriac, V.; Lequeux, N. Electroanalytical investigations on electrodeposited lead dioxide. J. Electroanal. Chem. 2004, 573, 227–239. [Google Scholar] [CrossRef]
- Wang, L.Z.; Zhao, Y.M.; Fu, J.F. The influence of TiO2 and aeration on the kinetics of electrochemical oxidation of phenol in packed bed reactor. J. Hazard. Mater. 2008, 160, 608–613. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, C.Z.; Lu, J.; Hu, C.J.; Peng, S.C.; Chen, T.H. Electro-catalytic degradation of bisphenol A with modified Co3O4/beta-PbO2/Ti electrode. Electrochim. Acta 2014, 118, 169–175. [Google Scholar] [CrossRef]
- Su, J.Q.; Wei, B.; Ou-Yang, W.Y.; Huang, F.Y.; Zhao, Y.; Xu, H.J.; Zhu, Y.G. Antibiotic resistome and its association with bacterial communities during sewage sludge composting. Environ. Sci. Technol. 2015, 49, 7356–7363. [Google Scholar] [CrossRef]
- Mao, D.Q.; Luo, Y.; Mathieu, J.; Wang, Q.; Feng, L.; Mu, Q.H.; Feng, C.Y.; Alvarez, P.J.J. Persistence of extracellular DNA in river sediment facilitates antibiotic resistance gene propagation. Environ. Sci. Technol. 2014, 48, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Mazel, D. Integrons: Agents of bacterial evolution. Nat. Rev. Microbiol. 2006, 4, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Chakraborty, R. Incidence of class 1 integrons in multiple antibiotic-resistant Gram-negative copiotrophic bacteria from the River Torsa in India. Res. Microbiol. 2006, 157, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Karkman, A.; Parnanen, K.; Larsson, D.G.J. Fecal pollution can explain antibiotic resistance gene abundances in anthropogenically impacted environments. Nat. Commun. 2019, 10, 80. [Google Scholar] [CrossRef]
- Wu, D.; Su, Y.; Xi, H.; Chen, X.; Xie, B. Urban and agriculturally influenced water contribute differently to the spread of antibiotic resistance genes in a mega-city river network. Water Res. 2019, 158, 11–21. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhai, L.; Wang, P.; Li, Y.; Gu, Y. Ti/PbO2 Electrode Efficiency in Catalytic Chloramphenicol Degradation and Its Effect on Antibiotic Resistance Genes. Int. J. Environ. Res. Public Health 2022, 19, 15632. https://doi.org/10.3390/ijerph192315632
Liu H, Zhai L, Wang P, Li Y, Gu Y. Ti/PbO2 Electrode Efficiency in Catalytic Chloramphenicol Degradation and Its Effect on Antibiotic Resistance Genes. International Journal of Environmental Research and Public Health. 2022; 19(23):15632. https://doi.org/10.3390/ijerph192315632
Chicago/Turabian StyleLiu, Hao, Luwei Zhai, Pengqi Wang, Yanfeng Li, and Yawei Gu. 2022. "Ti/PbO2 Electrode Efficiency in Catalytic Chloramphenicol Degradation and Its Effect on Antibiotic Resistance Genes" International Journal of Environmental Research and Public Health 19, no. 23: 15632. https://doi.org/10.3390/ijerph192315632
APA StyleLiu, H., Zhai, L., Wang, P., Li, Y., & Gu, Y. (2022). Ti/PbO2 Electrode Efficiency in Catalytic Chloramphenicol Degradation and Its Effect on Antibiotic Resistance Genes. International Journal of Environmental Research and Public Health, 19(23), 15632. https://doi.org/10.3390/ijerph192315632






