Effect of Normalization Methods on Accuracy of Estimating Low- and High-Molecular Weight PAHs Distribution in the Soils of a Coking Plant
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Sampling Procedure
2.2. Analytical Procedures
2.3. Geostatistical Normalization
2.3.1. Box-Cox Transformation
2.3.2. Normal Score Transformation
2.3.3. Johnson Transformation
2.4. Spatial Interpolation
2.4.1. Kriging Methods
2.4.2. Evaluation of Interpolation Method
2.5. Software
3. Results
3.1. Soil Ace, Nap, BaP, and BbF Concentrations
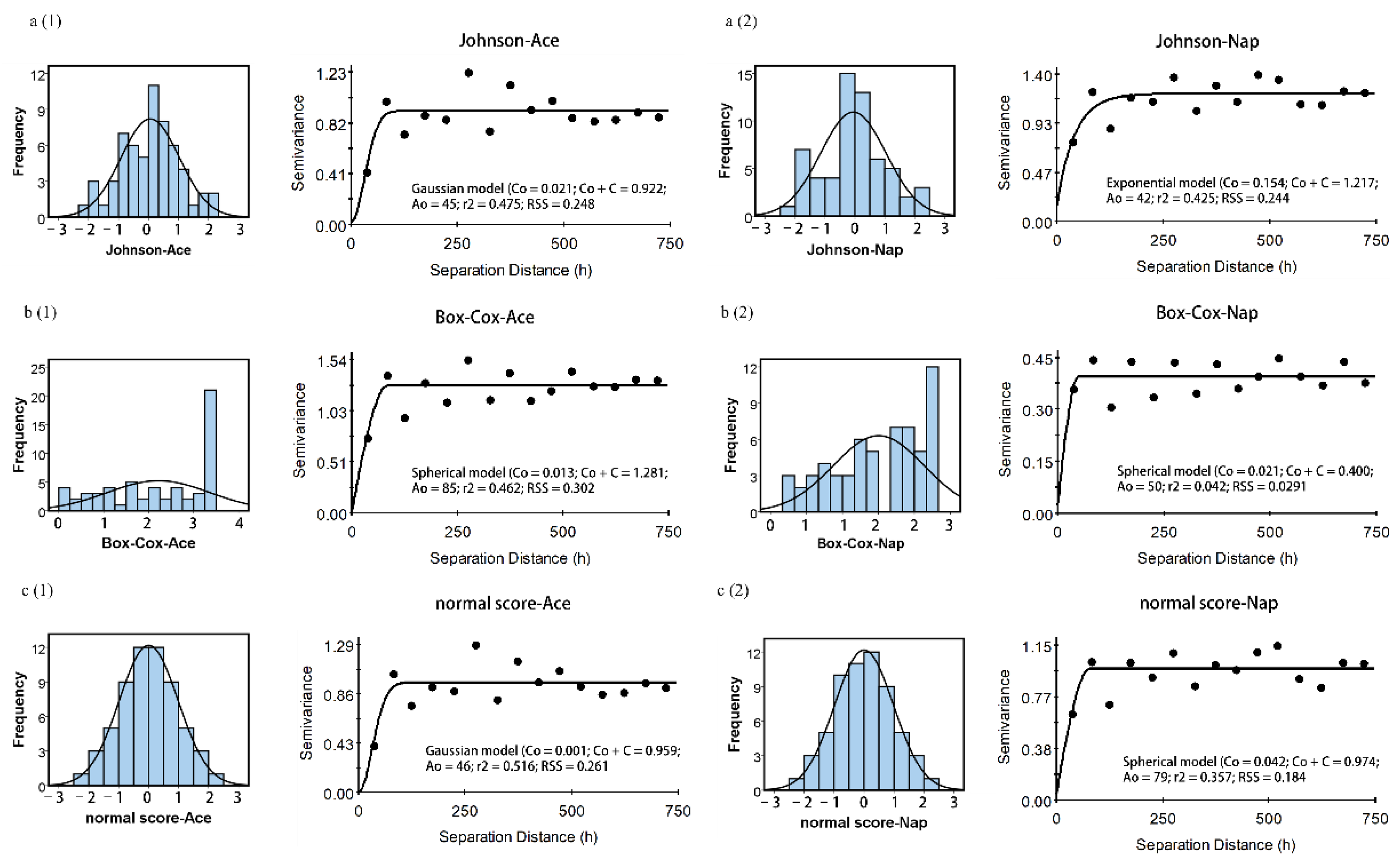
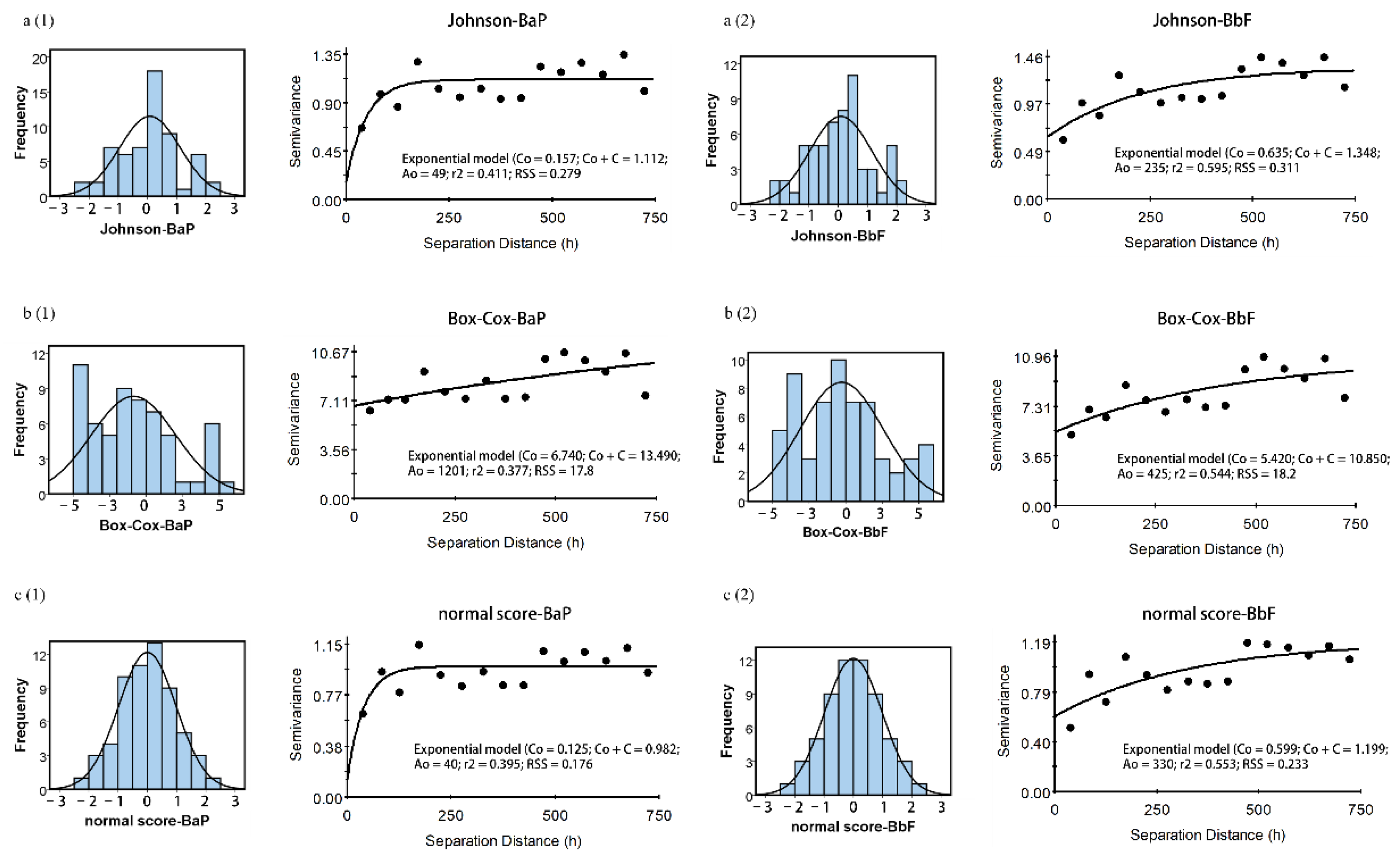
3.2. Spatial Structure of Soil Ace, Nap, BaP, and BbF Concentrations
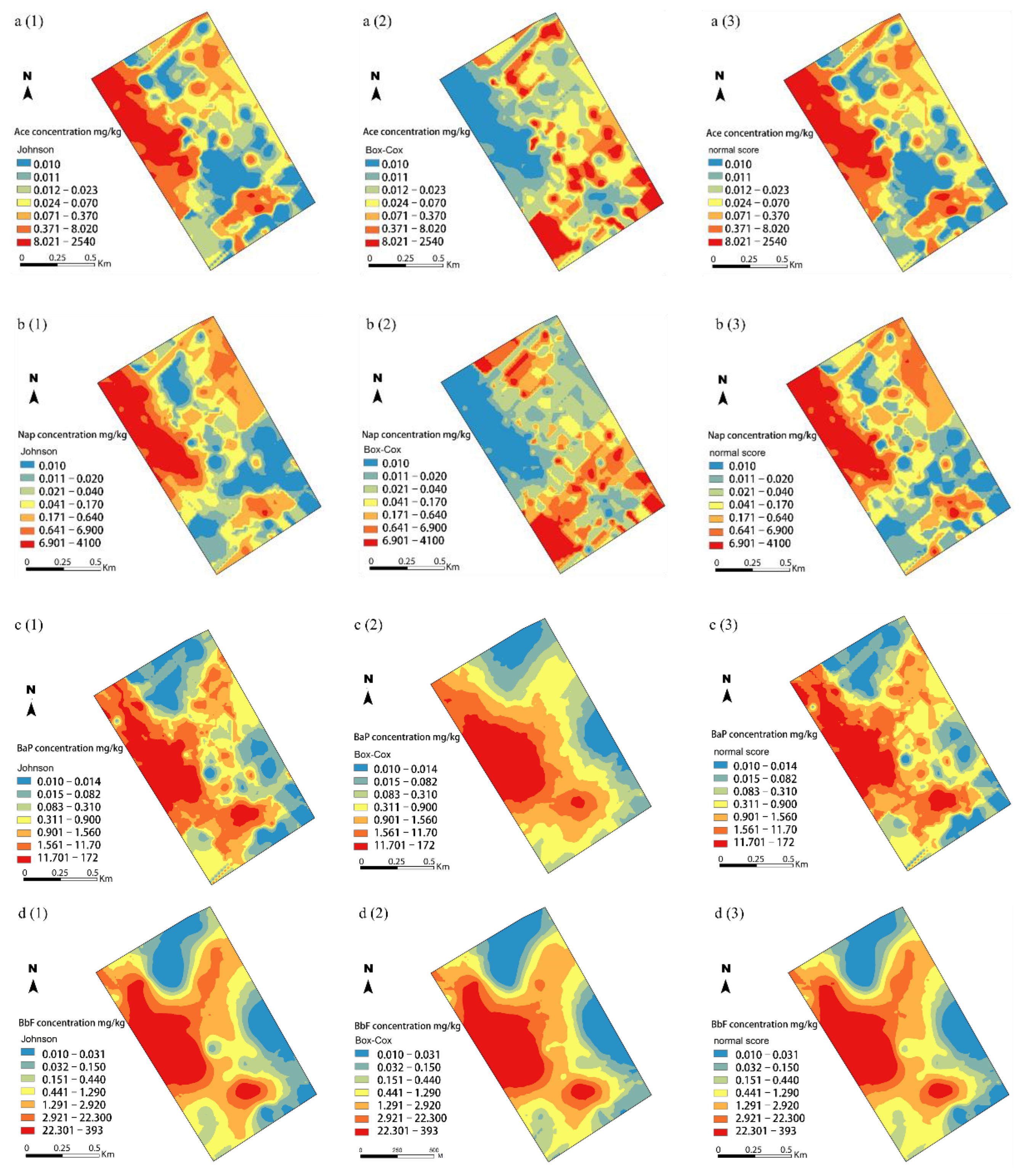
3.3. Spatial Distribution of Soil Ace, Nap, BaP, and BbF Concentrations
3.4. Interpolation Accuracy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- US Department of Health and Human Services. Public Health Service policies on research misconduct. Final rule. Fed. Regist. 2005, 70, 28369–28400. [Google Scholar]
- Tao, S.; Li, X.; Yang, Y.; Coveney, R.M.; Lu, X.; Chen, H.; Shen, W. Dispersion modeling of polycyclic aromatic hydrocarbons from combustion of biomass and fossil fuels and production of coke in Tianjin, China. Environ. Sci. Technol. 2006, 40, 4586–4591. [Google Scholar] [CrossRef] [PubMed]
- Seopela, M.P.; McCrindle, R.I.; Combrinck, S.; Augustyn, W. Occurrence, distribution, spatio-temporal variability and source identification of n-alkanes and polycyclic aromatic hydrocarbons in water and sediment from Loskop dam, South Africa. Water Res. 2020, 186, 116350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, L.; Bi, J.; Liu, Y.; Toriba, A.; Hayakawa, K.; Nagao, S.; Tang, N. Characteristics and unique sources of polycyclic aromatic hydrocarbons and nitro-polycyclic aromatic hydrocarbons in PM2.5 at a highland background site in northwestern China. Environ. Pollut. 2021, 274, 116527. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Niu, J.; Zhang, C.; Guo, G. Accuracy and uncertainty analysis of soil BbF spatial distribution estimation at a coking plant-contaminated site based on normalization geostatistical technologies. Environ. Sci. Pollut. R 2015, 22, 20121–20130. [Google Scholar] [CrossRef]
- Idowu, O.; Semple, K.T.; Ramadass, K.; O’Connor, W.; Hansbro, P.; Thavamani, P. Analysis of polycyclic aromatic hydrocarbons (PAHs) and their polar derivatives in soils of an industrial heritage city of Australia. Sci. Total Environ. 2020, 699, 134303. [Google Scholar] [CrossRef] [PubMed]
- Zang, T.; Wu, H.; Yan, B.; Zhang, Y.; Wei, C. Enhancement of PAHs biodegradation in biosurfactant/phenol system by increasing the bioavailability of PAHs. Chemosphere 2021, 266, 128941. [Google Scholar] [CrossRef]
- Chu, M.; Chen, C. Evaluation and Estimation of Potential Carcinogenic Risks of Polynuclear Aromatic Hydrocarbons (PAH); U.S. Environmental Protection Agency: Washington, DC, USA, 1985.
- Huo, X.; Li, H.; Sun, D.; Zhou, L.; Li, B. Combining Geostatistics with Moran’s I Analysis for Mapping Soil Heavy Metals in Beijing, China. Int. J. Environ. Res. Public Health 2012, 9, 995–1017. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, X.; Yu, D.; Zhang, L.; Wang, J.; Lv, J. Source apportionment and spatial distribution of potentially toxic elements in soils: A new exploration on receptor and geostatistical models. Sci. Total Environ. 2021, 759, 143428. [Google Scholar] [CrossRef]
- Webster, R.; Oliver, M.A. Geostatistics for Environmental Scientists; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Zawadzki, J.; Magiera, T.; Fabijańczyk, P. Geostatistical evaluation of magnetic indicators of forest soil contamination with heavy metals. Stud. Geophys. Geod. 2009, 53, 133–149. [Google Scholar] [CrossRef]
- Qu, M.; Guang, X.; Zhao, Y.; Huang, B. Spatially apportioning the source-oriented ecological risks of soil heavy metals using robust spatial receptor model with land-use data and robust residual kriging. Environ. Pollut. 2021, 285, 117261. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; He, B.; Wu, X.; Simonich, S.L.M.; Liu, H.; Fu, J.; Chen, A.; Liu, H.; Wang, Q. Polycyclic aromatic hydrocarbons (PAHs) in urban stream sediments of Suzhou Industrial Park, an emerging eco-industrial park in China: Occurrence, sources and potential risk. Ecotox Environ. Safe 2021, 214, 112095. [Google Scholar] [CrossRef] [PubMed]
- McBratney, A.B.; Webster, R.; McLaren, R.G.; Spiers, R.B. Regional variation of extractable copper and cobalt in the topsoil of southeast Scotland. Agron. Sci. Des Prod. Veg. L’environnement 1982, 2, 969–982. [Google Scholar]
- Li, H.; Wu, L.; Ma, T. Variable selection in joint location, scale and skewness models of the skew-normal distribution. J. Syst. Sci. Complex 2017, 30, 694–709. [Google Scholar] [CrossRef]
- Raymaekers, J.; Rousseeuw, P.J. Transforming variables to central normality. Mach Learn 2021, 1–23. [Google Scholar] [CrossRef]
- Eleni, T.; Constantini, S. Gas-Particle Partitioning of Polycyclic Aromatic Hydrocarbons in Urban, Adjacent Coastal, and Continental Background Sites of Western Greece. Environ. Sci. Technol. 2004, 38, 4973–4978. [Google Scholar]
- Hudson-Hanley, B.; Smit, E.; Branscum, A.; Hystad, P.; Kile, M.L. Trends in urinary metabolites of polycyclic aromatic hydrocarbons (PAHs) in the non-smoking U.S. population, NHANES 2001–2014. Chemosphere 2021, 276, 130211. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, N.; Xue, M.; Tao, S. Impact of soil organic matter on the distribution of polycyclic aromatic hydrocarbons (PAHs) in soils. Environ. Pollut. 2010, 158, 2170–2174. [Google Scholar] [CrossRef]
- Grimalt, J.O.; Borghini, F.; Sanchez-Hernandez, J.C.; Barra, R.; Torres García, C.J.; Focardi, S. Temperature Dependence of the Distribution of Organochlorine Compounds in the Mosses of the Andean Mountains. Environ. Sci. Technol. 2004, 38, 5386–5392. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. B 1964, 26, 211–243. [Google Scholar] [CrossRef]
- Bogunovic, I.; Filipovic, L.; Filipovic, V.; Pereira, P. Spatial mapping of soil chemical properties using multivariate geostatistics. A study from cropland in eastern Croatia. J. Cent. Eur. Agric. 2021, 22, 201–210. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, L.; Xu, W.; Ledwith, V. Use of local Moran’s I and GIS to identify pollution hotspots of Pb in urban soils of Galway, Ireland. Sci. Total Environ. 2008, 398, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Tepanosyan, G.; Sahakyan, L.; Zhang, C.; Saghatelyan, A. The application of Local Moran’s I to identify spatial clusters and hot spots of Pb, Mo and Ti in urban soils of Yerevan. Appl. Geochem. 2019, 104, 116–123. [Google Scholar] [CrossRef]
- Huang, S.; Shao, G.; Wang, L.; Wang, L.; Tang, L. Distribution and Health Risk Assessment of Trace Metals in Soils in the Golden Triangle of Southern Fujian Province, China. Int. J. Environ. Res. Public Health 2019, 16, 97. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Hill, R.; Holder, R.L. Algorithmas 99: Fitting Johnson curves by moments. Appl. Stat. 1976, 25, 180–189. [Google Scholar] [CrossRef]
- Slifker, J.F.; Shapiro, S.S. The johnson system: Selection and parameter estimation. Technometrics 1980, 22, 239–246. [Google Scholar] [CrossRef]
- Krige, D.G. A statistical approach to some basic mine valuation problems on the Witwatersrand, by D.G. Krige, published in the Journal, December 1951: Introduction by the author. J. S. Afr. Inst. Min. Metall. 1951, 52, 201–203. [Google Scholar]
- Adhikary, S.K.; Muttil, N.; Yilmaz, A.G. Genetic Programming-Based Ordinary Kriging for Spatial Interpolation of Rainfall. J. Hydrol. Eng. 2016, 21, 4015062. [Google Scholar] [CrossRef]
- Matheron, G. The internal consistency of models in geostatistics. In Geostatistics; Springer: Dordrecht, The Netherlands, 1989; pp. 21–38. [Google Scholar]
- Clark, I. Practical Geostatistics; Applied Science Publishers: London, UK, 1979. [Google Scholar]
- Mueller, T.G.; Pusuluri, N.B.; Mathias, K.K.; Cornelius, P.L.; Barnhisel, R.I.; Shearer, S.A. Map Quality for Ordinary Kriging and Inverse Distance Weighted Interpolation. Soil Sci. Soc. Am. J. 2004, 68, 2042–2047. [Google Scholar] [CrossRef]
- Stone, M. Cross-Validatory Choice and Assessment of Statistical Predictions. J. R. Stat. Soc. B 1974, 36, 111–133. [Google Scholar] [CrossRef]
- Zhang, C.; Manheim, F.T.; Hinde, J.P. Grossman, J.N. Statistical characterization of a large geochemical database and effect of sample size. Appl. Geochem. 2005, 20, 1857–1874. [Google Scholar] [CrossRef]
- Shamsudduha, M. Spatial variability and prediction modeling of groundwater arsenic distributions in the shallowest alluvial aquifers in Bangladesh. J. Spat. Hydrol. 2008, 7, 33–46. [Google Scholar]
- Gong, G.; Mattevada, S.; O’Bryant, S.E. Comparison of the accuracy of kriging and IDW interpolations in estimating groundwater arsenic concentrations in Texas. Environ. Res. 2014, 130, 59–69. [Google Scholar] [CrossRef]
- Cao, W.; Yin, L.; Zhang, D.; Wang, Y.; Yuan, J.; Zhu, Y.; Dou, J. Contamination, sources, and health risks associated with soil PAHs in rebuilt land from a Coking Plant, Beijing, China. Int. J. Environ. Res. Public Health 2019, 16, 670. [Google Scholar] [CrossRef] [PubMed]

| PAH | Minimum | Maximum | Mean | Median | Skewness | Kurtosis | CV | SD | K-S Test | RSV a |
|---|---|---|---|---|---|---|---|---|---|---|
| Ace | 0.01 | 2540 | 80.86 | 0.03 | 5.64 | 34.42 | 4.59 | 371.45 | Non-normal | -- |
| Nap | 0.01 | 4100 | 122.84 | 0.05 | 5.95 | 38.33 | 4.75 | 583.49 | Non-normal | 70 |
| BaP | 0.01 | 172 | 13.32 | 0.33 | 3.05 | 9.25 | 2.63 | 34.98 | Non-normal | 1.5 |
| BbF | 0.01 | 393 | 23.10 | 0.61 | 3.89 | 17.36 | 2.86 | 66.06 | Non-normal | 15 |
| PAH | Transformation | Skewness | Kurtosis | p | K-S Test |
|---|---|---|---|---|---|
| Ace | Johnson | −0.034 | −0.144 | 0.997 | Normal |
| Box-Cox | −0.461 | −1.264 | 0.038 | Normal | |
| Normal score | 0.005 | −0.242 | 0.010 | Normal | |
| Nap | Johnson | 0.061 | −0.126 | 0.743 | Normal |
| Box-Cox | −0.471 | −1.052 | 0.167 | Normal | |
| Normal score | 0.002 | −0.233 | 0.393 | Normal | |
| BaP | Johnson | −0.12 | −0.168 | 0.880 | Normal |
| Box-Cox | 0.493 | −0.614 | 0.620 | Normal | |
| Normal score | 0.001 | −0.233 | 0.999 | Normal | |
| BbF | Johnson | 0.006 | −0.347 | 0.972 | Normal |
| Box-Cox | 0.438 | −0.559 | 0.923 | Normal | |
| Normal score | 0.002 | −0.236 | 0.999 | Normal |
| PAH | Model | Nugget (C0) | Sill (C0 + C) | Proportion [C0/(C0 + C)] | Range (A0) | r2 | Residual SS |
|---|---|---|---|---|---|---|---|
| J-Ace | Gaussian | 0.021 | 0.922 | 2.28 | 45 | 0.475 | 0.248 |
| J-Nap | Exponential | 0.154 | 1.217 | 12.65 | 42 | 0.425 | 0.244 |
| J-BaP | Exponential | 0.157 | 1.112 | 14.12 | 49 | 0.411 | 0.279 |
| J-BbF | Exponential | 0.635 | 1.348 | 47.11 | 235 | 0.596 | 0.311 |
| B-Ace | Spherical | 0.013 | 1.281 | 1.01 | 85 | 0.462 | 0.302 |
| B-Nap | Spherical | 0.021 | 0.400 | 5.25 | 50 | 0.042 | 0.029 |
| B-BaP | Exponential | 6.740 | 13.490 | 49.96 | 1201 | 0.377 | 17.800 |
| B-BbF | Exponential | 5.420 | 10.850 | 49.95 | 425 | 0.544 | 18.100 |
| N-Ace | Gaussian | 0.001 | 0.959 | 0.10 | 46 | 0.516 | 0.261 |
| N-Nap | Spherical | 0.042 | 0.974 | 4.31 | 79 | 0.357 | 0.184 |
| N-BaP | Exponential | 0.125 | 0.982 | 12.73 | 40 | 0.395 | 0.176 |
| N-BbF | Exponential | 0.599 | 1.199 | 49.96 | 330 | 0.553 | 0.233 |
| PAH | Prediction Model | ME | RMSE | ASE | RMSSE |
|---|---|---|---|---|---|
| Ace | Normal score-ordinary kriging | 0.003 | 0.863 | 1.026 | 0.838 |
| Johnson-ordinary kriging | 0.002 | 0.855 | 1.019 | 0.836 | |
| Box-Cox-ordinary kriging | −0.024 | 1.007 | −0.005 | 0.886 | |
| Nap | Normal score-ordinary kriging | 0.014 | 0.951 | 1.032 | 0.921 |
| Johnson-ordinary kriging | −0.032 | 1.036 | 1.247 | 0.833 | |
| Box-Cox-ordinary kriging | 0.009 | 0.589 | 0.687 | 0.856 | |
| BaP | Normal score-ordinary kriging | −0.013 | 0.908 | 1.121 | 0.815 |
| Johnson-ordinary kriging | −0.006 | 0.970 | 1.196 | 0.817 | |
| Box-Cox-ordinary kriging | 0.100 | 2.647 | 3.332 | 0.811 | |
| BbF | Normal score-ordinary kriging | 0.046 | 0.904 | 1.212 | 0.758 |
| Johnson-ordinary kriging | 0.057 | 0.980 | 1.336 | 0.744 | |
| Box-Cox-ordinary kriging | 0.158 | 2.591 | 3.500 | 0.757 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Yang, K.; Cheng, L.; Bai, Y.; Wang, Y.; Hou, Y.; Ding, A. Effect of Normalization Methods on Accuracy of Estimating Low- and High-Molecular Weight PAHs Distribution in the Soils of a Coking Plant. Int. J. Environ. Res. Public Health 2022, 19, 15470. https://doi.org/10.3390/ijerph192315470
Yuan Y, Yang K, Cheng L, Bai Y, Wang Y, Hou Y, Ding A. Effect of Normalization Methods on Accuracy of Estimating Low- and High-Molecular Weight PAHs Distribution in the Soils of a Coking Plant. International Journal of Environmental Research and Public Health. 2022; 19(23):15470. https://doi.org/10.3390/ijerph192315470
Chicago/Turabian StyleYuan, Yumin, Kai Yang, Lirong Cheng, Yijuan Bai, Yingying Wang, Ying Hou, and Aizhong Ding. 2022. "Effect of Normalization Methods on Accuracy of Estimating Low- and High-Molecular Weight PAHs Distribution in the Soils of a Coking Plant" International Journal of Environmental Research and Public Health 19, no. 23: 15470. https://doi.org/10.3390/ijerph192315470
APA StyleYuan, Y., Yang, K., Cheng, L., Bai, Y., Wang, Y., Hou, Y., & Ding, A. (2022). Effect of Normalization Methods on Accuracy of Estimating Low- and High-Molecular Weight PAHs Distribution in the Soils of a Coking Plant. International Journal of Environmental Research and Public Health, 19(23), 15470. https://doi.org/10.3390/ijerph192315470







