Corticospinal Responses Following Gait-Specific Training in Stroke Survivors: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Literature Source
2.2. Eligibility Criteria
2.3. Screening of Studies
2.4. Methodological Quality and Risk of Bias
2.5. Data Extraction
3. Results
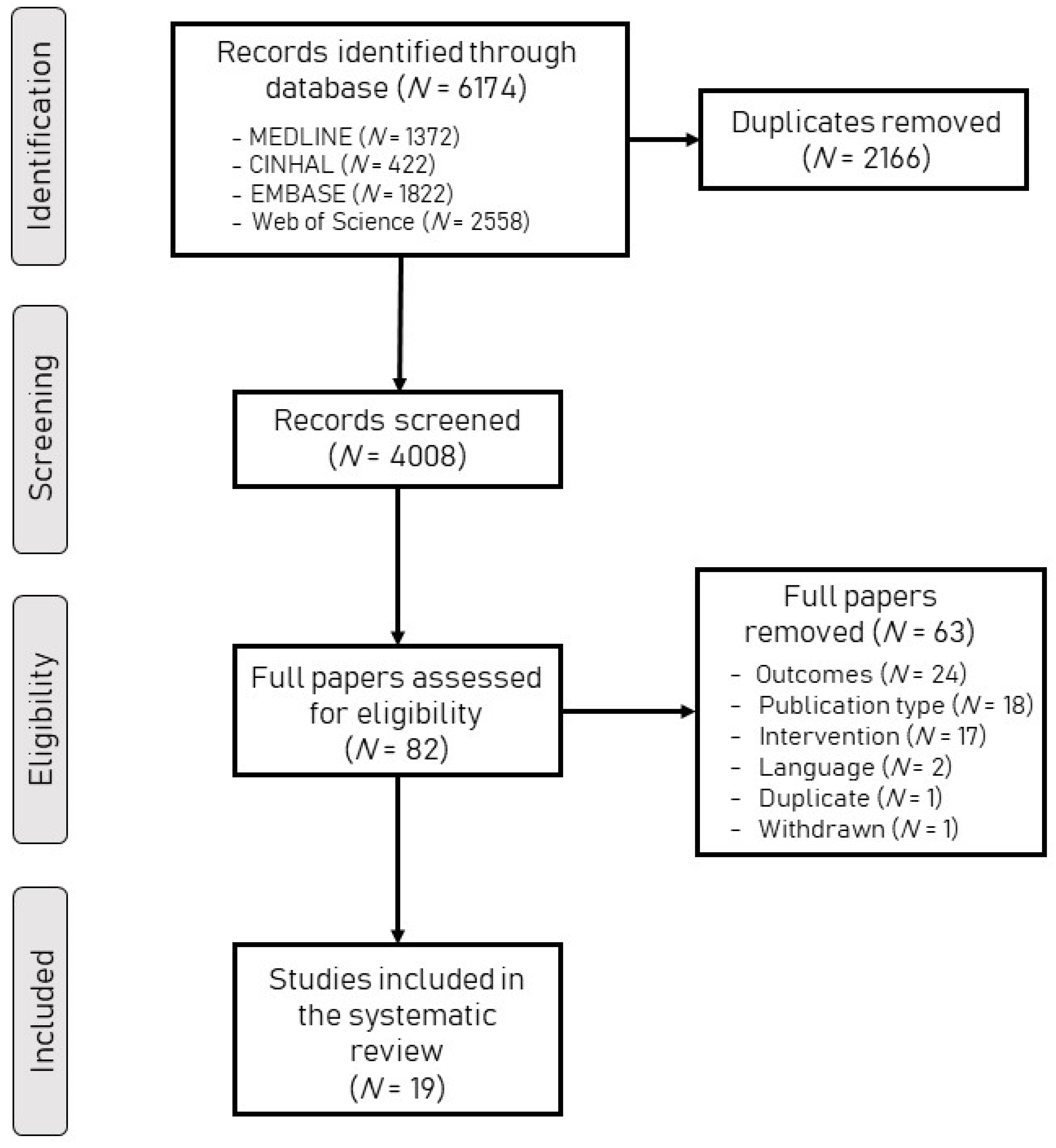
3.1. Search Results
3.2. Risk of Bias
3.3. Characteristics of the Participants
3.4. Gait Training Protocols
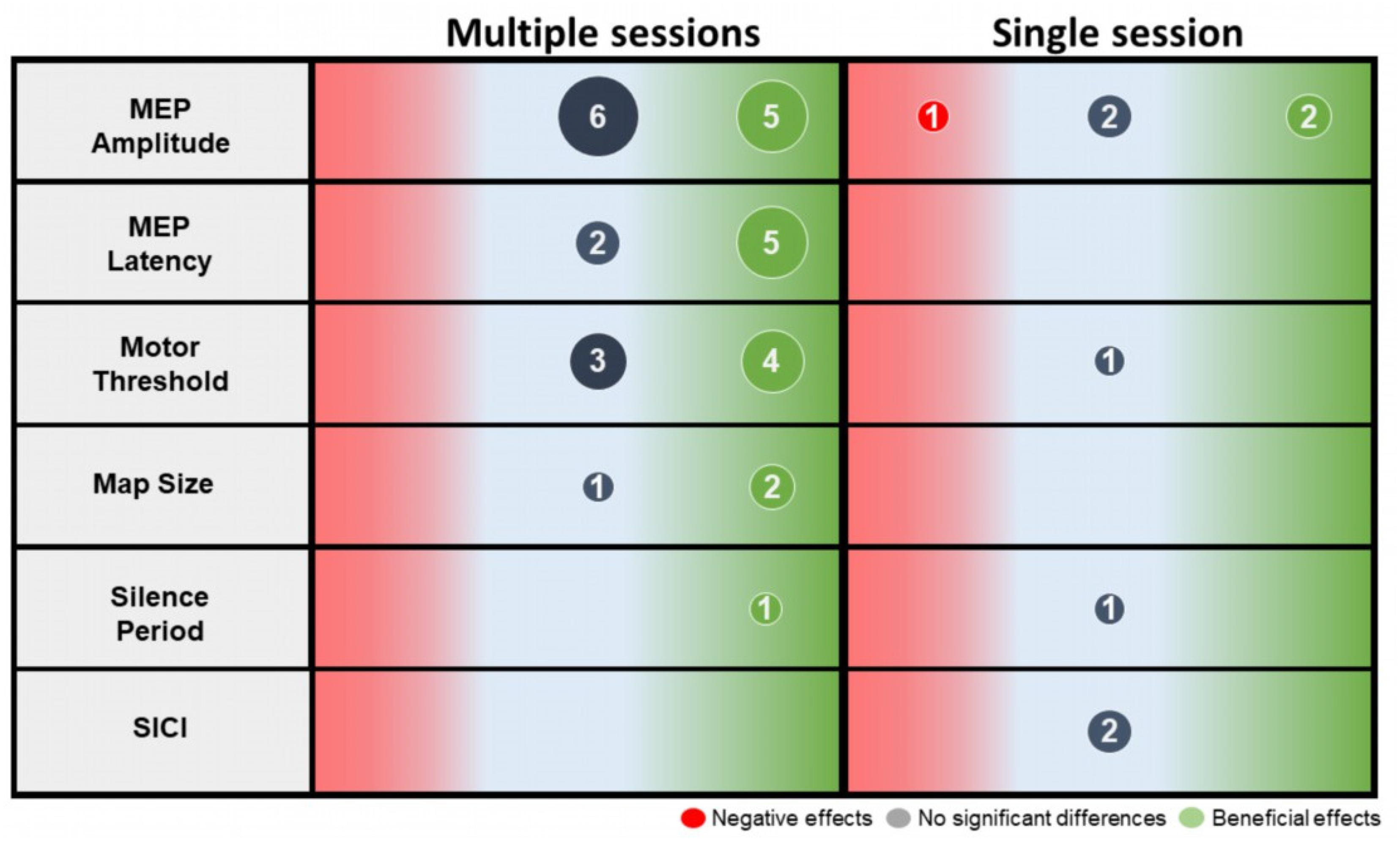
3.5. Effect of Gait-Specific Training on Corticospinal Excitability
4. Discussion
4.1. Effect of Gait-Specific Training on Corticospinal Excitability
4.2. Clinical Recommendations
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Strong, K.; Mathers, C.; Bonita, R. Preventing stroke: Saving lives around the world. Lancet Neurol. 2007, 6, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, Y.; Otani, T.; Morioka, S. Agency judgments in post-stroke patients with sensorimotor deficits. PLoS ONE 2020, 15, e0230603. [Google Scholar] [CrossRef] [PubMed]
- Kessner, S.S.; Schlemm, E.; Cheng, B.; Bingel, U.; Fiehler, J.; Gerloff, C.; Thomalla, G. Somatosensory Deficits after Ischemic Stroke. Stroke 2019, 50, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Francisco, G.E.; Zhou, P. Post-stroke Hemiplegic Gait: New Perspective and Insights. Front. Physiol. 2018, 9, 1021. [Google Scholar] [CrossRef]
- Barthélemy, D.; Knudsen, H.; Willerslev-Olsen, M.; Lundell, H.; Nielsen, J.B.; Biering-Sørensen, F. Functional implications of corticospinal tract impairment on gait after spinal cord injury. Spinal Cord 2013, 51, 852–856. [Google Scholar] [CrossRef][Green Version]
- Barthélemy, D.; Grey, M.J.; Nielsen, J.B.; Bouyer, L. Involvement of the corticospinal tract in the control of human gait. Prog. Brain Res. 2011, 192, 181–197. [Google Scholar] [CrossRef]
- Groppa, S.; Oliviero, A.; Eisen, A.; Quartarone, A.; Cohen, L.; Mall, V.; Kaelin-Lang, A.; Mima, T.; Rossi, S.; Thickbroom, G.; et al. A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clin. Neurophysiol. 2012, 123, 858–882. [Google Scholar] [CrossRef]
- Cleland, B.T.; Sisel, E.; Madhavan, S. Motor evoked potential latency and duration from tibialis anterior in individuals with chronic stroke. Exp. Brain Res. 2021, 239, 2251–2260. [Google Scholar] [CrossRef]
- Ahonen, J.-P.; Jehkonen, M.; Dastidar, P.; Molnár, G.; Häkkinen, V. Cortical silent period evoked by transcranial magnetic stimulation in ischemic stroke. Electroencephalogr. Clin. Neurophysiol. Mot. Control 1998, 109, 224–229. [Google Scholar] [CrossRef]
- Duncan, P. Stroke Disability. Phys. Ther. 1994, 74, 399–407. [Google Scholar] [CrossRef]
- Palmer, J.A.; Needle, A.R.; Pohlig, R.T.; Binder-Macleod, S.A. Atypical cortical drive during activation of the paretic and nonparetic tibialis anterior is related to gait deficits in chronic stroke. Clin. Neurophysiol. 2016, 127, 716–723. [Google Scholar] [CrossRef]
- Cherni, Y.; Bouyer, L.; Bretheau, F.; Mercier, C. Effect of a complex walking task on corticospinal excitability and muscle activity in individuals with cerebral palsy: A multiple-case study. Gait Posture 2022, 97, S324–S325. [Google Scholar] [CrossRef]
- Meijer, R.; Plotnik, M.; Zwaaftink, E.G.; Van Lummel, R.C.; Ainsworth, E.; Martina, J.D.; Hausdorff, J.M. Markedly impaired bilateral coordination of gait in post-stroke patients: Is this deficit distinct from asymmetry? A cohort study. J. Neuroeng. Rehabil. 2011, 8, 23. [Google Scholar] [CrossRef]
- Jonkers, I.; Delp, S.; Patten, C. Capacity to increase walking speed is limited by impaired hip and ankle power generation in lower functioning persons post-stroke. Gait Posture 2009, 29, 129–137. [Google Scholar] [CrossRef]
- Wei, W.E.; De Silva, D.A.; Chang, H.M.; Yao, J.; Matchar, D.B.; Young, S.H.Y.; See, S.J.; Lim, G.H.; Wong, T.H.; Venketasubramanian, N. Post-stroke patients with moderate function have the greatest risk of falls: A National Cohort Study. BMC Geriatr. 2019, 19, 373. [Google Scholar] [CrossRef]
- Pohl, P.S.; Duncan, P.W.; Perera, S.; Liu, W.; Lai, S.M.; Studenski, S.; Long, J. Influence of stroke-related impairments on performance in 6-minute walk test. J. Rehabil. Res. Dev. 2002, 39, 439–444. [Google Scholar]
- Michael, K.M.; Allen, J.K.; Macko, R.F. Reduced Ambulatory Activity after Stroke: The Role of Balance, Gait, and Cardiovascular Fitness. Arch. Phys. Med. Rehabil. 2005, 86, 1552–1556. [Google Scholar] [CrossRef]
- Wall, J.C.; Ashburn, A. Assessment of gait disability in hemiplegics. Hemiplegic gait. Scand. J. Rehabil. Med. 1979, 11, 95–103. [Google Scholar]
- Bohannon, R.W.; Kloter, K.; Cooper, J. Recovery and outcome of patients with stroke treated in an acute care hospital. J. Stroke Cerebrovasc. Dis. 1991, 1, 190–195. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Morton, M.G.; Wikholm, J.B. Importance of four variables of walking to patients with stroke. Int. J. Rehabil. Res. 1991, 14, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-M.; Lee, Y.-S.; Moon, B.-M.; Sim, S.-M. A Comparison of the Effects of Overground Gait Training and Treadmill Gait Training According to Stroke Patients’ Gait Velocity. J. Phys. Ther. Sci. 2013, 25, 379–382. [Google Scholar] [CrossRef]
- Polese, J.C.; Ada, L.; Dean, C.; Nascimento, L.R.; Teixeira-Salmela, L.F. Treadmill training is effective for ambulatory adults with stroke: A systematic review. J. Physiother. 2013, 59, 73–80. [Google Scholar] [CrossRef]
- Cherni, Y.; Gagné-Pelletier, L.; Bouyer, L.; Mercier, C. Lower-Body Positive Pressure Treadmill Training for Pediatric Gait Disorders: A Scoping Review. Appl. Sci. 2021, 12, 323. [Google Scholar] [CrossRef]
- Cherni, Y.; Ballaz, L.; Lemaire, J.; Maso, F.D.; Begon, M. Effect of low dose robotic-gait training on walking capacity in children and adolescents with cerebral palsy. Neurophysiol. Clin. Clin. Neurophysiol. 2020, 50, 507–519. [Google Scholar] [CrossRef]
- Cherni, Y.; Ziane, C. A Narrative Review on Robotic-Assisted Gait Training in Children and Adolescents with Cerebral Palsy: Training Parameters, Choice of Settings, and Perspectives. Disabilities 2022, 2, 293–303. [Google Scholar] [CrossRef]
- Nam, K.Y.; Kim, H.J.; Kwon, B.S.; Park, J.-W.; Lee, H.J.; Yoo, A. Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: A systematic review. J. Neuroeng. Rehabil. 2017, 14, 24. [Google Scholar] [CrossRef]
- Moreau, N.G.; Bodkin, A.W.; Bjornson, K.; Hobbs, A.; Soileau, M.; Lahasky, K. Effectiveness of Rehabilitation Interventions to Improve Gait Speed in Children with Cerebral Palsy: Systematic Review and Meta-analysis. Phys. Ther. 2016, 96, 1938–1954. [Google Scholar] [CrossRef]
- Enzinger, C.; Dawes, H.; Johansen-Berg, H.; Wade, D.; Bogdanovic, M.; Collett, J.; Guy, C.; Kischka, U.; Ropele, S.; Fazekas, F.; et al. Brain Activity Changes Associated with Treadmill Training after Stroke. Stroke 2009, 40, 2460–2467. [Google Scholar] [CrossRef]
- Luft, A.R.; Macko, R.F.; Forrester, L.W.; Villagra, F.; Ivey, F.; Sorkin, J.D.; Whitall, J.; McCombe-Waller, S.; Katzel, L.; Goldberg, A.P.; et al. Treadmill Exercise Activates Subcortical Neural Networks and Improves Walking after Stroke. Stroke 2008, 39, 3341–3350. [Google Scholar] [CrossRef]
- Jones, T.A. Motor compensation and its effects on neural reorganization after stroke. Nat. Rev. Neurosci. 2017, 18, 267–280. [Google Scholar] [CrossRef]
- Nudo, R.J. Postinfarct Cortical Plasticity and Behavioral Recovery. Stroke 2007, 38, 840–845. [Google Scholar] [CrossRef]
- Kleim, J.A.; Hogg, T.M.; Vandenberg, P.M.; Cooper, N.R.; Bruneau, R.; Remple, M. Cortical Synaptogenesis and Motor Map Reorganization Occur during Late, But Not Early, Phase of Motor Skill Learning. J. Neurosci. 2004, 24, 628–633. [Google Scholar] [CrossRef]
- Forrester, L.W.; Hanley, D.F.; Macko, R.F. Effects of Treadmill Exercise on Transcranial Magnetic Stimulation−Induced Excitability to Quadriceps after Stroke. Arch. Phys. Med. Rehabil. 2006, 87, 229–234. [Google Scholar] [CrossRef]
- Yen, C.-L.; Wang, R.-Y.; Liao, K.-K.; Huang, C.-C.; Yang, Y.-R. Gait Training—Induced Change in Corticomotor Excitability in Patients with Chronic Stroke. Neurorehabilit. Neural Repair 2007, 22, 22–30. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef]
- Chipchase, L.; Schabrun, S.; Cohen, L.; Hodges, P.; Ridding, M.; Rothwell, J.; Taylor, J.; Ziemann, U. A checklist for assessing the methodological quality of studies using transcranial magnetic stimulation to study the motor system: An international consensus study. Clin. Neurophysiol. 2012, 123, 1698–1704. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Naro, A.; Russo, M.; Bramanti, P.; Carioti, L.; Balletta, T.; Buda, A.; Manuli, A.; Filoni, S.; Bramanti, A. Shaping neuroplasticity by using powered exoskeletons in patients with stroke: A randomized clinical trial. J. Neuroeng. Rehabil. 2018, 15, 35. [Google Scholar] [CrossRef]
- Yang, Y.-R.; Chen, I.-H.; Liao, K.-K.; Huang, C.-C.; Wang, R.-Y. Cortical Reorganization Induced by Body Weight–Supported Treadmill Training in Patients with Hemiparesis of Different Stroke Durations. Arch. Phys. Med. Rehabil. 2010, 91, 513–518. [Google Scholar] [CrossRef]
- Jayaraman, A.; O’Brien, M.K.; Madhavan, S.; Mummidisetty, C.K.; Roth, H.R.; Hohl, K.; Tapp, A.; Brennan, K.; Kocherginsky, M.; Williams, K.J.; et al. Stride management assist exoskeleton vs functional gait training in stroke. Neurology 2018, 92, e263–e273. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wei, J.; Huang, X.; Duan, Q.; Zhang, T. Effects of a Brain-Computer Interface-Operated Lower Limb Rehabilitation Robot on Motor Function Recovery in Patients with Stroke. J. Healthc. Eng. 2021, 2021, 4710044. [Google Scholar] [CrossRef]
- Shahine, E.M.; Shafshak, T.S. Central neuroplasticity and lower limbs functional outcome following repetitive locomotor training in stroke patients. Egypt. Rheumatol. Rehabil. 2014, 41, 85–91. [Google Scholar] [CrossRef]
- Palmer, J.A.; Hsiao, H.; Wright, T.; Binder-Macleod, S.A. Single Session of Functional Electrical Stimulation-Assisted Walking Produces Corticomotor Symmetry Changes Related to Changes in Poststroke Walking Mechanics. Phys. Ther. 2017, 97, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Charalambous, C.C.; Reisman, D.S.; Morton, S.M. A short bout of high-intensity exercise alters ipsilesional motor cortical excitability post-stroke. Top. Stroke Rehabil. 2019, 26, 405–411. [Google Scholar] [CrossRef]
- Wang, R.-Y.; Tseng, H.-Y.; Liao, K.-K.; Wang, C.-J.; Lai, K.-L.; Yang, Y.-R. rTMS Combined with Task-Oriented Training to Improve Symmetry of Interhemispheric Corticomotor Excitability and Gait Performance after Stroke. Neurorehabilit. Neural Repair 2011, 26, 222–230. [Google Scholar] [CrossRef]
- Wang, R.-Y.; Wang, F.-Y.; Huang, S.-F.; Yang, Y.-R. High-frequency repetitive transcranial magnetic stimulation enhanced treadmill training effects on gait performance in individuals with chronic stroke: A double-blinded randomized controlled pilot trial. Gait Posture 2018, 68, 382–387. [Google Scholar] [CrossRef]
- Chang, M.C.; Kim, D.Y.; Park, D.H. Enhancement of Cortical Excitability and Lower Limb Motor Function in Patients with Stroke by Transcranial Direct Current Stimulation. Brain Stimul. 2015, 8, 561–566. [Google Scholar] [CrossRef]
- Gil Seo, H.; Lee, W.H.; Lee, S.H.; Yi, Y.; Kim, K.D.; Oh, B.-M. Robotic-assisted gait training combined with transcranial direct current stimulation in chronic stroke patients: A pilot double-blind, randomized controlled trial. Restor. Neurol. Neurosci. 2017, 35, 527–536. [Google Scholar] [CrossRef]
- Wong, P.-L.; Yang, Y.-R.; Tang, S.-C.; Huang, S.-F.; Wang, R.-Y. Comparing different montages of transcranial direct current stimulation on dual-task walking and cortical activity in chronic stroke: Double-blinded randomized controlled trial. BMC Neurol. 2022, 22, 119. [Google Scholar] [CrossRef]
- Koganemaru, S.; Kitatani, R.; Fukushima-Maeda, A.; Mikami, Y.; Okita, Y.; Matsuhashi, M.; Ohata, K.; Kansaku, K.; Mima, T. Gait-Synchronized Rhythmic Brain Stimulation Improves Poststroke Gait Disturbance. Stroke 2019, 50, 3205–3212. [Google Scholar] [CrossRef]
- Madhavan, S.; Stinear, J.W.; Kanekar, N. Effects of a Single Session of High Intensity Interval Treadmill Training on Corticomotor Excitability following Stroke: Implications for Therapy. Neural Plast. 2016, 2016, 1686414. [Google Scholar] [CrossRef]
- Krishnan, C.; Ranganathan, R.; Kantak, S.S.; Dhaher, Y.Y.; Rymer, W.Z. Active robotic training improves locomotor function in a stroke survivor. J. Neuroeng. Rehabil. 2012, 9, 57. [Google Scholar] [CrossRef]
- Peurala, S.H.; Tarkka, I.M.; Juhakoski, M.; Könönen, M.; Karhu, J.; Jäkälä, P.; Vanninen, R.; Sivenius, J. Restoration of Normal Cortical Excitability and Gait Ability in Acute Stroke after Intensive Rehabilitation. Cerebrovasc. Dis. 2008, 26, 208–209. [Google Scholar] [CrossRef]
- Poydasheva, A.G.; Saenko, I.A.; Chervyakov, A.V.; Zmeykina, E.A.; Lukmanov, R.H.; Chernikova, L.A.; Suponeva, N.A.; Piradov, M.A.; Kozlovskaya, I.B. Evaluation of changes in the cortical gait control in post-stroke patients induced by the use of the “Regent” soft exoskeleton complex (SEC) by navigated transcranial magnetic stimulation. Fiziol. Cheloveka. 2016, 42, 25–31. [Google Scholar] [CrossRef]
- Keci, A.; Tani, K.; Xhema, J. Role of Rehabilitation in Neural Plasticity. Open Access Maced. J. Med. Sci. 2019, 7, 1540–1547. [Google Scholar] [CrossRef]
- Nudo, R.J.; Wise, B.M.; SiFuentes, F.; Milliken, G.W. Neural Substrates for the Effects of Rehabilitative Training on Motor Recovery after Ischemic Infarct. Science 1996, 272, 1791–1794. [Google Scholar] [CrossRef]
- Nudo, R.J.; Milliken, G.W. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J. Neurophysiol. 1996, 75, 2144–2149. [Google Scholar] [CrossRef]
- Pinter, D.; Landsmann, B.; Pirker, E.; Pichler, G.; Schippinger, W.; Weiss, E.M.; Mathie, G.; Gattringer, T.; Fazekas, F.; Enzinger, C. An exploratory intervention study suggests clinical benefits of training in chronic stroke to be paralleled by changes in brain activity using repeated fMRI. Clin. Interv. Aging 2016, 11, 97–103. [Google Scholar] [CrossRef][Green Version]
- Su, B.; Jia, Y.; Zhang, L.; Li, D.; Shen, Q.; Wang, C.; Chen, Y.; Gao, F.; Wei, J.; Huang, G.; et al. Reliability of TMS measurements using conventional hand-hold method with different numbers of stimuli for tibialis anterior muscle in healthy adults. Front. Neural Circuits 2022, 16, 986669. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.; Barker, A.; Berardelli, A.; Caramia, M.; Caruso, G.; Cracco, R.; Dimitrijević, M.; Hallett, M.; Katayama, Y.; Lücking, C.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Leone, A.; Nguyet, D.; Cohen, L.G.; Brasil-Neto, J.P.; Cammarota, A.; Hallett, M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J. Neurophysiol. 1995, 74, 1037–1045. [Google Scholar] [CrossRef]
- Peters, H.T.; Dunning, K.; Belagaje, S.; Kissela, B.M.; Ying, J.; Laine, J.; Page, S.J. Navigated Transcranial Magnetic Stimulation: A Biologically Based Assay of Lower Extremity Impairment and Gait Velocity. Neural Plast. 2017, 2017, 6971206. [Google Scholar] [CrossRef] [PubMed]
- Kesar, T.M.; Stinear, J.W.; Wolf, S.L. The use of transcranial magnetic stimulation to evaluate cortical excitability of lower limb musculature: Challenges and opportunities. Restor. Neurol. Neurosci. 2018, 36, 333–348. [Google Scholar] [CrossRef]
- Kleim, J.A.; Jonest, T. Principles of Experience-Dependent Neural Plasticity: Implications for Rehabilitation after Brain Damage. J. Speech, Lang. Hear. Res. 2008, 51, S225–S239. [Google Scholar] [CrossRef]
- Lundbye-Jensen, J.; Marstrand, P.C.D.; Nielsen, J.B. Motor skill training and strength training are associated with different plastic changes in the central nervous system. J. Appl. Physiol. 2005, 99, 1558–1568. [Google Scholar] [CrossRef]
- Woldag, H.; Hummelsheim, H. Evidence-based physiotherapeutic concepts for improving arm and hand function in stroke patients. J. Neurol. 2002, 249, 518–528. [Google Scholar] [CrossRef]
- Barreca, S.; Wolf, S.L.; Fasoli, S.; Bohannon, R. Treatment Interventions for the Paretic Upper Limb of Stroke Survivors: A Critical Review. Neurorehabilit. Neural Repair 2003, 17, 220–226. [Google Scholar] [CrossRef]
- Kwakkel, G. Impact of intensity of practice after stroke: Issues for consideration. Disabil. Rehabil. 2006, 28, 823–830. [Google Scholar] [CrossRef]
- Lohse, K.R.; Lang, C.; Boyd, L.A. Is More Better? Using Metadata to Explore Dose–Response Relationships in Stroke Rehabilitation. Stroke 2014, 45, 2053–2058. [Google Scholar] [CrossRef]
- Bernhardt, J.; Indredavik, B.; Langhorne, P. When Should Rehabilitation Begin after Stroke? Int. J. Stroke 2012, 8, 5–7. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, Y.; Liu, H. Corticomuscular Coherence and Its Applications: A Review. Front. Hum. Neurosci. 2019, 13, 100. [Google Scholar] [CrossRef]
| Authors | Design | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | Item 9 | Item 10 | Item 11 | Total/10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calabrò [40] | RCT | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Yang [41] | RCT | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Jayaraman [42] | RCT | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Li [43] | RCT | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Shahine [44] | RCT | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Yen [35] | RCT | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Forrester [34] | Cross sectional | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Palmer [45] | Crossover study | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Li [46] | Crossover study | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 5 |
| Wang [47] * | Pre/Post study * | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 4 |
| Chang [49] * | Pre/Post study * | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 3 |
| Seo [50] * | Pre/Post study * | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 3 |
| Wang [48] * | Pre/Post study * | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 3 |
| Wong [51] | Pre/Post study * | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 3 |
| Koganemaru [52] * | Pre/Post study * | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 3 |
| Madhavan [53] * | Pre/Post study * | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 3 |
| Poydasheva [56] | Pre/Post study | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 2 |
| Krishnan [54] | Case study | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 |
| Peurala [55] | Case study | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Studies | Participant Factors | Methodological Factors | Total | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | /26 | |
| Calabrò [40] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 13 |
| Yang [41] | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 14 |
| Jayaraman [42] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 20 |
| Li [43] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 19 |
| Shahine [44] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 13 |
| Yen [35] | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 13 |
| Forrester [34] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 17 |
| Palmer [45] | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 17 |
| Li [46] | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 14 |
| Chang [49] | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 16 |
| Seo [50] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 12 |
| Wang [47] | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 17 |
| Wang [48] | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 14 |
| Wong [51] | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 15 |
| Koganemaru [52] | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 14 |
| Madhavan [53] | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 17 |
| Poydasheva [56] | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 5 |
| Krishnan [54] | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 12 |
| Peurala [55] | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 15 |
| Authors | N | Age (Years) (Mean ± SD or Median [Quartiles]) | Time Poststroke (Mean ± SD or Median [Quartiles]) | Interventions | Key Outcomes | Results | |
|---|---|---|---|---|---|---|---|
| Experimental | Control | ||||||
| Calabrò [40] | EG = 20 CG = 20 | EG = 69 ± 4 CG = 67 ± 6 | EG = 10 ± 3 months CG = 11 ± 3 months | Exoskeleton (Ekso) training | Conventional overground gait training | MEP amplitudes (peak to peak) in HA in both sides. Stim intensity: N/S | In both groups: ↑ MEP amplitude on the paretic side. Greater change in EG. ↓ MEP amplitude in non-paretic side in EG. |
| 40 sessions: 8weeks—5x/week | |||||||
| Chang [49] | EG = 12 | EG = 59.9 ± 10.2 | EG = 16.0 ± 6.2 days | Overground gait training including postural control, motor function, and movement patterns. | None | MEP amplitudes (peak to peak) and latency in TA in affected side at rest. Stim intensity: 100% MSO | ↑ MEP amplitude (+127%) and ↓ MEP latency (−3%) after training. |
| 10 sessions: 2 weeks—5x/week | |||||||
| Forrester [34] | EG = 3 CG = 8 | EG = 65.3 ± 6.3 CG = 62.2 ± 1.7 | EG = 31.2 ± 20.4 months CG = 31.2 ± 20.4 months | A group previously trained with treadmill received a submaximal effort (60% heart rate reserve) treadmill training. | A non-trained treadmill group received a submaximal effort (60% heart rate reserve) treadmill training. | RMT, MEP amplitudes (peak to peak) and latency in VM on both sides at rest. Stim intensity: 110% RMT | In CG: ↓ MEP latencies in the paretic and non-paretic side (−7%). No significant change in the EG. No significant difference between groups. |
| 72 sessions: 24 weeks—3x/week | |||||||
| Jayaraman [42] | EG = 25 CG = 25 | EG = 59.5 ± 9.7 CG = 61.6 ± 12.6 | EG = 85.2 ± 74.4 months CG = 64.8 ± 36.0 months | Exoskeleton (Honda Stride Management Assist) training | Treadmill gait training + patients’ goals-oriented tasks | MEP amplitudes (Slope) in RF, MH and TA in both sides. Stim intensity: Recruitment curves for each muscle were obtained by collecting MEPs for a range of stimulus intensities from 80% to 140% of AMT, in increments of 10%, resulting in 7 total intensities. | In both groups: ↑ MEP amplitudes of paretic RF. Greater change in EG (+178% ± 75%) vs. in CG (33% ± 32%). In CG: ↑ MEP amplitude in MH (110%) and TA (214%). |
| 18 sessions: 6–8 weeks s; 3x/week | |||||||
| Koganemaru [52] | N = 11 | 65.7 ± 3.6 | 74.4 ± 32.3 months | Gait training on a treadmill and FES to assist paretic ankle. | None | AMT in TA and gastrocnemius muscles in both sides. Stim intensity: N/S | No significant differences. |
| 12 sessions: 4 weeks—3x/week | |||||||
| Krishnan [54] | N = 1 | 52 | 7.0 months | RAGT (Lokomat) | None | MEP amplitudes during gait in GM, RF, VM, MG, SOL (during stance) MH, LH, TA (during swing). Stim intensity: N/S | ↓ MEP amplitude of the VM (−55%), MH (−72%) and GM (−66%) muscles after training. |
| 1 session | |||||||
| Li [46] | N = 13 | 65.8 ± 7.2 | 39.5 ± 33.7 months | Phase 1: high-intensity exercise priming (i.e., fast treadmill walking); Phase 2: rest | None | MEP amplitudes (peak to peak) in extensor carpi radialis and SICI in both sides. Stim intensity: 120% RMT | ↑ MEP post-exercise in paretic side compared to rest in paretic side (+0.35%). |
| 1 session | |||||||
| Li [43] | EG = 12 CG = 13 | EG = 51.2 ± 7.8 CG = 49.6 ± 8.4 | N/A | Exoskeleton (BCI-LLRR) + routine rehabilitation | Routine rehabilitation interventions (pulsed electrical therapy, partial hemiplegia comprehensive training) | MEP amplitudes (peak to peak) and latency in TA in both sides. Stim intensity: 90% TA muscle AMT. | In both groups: ↓ MEP latencies and ↑ MEP amplitude. Greater change in EG. |
| 30 sessions: around 4 weeks | |||||||
| Madhavan [53] | N = 11 | 58 ± 2.7 | 108.0 ± 21.6 months | High intensity interval treadmill training. | None | MEP amplitudes (peak to peak) in TA in both sides. Stim intensity: N/S | No significant differences. |
| 1 session | |||||||
| Palmer [45] | N = 20 | 59.5 ± 12.0 | 42.0 ± 2.05 months | Phase 1: Walking with FES; Phase 2: Walking without FES. | None | MEPs amplitudes in TA and SOL in both sides. Stim intensity: N/S | ↑ MEP amplitudes in the paretic SOL (+30%) following gait training with FES. |
| 1 session/intervention—1week apart | |||||||
| Peurala [55] | N = 1 | 76.0 | Acute phase | Conventional gait training (standing and overground exercises) | None | RMT, MEP amplitudes and silent period in TA in both sides. Stim intensity: N/S | ↓ RMT and silent period in the non-paretic side. |
| 15 sessions: 3 weeks—5x/week | |||||||
| Poydasheva [56] | N = 14 | 53.0 yrs [49.0; 62.0]; | 14.2 [7.0; 2.0] months | Standard rehabilitation + rehabilitation exercises with Exoskeleton | None | MEP amplitudes and latency in TA and map size in both sides. Stim intensity: N/S | ↓ MEP latency (−8.5%) in the paretic side. |
| 10 sessions: 2 weeks s—5x/week | |||||||
| Seo [50] | N = 10 | 62.9 ± 8.9 | 152.5 ± 122.8 months | RAGT on a treadmill (Walkbot_S) + sham tDCS | None | RMT, MEP amplitudes (peak to peak) and latency in HA in both sides. Stim intensity: N/S | No significant differences. |
| 10 sessions: 2 weeks—5x/week | |||||||
| Shahine [44] | EG = 25 CG = 25 | EG = 58.3 ± 8.6 CG = 59.7 ± 7.4 | EG = 30.3 ± 21.8 months CG = 28.4 ± 19.8 months | Electromechanical gait training (GT): movements of lower limb are assisted. | BWSTT: weight support with free movements of lower limb. | RMT, MEP amplitudes (peak to peak) and latency in RF, TA, MG. Stim intensity: N/S | In both groups: ↓ RMT in RF, TA, MG; ↑ MEP amplitude in RF, TA, MG; ↓ MEP latency in RF, TA, MG. No significant differences between groups. |
| 48 sessions: 8 weeks—6x/week | |||||||
| Wang [47] | N = 12 | 62.9 ± 10.9 | 24.0 ± 14.0 months | Sham rTMS, followed by functional task-oriented training (standing and walking) | None | MEP amplitudes (peak to peak) and latency in RF sides at rest. Stim intensity: 110% of RMT | No significant differences. |
| 10 sessions: 2 weeks—5x/week | |||||||
| Wang [48] | N = 6 | 54.7 ± 12.2 | 31.8 ± 24 months | Regular physical therapy + Sham rTMS, followed by treadmill training | None | MEP amplitudes (peak to peak) in TA in both sides at rest. Stim intensity: 120% of RMT | No significant differences. |
| 9 sessions: 3 weeks—3x/week | |||||||
| Wong [51] | N = 12 | 57.3 [46.1; 62.8] | 54.0 [24.0; 93.4] months | Walking under 3 conditions: cognitive dual task walking, motor dual task walking, and single walking. | None | RMT, MEP amplitudes (peak to peak), cortical silent period duration, and SICI in the paretic TA during contraction. Stim intensity: 120% RMT | No significant differences. |
| 1 session of 20 min each exercise | |||||||
| Yang [41] | EG Chro = 5 EG Sub = 5 CG Chro = 4 CG Sub = 4 | EG Chro = 57.5 ± 6.1 EG Sub = 56.8 ± 1.3 CG Chro = 48.1 ± 3.7 CG Sub = 61.8 ± 3.8 | EG Chro = 25.2 ± 3.6 months EG Sub = 3.0 ± 1.0 months CG Chro = 34.8 ± 6.0 months CG Sub = 3.0 ± 1.0 months | BWSTT + General exercise program (stretching, strengthening, endurance, overground walking training) | General exercise program (stretching, strengthening, endurance, overground walking training) | RMT and map size of HA at rest. Stim intensity: 110% of RMT | In EG: ↓ RMT in subacute patients (−23%). ↑ map size in subacute (+134%) and chronic patients (+38%). |
| 12 sessions: 4 week—3x/week | |||||||
| Yen [35] | EG = 7 CG = 7 | EG = 57.3 ± 16.4 CG = 56.0 ± 12.7 | EG = 22.8 ± 7.32 months CG = 22.8 ± 7.32 months | BWSTT + General exercise program (stretching, strengthening, endurance, overground walking training) | General exercise program (stretching, strengthening, endurance, overground walking training) | RMT, map size of TA and HA in both sides at rest. Stim intensity: 110% of RMT | In EG: ↓ RMT for TA in the non-paretic side (−9%). ↑ map size for TA in the paretic (+24%) and non-paretic (+35%) sides. ↑ map size of AH in the paretic side (500%). |
| 12 sessions: 3x/week of BWSTT; 2 to 5x/week of general exercise. | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherni, Y.; Tremblay, A.; Simon, M.; Bretheau, F.; Blanchette, A.K.; Mercier, C. Corticospinal Responses Following Gait-Specific Training in Stroke Survivors: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 15585. https://doi.org/10.3390/ijerph192315585
Cherni Y, Tremblay A, Simon M, Bretheau F, Blanchette AK, Mercier C. Corticospinal Responses Following Gait-Specific Training in Stroke Survivors: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(23):15585. https://doi.org/10.3390/ijerph192315585
Chicago/Turabian StyleCherni, Yosra, Alexia Tremblay, Margaux Simon, Floriane Bretheau, Andréanne K. Blanchette, and Catherine Mercier. 2022. "Corticospinal Responses Following Gait-Specific Training in Stroke Survivors: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 23: 15585. https://doi.org/10.3390/ijerph192315585
APA StyleCherni, Y., Tremblay, A., Simon, M., Bretheau, F., Blanchette, A. K., & Mercier, C. (2022). Corticospinal Responses Following Gait-Specific Training in Stroke Survivors: A Systematic Review. International Journal of Environmental Research and Public Health, 19(23), 15585. https://doi.org/10.3390/ijerph192315585








