Recreational Running Motivations among Breast Cancer Survivors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Research Tool
- Physical health: general health orientation (six items) and weight concern (four items).
- Achievement: personal goal achievement (six items) and competition (four items).
- Social motives: recognition (six items) and affiliation (six items).
- Psychological motives: psychological coping (nine items), self-esteem (eight items), and life meaning (seven items).
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masters, K.S.; Ogles, B.M.; Jolton, J.A. The development of an instrument to measure motivation for marathon running: The Motivations of Marathoners Scales (MOMS). Res. Q. Exerc. Sport 1993, 64, 134–143. [Google Scholar] [CrossRef]
- Nikolaidis, P.T.; Chalabaev, A.; Rosemann, T.; Knechtle, B. Motivation in the Athens Classic Marathon: The Role of Sex, Age, and Performance Level in Greek Recreational Marathon Runners. Int. J. Environ. Res. Public Health 2019, 16, 2549. [Google Scholar] [CrossRef] [PubMed]
- Ogles, B.M.; Masters, K.S.; Richardson, S.A. Obligatory running and gender: An analysis of participative motives and training habits. Int. J. Sport Psychol. 1995, 26, 233–248. [Google Scholar]
- Waśkiewicz, Z.; Nikolaidis, P.T.; Gerasimuk, D.; Borysiuk, Z.; Rosemann, T.; Knechtle, B. What Motivates Successful Marathon Runners? The Role of Sex, Age, Education, and Training Experience in Polish Runners. Front. Psychol. 2019, 10, 1671. [Google Scholar] [CrossRef] [PubMed]
- Knechtle, B.; Rüst, C.A.; Rosemann, T. The aspect of nationality in participation and performance in ultra-marathon running—A comparison between ‘Badwater’ and ‘Spartathlon’. OA Sport. Med. 2013, 1, 1. [Google Scholar] [CrossRef]
- Malchrowicz-Mośko, E.; Gravelle, F.; Dąbrowska, A.; León-Guereño, P. Do Years of Running Experience Influence the Motivations of Amateur Marathon Athletes? Int. J. Environ. Res. Public Health 2020, 17, 585. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.T. Significantly greater reduction in breast cancer mortality from post-diagnosis running than walking. Epidemiology 2014, 135, 1195–1202. [Google Scholar] [CrossRef]
- Jochem, C.; Leitzmann, M. Physical Activity and Sedentary Behavior in Relation to Cancer Survival: A Narrative Review. Cancers 2022, 14, 1720. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martinez, O.; Garcia-Montero, C.; Pekarek, L.; Guijarro, L.G.; Castellanos, A.J.; Sanchez-Trujillo, L.; Garcia-Honduvilla, N.; Alvarez-Mon, M.; Bujan, J.; et al. Physical Activity as an Imperative Support in Breast Cancer Management. Cancers 2021, 13, 55. [Google Scholar] [CrossRef]
- Irwin, M.L.; McTiernan, A.; Bernstein, L.; Gilliland, F.D.; Baumgartner, R.; Baumgartner, K.; Ballard-Barbash, R. Physical activity levels among breast cancer survivors. Med. Sci. Sports Exerc. 2004, 36, 1484–1491. [Google Scholar]
- Betof, A.S.; Dewhirst, M.W.; Jones, L.W. Effects and potential mechanisms of exercise training on cancer progression: A translational perspective. Brain Behav. Immun. 2012, 30, S75–S87. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.W.; Peppercom, J.; Scott, J.M.; Battaglini, C. Exercise therapy in the management of solid tumors. Curr. Treat Options Oncol. 2010, 11, 45–58. [Google Scholar] [CrossRef][Green Version]
- Schmitz, K.H.; Ahmed, R.L.; Troxel, A.; Cheville, A.; Smith, R.; Lewis-Grant, L.; Bryan, C.J.; Williams-Smith, C.T.; Greene, Q.P. Weight lifting in women with breast-cancer-related lymphedema. N. Engl. J. Med. 2009, 361, 664–673. [Google Scholar] [CrossRef]
- Torres Lacomba, M.; Yuste Sanchez, M.J.; Zapico Goni, A.; Prieto Merino, D.; Mayoral del Moral, O.; Cerezo Tellez, E.; Minayo Mogollón, E. Effectiveness of early physiotherapy to prevent lymphedema after surgery for breast cancer: Randomized, single blinded, clinical trial. BMJ 2010, 340, b5396. [Google Scholar] [CrossRef]
- Todd, J.; Scally, A.; Dodwell, D.; Horgaan, K.; Topping, A. A randomized controlled trial of two programmes of shoulder exercise following axillary lymph node dissection for invasive breast cancer. Physiotherapy 2008, 94, 265–273. [Google Scholar] [CrossRef]
- Tredan, O.; Bajard, A.; Meunier, A.; Roux, P.; Fiorletta, I.; Gargi, T.; Bachelot, T.; Guastalla, J.P.; Lallemand, Y.; Faure, C.; et al. Body weight change in women receiving chemotherapy for breast cancer: A French prospective study. Clin. Nutr. 2010, 29, 187–191. [Google Scholar] [CrossRef]
- Casla, S.; Hojman, P.; Marquez-Rodas, I.; Lopez-Tarruella, S.; Jerez, Y.; Barakat, R.; Martin, M. Running away from side effects: Physical exercise as a complementary intervention for breast cancer patients. Clin. Transl. Oncol. 2015, 17, 180–196. [Google Scholar] [CrossRef]
- Galanti, G.; Stefani, L.; Gensini, G. Exercise as a prescription therapy for breast and colon cancer survivors. Int. J. Gen. Med. 2013, 6, 245–251. [Google Scholar]
- Johansson, A.; Johansson, A.; Johansson, K. Physical activity during and after adjuvant chemotherapy in patients with breast cancer. Physiotherapy 2013, 99, 221–227. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Wiskemann, J.; Ulrich, C.M.; Schneeweiss, A.; Steindorf, K. Self-reported physical activity behavior of breast cancer survivors during and after adjuvant therapy: 12 months follow-up of two randomized exercise intervention trials. Acta Oncol. 2017, 56, 618–627. [Google Scholar] [CrossRef]
- Elshahat, S.; Treanor, C.; Donnelly, M. Factors influencing physical activity participation among people living with or beyond cancer: A systematic scoping review. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Lynch, A.; Merdjanoff, A.; Wilson, D.; Chiarello, L.; Hay, J.; Mao, J.J. Moving Forward: Older Adult Motivations for Group-Based Physical Activity After Cancer Treatment. Int. J. Behav. Med. 2022, 29, 286–298. [Google Scholar] [CrossRef]
- World Health Organization Guidelines on Physical Activity and Sedentary Behavior; World Health Organization: Geneva, Switzerland, 2020.
- Kiebert, G.M. Quality of life as a result of clinical trials in oncology—Selected issues. In Quality of Life with Cancer; Meyza, J., Ed.; M. Sklodowska-Curie Institute of Oncology: Warsaw, Poland, 1997; pp. 43–57. [Google Scholar]
- Courney, K.S.; Friedenreich, C.M. Physical exercise and quality of life following cancer diagnosis: A literature review. Ann. Behav. Med. 1999, 21, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Courney, K.S.; McKey, J.R.; Jones, L.W. Coping with cancer: Can exercise help? Phys. Sportsmed. 2003, 28, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Czerniak, U.; Demuth, A. Relationship between life quality perception and physical activity of females after mastectomy. Pol. J. Sport. Med. 2010, 26, 98–105. [Google Scholar]
- Czerniak, U.; Demuth, A.; Krzykała, M.; Ziółkowska-Łajp, E. Body fat and quality of life in women treated for breast cancer. Stud. Phys. Cult. Tour. 2012, 19, 21–24. [Google Scholar]
- Sander, A.P.; Wilson, J.; Izzo, N.; Mountford, S.A.; Hayes, K.W. Factors That Affect Decisions About Physical Activity and Exercise in Survivors of Breast Cancer: A Qualitative Study. Phys. Ther. 2012, 92, 525–536. [Google Scholar] [CrossRef]
- Segal, R.; Zwaal, C.; Green, E.; Tomasone, J.R.; Loblaw, A.; Petrella, T.; Exercise for People with Cancer Guideline Development Group. Exercise for people with cancer: A clinical practice guideline. Curr. Oncol. 2017, 24, 40–46. [Google Scholar] [CrossRef]
- McNeely, M.L.; Campbell, K.L.; Rowe, B.H.; The Exercise for People with Cancer Guideline Development Group. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. CMAJ 2006, 175, 34–41. [Google Scholar] [CrossRef]
- Valenti, M.; Porzio, G.; Aielli, F.; Verna, L.; Cannita, K.; Manno, R.; Masedu, F.; Marchetti, P.; Ficorella, C. Physical Exercise and Quality of life in breast cancer survivors. Int. J. Med. Sci. 2008, 5, 24–28. [Google Scholar] [CrossRef]
- Yuan-Yuan, L.; Ho, S.C.; Cheung, K.L.; Yeo, V.A.; Lee, R.; Kwok, C.; Cheng, A.; Mo, F.; Yeo, W. Higher Level of Sports Activities Participation during Five-Year Survival Is Associated with Better Quality of Life among Chinese Breast Cancer Survivors. Cancers 2021, 13, 6056. [Google Scholar] [CrossRef]
- Blanchard, C.M.; Courneya, K.S.; Laing, D. Effects of acute exercise on state anxiety in breast cancer survivors. Oncol. Nurs. Forum. 2001, 28, 1617–1621. [Google Scholar] [PubMed]
- Friedenreich, C.M.; Kopciuk, G.J.; Kopciuk, K.A.; Mackey, J.R.; Courneya, K.S. Prospective cohort study of lifetime physical activity and breast cancer survivors. Int. J. Cancer 2009, 124, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nagpal, R.; Hemalatha, R.; Verma, V.; Kumar, A.; Singh, S.; Marotta, F.; Jain, S.; Yadav, H. Targeted cancer therapies: The future of cancer treatment. Acta Biomed. Atenei Parm. 2012, 83, 220–233. [Google Scholar]
- American Cancer Society. Available online: www.cancer.org (accessed on 15 July 2022).
- Andrykowski, M.A.; Curran, S.L.; Lightner, R. Off-treatment fatigue in breast cancer survivors: A controlled comparison. J. Behav. Med. 1998, 21, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Avancini, A.; Skrocke, K.; Tregnano, D.; Frada, P.; Trestini, I.; Cercato, M.C.; Bonaiuto, C.; Tarperi, C.; Schena, F.; Milella, M.; et al. Running with cancer: A qualitative study to evaluate barriers and motivations in running for female oncological patients. PLoS ONE 2020, 15, e0227846. [Google Scholar] [CrossRef] [PubMed]
- Huy, C.; Schmidt, M.E.; Vrieling, A.; Chang-Claude, J.; Steindorf, K. Physical activity in a German breast cancer patient cohort: One-year trends and characteristics associated with change in activity level. Eur. J. Cancer 2012, 48, 297–304. [Google Scholar] [CrossRef]
- Malchrowicz-Mośko, E.; Poczta, J. Running as a form of therapy—Socio-psychological functions of mass running events for men and women. Int. J. Environ. Res. Public Health 2018, 15, 2262. [Google Scholar] [CrossRef]
- Dybała, M. Polska Adaptacja Kwestionariusza Motywów Biegaczy do Biegania/The Polish adaptation of the Motives of Runners for Running Questionnaire. Rozpr. Nauk. 2013, 40, 118–128. [Google Scholar]
- Friedenreich, C.M.; Shaw, E.; Neilson, H.K.; Brenner, D.R. Epidemiology and Biology of Physical Activity and Cancer Recurrence. J. Mol. Med. 2017, 95, 1029–1041. [Google Scholar] [CrossRef]
- Argolo, D.F.; Hudis, C.A.; Iyengar, N.M. The Impact of Obesity on Breast Cancer. Curr. Oncol. Rep. 2018, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, P.J.; Ennis, M.; Pritchard, K.; McCready, D.; Koo, J.; Sidlofsky, S.; Trudeau, M.; Hood, N.; Redwood, S. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. J. Clin. Oncol. 1999, 17, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Aiello, E.; McTiernan, A. Weight loss in breast cancer patient management. J. Clin. Oncol. 2002, 20, 1128–1143. [Google Scholar] [CrossRef]
- Bahri, N.; Fathi Najafi, T.; Homaei Shandiz, F.; Tohidinik, H.R.; Khajavi, A. The relation between stressful life events and breast cancer: A systematic review and meta-analysis of cohort studies. Breast Cancer Res. Treat 2019, 176, 53–61. [Google Scholar] [CrossRef]
- Cuevas, B.T.; Hughes, D.C.; Parma, D.; Trevino-Whitaker, R.A.; Ghosh, S.; Li, R.; Ramirez, A.G. Motivation, Exercise and Stress in Breast Cancer Survivors. Support Care Cancer 2014, 22, 911–917. [Google Scholar] [CrossRef]
- Blanchard, C.M.; Courneya, K.S.; Stein, K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: Results from the American Cancer Society’s SCS-II. J. Clin. Oncol. 2008, 26, 2198–2204. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Denmark-Wahnefried, W.; Galvao, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sports Exerc. 2010, 41, 1409–1426. [Google Scholar] [CrossRef]
- Stempień, J.R.; Stańczyk, M.; Tokarski, J.; Tkaczyk, M. Running for health? Polish long-distance leisure runners and the problem of health literacy. Pol. Sociol. Rev. 2020, 211, 379–392. [Google Scholar]
- Malchrowicz-Mośko, E. Kinesiophobia among Breast Cancer Survivors One Year after Hospital Treatment. Int. J. Environ. Res. Public Health 2022, 19, 14565. [Google Scholar] [CrossRef]
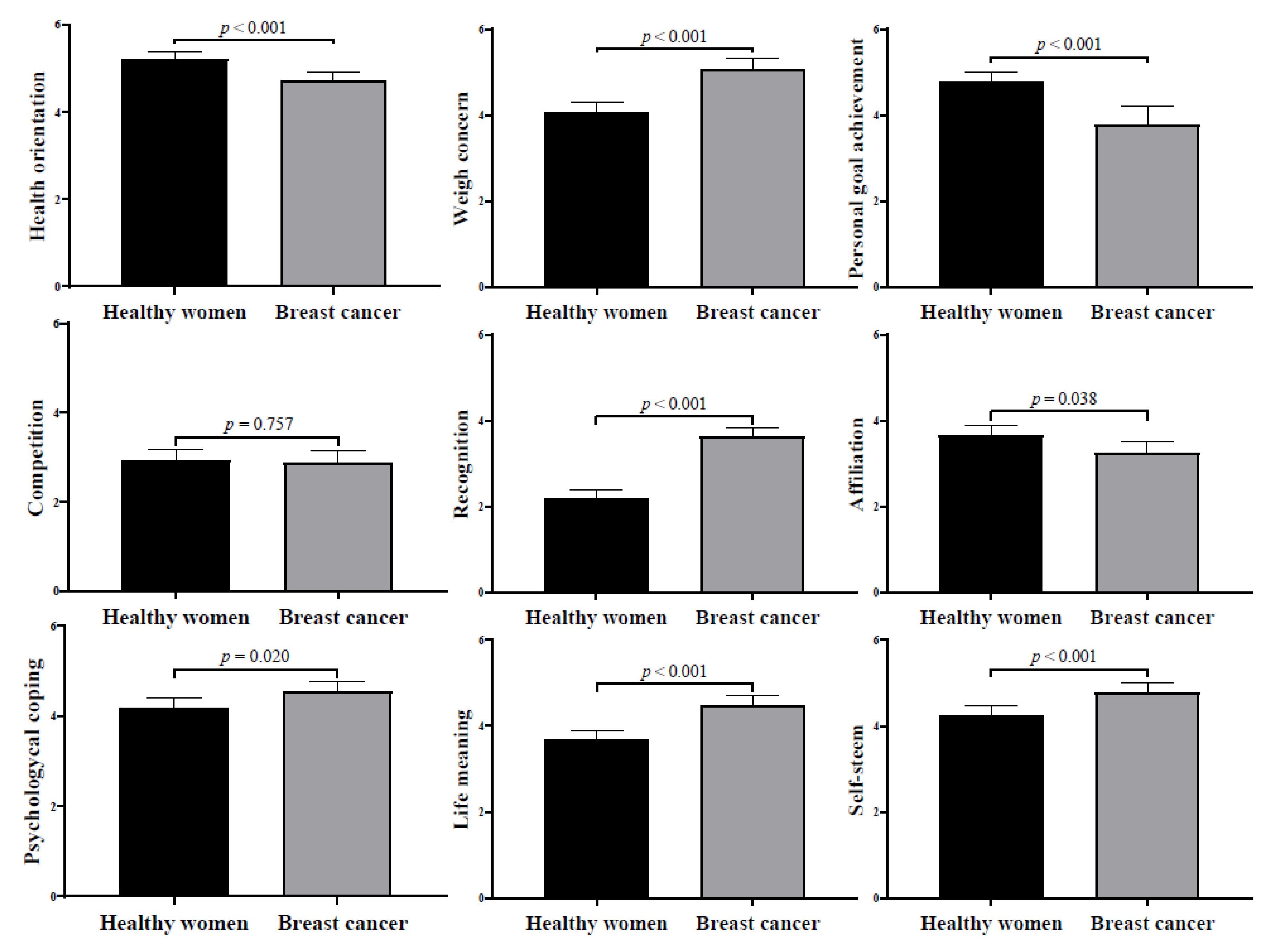
| Measurements | Healthy Runners | Breast Cancer Survivors |
|---|---|---|
| M ± SD | M ± SD | |
| n | 165 | 152 |
| Sociodemographic characteristics | ||
| Age (years) | 36.91 ± 9.68 | 46.49 ± 7.83 |
| Body mass index (kg/m2) | 23.41 ± 3.94 | 24.78 ± 3.50 |
| Health orientation (score: 1–7) | 5.15 ± 1.37 | 4.80 ± 0.65 |
| Weight concern (score: 1–7) | 4.08 ± 1.87 | 5.08 ± 0.72 |
| Personal goal achievement (score: 1–7) | 4.97 ± 1.55 | 3.81 ± 0.93 |
| Competition (score: 1–7) | 3.18 ± 1.76 | 2.63 ± 1.32 |
| Recognition (score: 1–7) | 2.41 ± 1.35 | 3.43 ± 1.06 |
| Affiliation (score: 1–7) | 3.79 ± 1.92 | 3.10 ± 1.03 |
| Psychological coping (score: 1–7) | 4.27 ± 1.61 | 4.47 ± 0.74 |
| Life meaning (score: 1–7) | 3.77 ± 1.56 | 4.39 ± 0.71 |
| Self-esteem (score: 1–7) | 4.40 ± 1.55 | 4.64 ± 0.76 |
| Measurements | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | - | ||||||||||
| 2. Body mass index | 0.387 *** | - | |||||||||
| 3. Health orientation | 0.031 | −0.018 | - | ||||||||
| 4. Weight concern | 0.151 ** | 0.124 * | 0.394 *** | - | |||||||
| 5. Personal goal achievement | −0.403 *** | −0.259 *** | 0.356 *** | 0.049 | - | ||||||
| 6. Competition | −0.341 *** | −0.249 *** | 0.195 *** | 0.020 | 0.727 *** | - | |||||
| 7. Recognition | −0.063 | −0.088 | 0.209 *** | 0.315 *** | 0.248 *** | 0.465 *** | - | ||||
| 8. Affiliation | −0.248 *** | −0.158 ** | 0.207 *** | −0.097 | 0.444 *** | 0.482 *** | 0.368 *** | - | |||
| 9. Psychological coping | −0.071 | −0.091 | 0.385 *** | 0.280 *** | 0.273 *** | 0.252 *** | 0.473 *** | 0.360 *** | - | ||
| 10. Life meaning | −0.006 | −0.028 | 0.419 *** | 0.262 *** | 0.271 *** | 0.304 *** | 0.590 *** | 0.465 *** | 0.808 *** | - | |
| 11. Self-esteem | −0.137 * | −0.101 | 0.483 *** | 0.366 *** | 0.458 *** | 0.428 *** | 0.645 *** | 0.411 *** | 0.745 *** | 0.783 *** | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malchrowicz-Mośko, E. Recreational Running Motivations among Breast Cancer Survivors. Int. J. Environ. Res. Public Health 2022, 19, 15500. https://doi.org/10.3390/ijerph192315500
Malchrowicz-Mośko E. Recreational Running Motivations among Breast Cancer Survivors. International Journal of Environmental Research and Public Health. 2022; 19(23):15500. https://doi.org/10.3390/ijerph192315500
Chicago/Turabian StyleMalchrowicz-Mośko, Ewa. 2022. "Recreational Running Motivations among Breast Cancer Survivors" International Journal of Environmental Research and Public Health 19, no. 23: 15500. https://doi.org/10.3390/ijerph192315500
APA StyleMalchrowicz-Mośko, E. (2022). Recreational Running Motivations among Breast Cancer Survivors. International Journal of Environmental Research and Public Health, 19(23), 15500. https://doi.org/10.3390/ijerph192315500






