Clinical Use of Lansoprazole and the Risk of Osteoporosis: A Nationwide Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Cohort
2.3. Identification of Exposure to PPIs
2.4. Ascertainment of Osteoporosis
2.5. Statistical Analysis
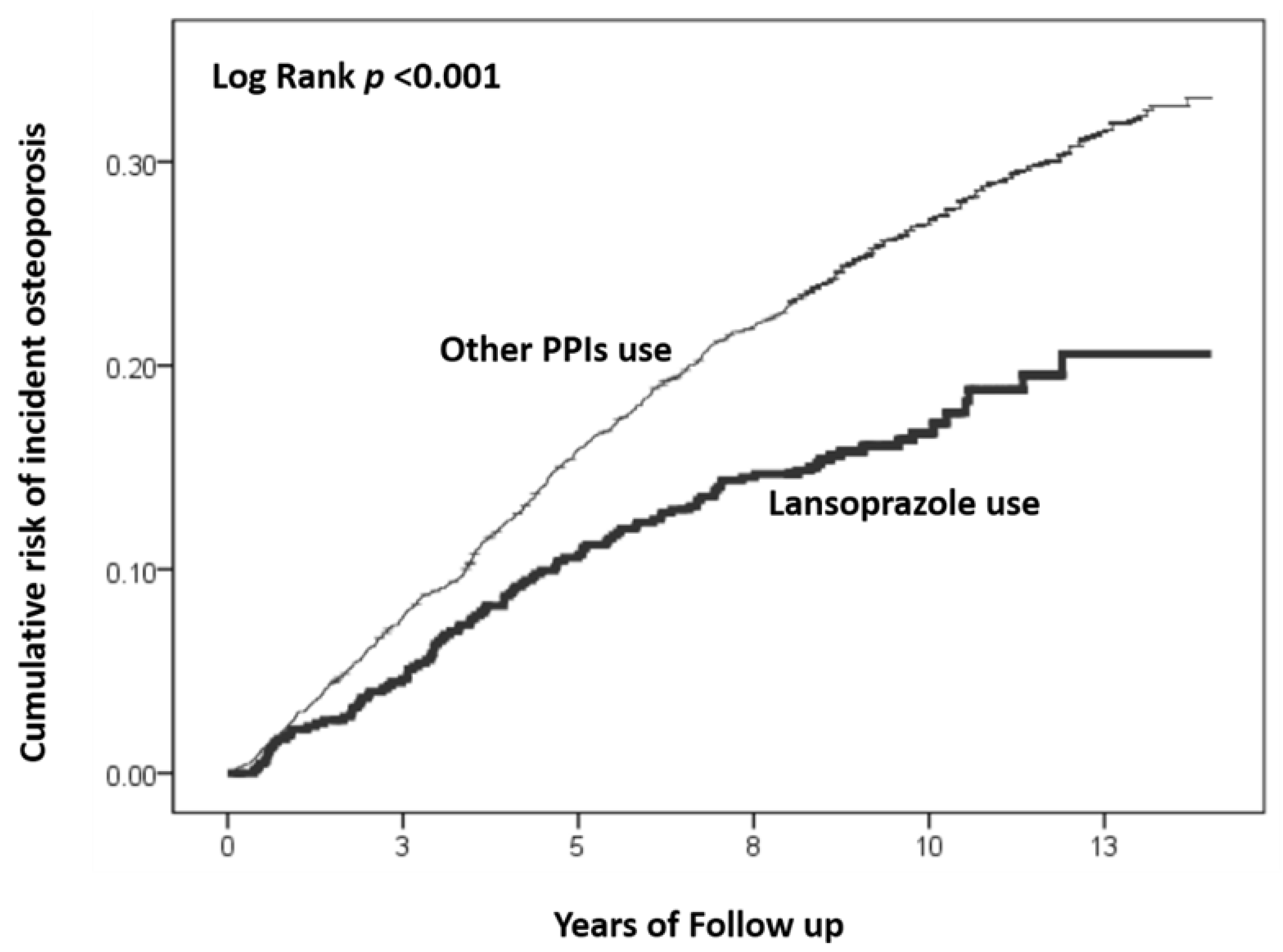
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The epidemiology of osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Pouresmaeili, F.; Kamalidehghan, B.; Kamarehei, M.; Goh, Y.M. A comprehensive overview on osteoporosis and its risk factors. Ther. Clin. Risk Manag. 2018, 14, 2029–2049. [Google Scholar] [CrossRef] [PubMed]
- Mazziotti, G.; Canalis, E.; Giustina, A. Drug-induced osteoporosis: Mechanisms and clinical implications. Am. J. Med. 2010, 123, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Kandulski, A.; Venerito, M. Proton-pump inhibitors: Understanding the complications and risks. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Jensen, R.T. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium. Curr. Gastroenterol. Rep. 2010, 12, 448–457. [Google Scholar] [CrossRef]
- Thong, B.K.S.; Ima-Nirwana, S.; Chin, K.-Y. Proton pump inhibitors and fracture risk: A review of current evidence and mechanisms involved. Int. J. Environ. Res. Public Health 2019, 16, 1571. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Reis, S.; Teixeira, S.; Lopes, S.; Fernandes, M.H. Dose-dependent inhibitory effects of proton pump inhibitors on human osteoclastic and osteoblastic cell activity. FEBS J. 2013, 280, 5052–5064. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, Y.; Ma, M.; Sun, S.; Ma, Z.; Wang, Y.; Yu, L.; Qian, X.; Sun, L.; Zhang, X. Lansoprazole-induced osteoporosis via the IP3R-and SOCE-mediated calcium signaling pathways. Mol. Med. 2022, 28, 21. [Google Scholar] [CrossRef]
- Vestergaard, P.; Rejnmark, L.; Mosekilde, L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif. Tissue Int. 2006, 79, 76–83. [Google Scholar] [CrossRef]
- Targownik, L.E.; Lix, L.M.; Metge, C.J.; Prior, H.J.; Leung, S.; Leslie, W.D. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ 2008, 179, 319–326. [Google Scholar] [CrossRef]
- Fraser, L.; Leslie, W.; Targownik, L.; Papaioannou, A.; Adachi, J. The effect of proton pump inhibitors on fracture risk: Report from the Canadian Multicenter Osteoporosis Study. Osteoporos. Int. 2013, 24, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Poly, T.; Islam, M.; Yang, H.-C.; Wu, C.; Li, Y.-C. Proton pump inhibitors and risk of hip fracture: A meta-analysis of observational studies. Osteoporos. Int. 2019, 30, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.W.; Blackwell, T.; Ensrud, K.E.; Hillier, T.A.; Lane, N.E.; Orwoll, E.; Bauer, D.C. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif. Tissue Int. 2008, 83, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Targownik, L.E.; Lix, L.M.; Leung, S.; Leslie, W.D. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology 2010, 138, 896–904. [Google Scholar] [CrossRef]
- Hove, K.D.; Færch, K.; Bödvarsdóttir, T.B.; Karlsen, A.E.; Petersen, J.S.; Vaag, A. Treatment with a proton pump inhibitor improves glycaemic control in type 2 diabetic patients-a retrospective analysis. Diabetes Res. Clin. Pract. 2010, 90, e72–e74. [Google Scholar] [CrossRef]
- Takebayashi, K.; Sakurai, S.; Suzuki, T.; Hori, K.; Terasawa, T.; Naruse, R.; Hara, K.; Suetsugu, M.; Tsuchiya, T.; Aoki, H. Effect of combination therapy with alogliptin and lansoprazole on glycemic control in patients with type 2 diabetes. Endocr. J. 2014, EJ14-0208. [Google Scholar] [CrossRef]
- Benchamana, A.; Mori, H.; MacDougald, O.A.; Soodvilai, S. Regulation of adipocyte differentiation and metabolism by lansoprazole. Life Sci. 2019, 239, 116897. [Google Scholar] [CrossRef]
- Shin, D.; Kim, S.; Kim, K.H.; Lee, K.; Park, S.M. Association between insulin resistance and bone mass in men. J. Clin. Endocrinol. Metab. 2014, 99, 988–995. [Google Scholar] [CrossRef]
- Kleyer, A.; Scholtysek, C.; Bottesch, E.; Hillienhof, U.; Beyer, C.; Distler, J.H.; Tuckermann, J.P.; Schett, G.; Krönke, G. Liver X receptors orchestrate osteoblast/osteoclast crosstalk and counteract pathologic bone loss. J. Bone Miner. Res. 2012, 27, 2442–2451. [Google Scholar] [CrossRef]
- Goel, D.; Vohora, D. Liver X receptors and skeleton: Current state-of-knowledge. Bone 2021, 144, 115807. [Google Scholar] [CrossRef]
- Cronican, A.A.; Fitz, N.F.; Pham, T.; Fogg, A.; Kifer, B.; Koldamova, R.; Lefterov, I. Proton pump inhibitor lansoprazole is a nuclear liver X receptor agonist. Biochem. Pharmacol. 2010, 79, 1310–1316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, C.-Y.; Chen, Y.-J.; Ho, H.J.; Hsu, Y.-C.; Kuo, K.N.; Wu, M.-S.; Lin, J.-T. Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. JAMA 2012, 308, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Kao, Y.H.Y.; Lin, S.J.; Lee, C.H.; Lai, M.L. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol. Drug Saf. 2011, 20, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.H.; Hong, J.H.; See, L.C.; Yu, H.P.; Hsu, J.T.; Chou, I.J.; Chou, W.C.; Chiou, M.J.; Wang, C.C.; Kuo, C.F. Validity of cancer diagnosis in the National Health Insurance database compared with the linked National Cancer Registry in Taiwan. Pharmacoepidemiol. Drug Saf. 2018, 27, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.A. Evaluating medication effects outside of clinical trials: New-user designs. Am. J. Epidemiol. 2003, 158, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Siris, E.; Adler, R.; Bilezikian, J.; Bolognese, M.; Dawson-Hughes, B.; Favus, M.; Harris, S.; Jan de Beur, S.; Khosla, S.; Lane, N.E. The clinical diagnosis of osteoporosis: A position statement from the National Bone Health Alliance Working Group. Osteoporos. Int. 2014, 25, 1439–1443. [Google Scholar] [CrossRef]
- Gill, R.; Schumacher, M. A simple test of the proportional hazards assumption. Biometrika 1987, 74, 289–300. [Google Scholar] [CrossRef]
- O’Connell, M.B.; Madden, D.M.; Murray, A.M.; Heaney, R.P.; Kerzner, L.J. Effects of proton pump inhibitors on calcium carbonate absorption in women: A randomized crossover trial. Am. J. Med. 2005, 118, 778–781. [Google Scholar] [CrossRef]
- Yang, Y.-X.; Lewis, J.D.; Epstein, S.; Metz, D.C. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006, 296, 2947–2953. [Google Scholar] [CrossRef]
- Kaye, J.A.; Jick, H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2008, 28, 951–959. [Google Scholar] [CrossRef]
- Gray, S.L.; LaCroix, A.Z.; Larson, J.; Robbins, J.; Cauley, J.A.; Manson, J.E.; Chen, Z. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: Results from the Women’s Health Initiative. Arch. Intern. Med. 2010, 170, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Corley, D.A.; Kubo, A.; Zhao, W.; Quesenberry, C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 2010, 139, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Huang, E.S.; Jacobson, B.C.; Camargo, C.A.; Feskanich, D.; Chan, A.T. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: A prospective cohort study. BMJ 2012, 344, e372. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Como, G.; Badalamenti, S.; Finazzi, S.; Malesci, A.; Gallieni, M.; Brancaccio, D.; Ponticelli, C. Effect of gastric acid secretion on intestinal phosphate and calcium absorption in normal subjects. Nephrol. Dial. Transplant. 1995, 10, 1376–1380. [Google Scholar]
- Graziani, G.; Badalamenti, S.; Como, G.; Gallieni, M.; Finazzi, S.; Angelini, C.; Brancaccio, D.; Ponticelli, C. Calcium and phosphate plasma levels in dialysis patients after dietary Ca-P overload. Nephron 2002, 91, 474–479. [Google Scholar] [CrossRef]
- Yu, E.M.; Bauer, S.R.; Bain, P.A.; Bauer, D.C. Proton pump inhibitors and risk of fractures: A meta-analysis of 11 international studies. Am. J. Med. 2011, 124, 519–526. [Google Scholar] [CrossRef]
- Hussain, S.; Siddiqui, A.N.; Habib, A.; Hussain, M.; Najmi, A.K. Proton pump inhibitors’ use and risk of hip fracture: A systematic review and meta-analysis. Rheumatol. Int. 2018, 38, 1999–2014. [Google Scholar] [CrossRef]
- Matuszewska, A.; Nowak, B.; Rzeszutko, M.; Zduniak, K.; Szandruk, M.; Jedrzejuk, D.; Landwojtowicz, M.; Bolanowski, M.; Piesniewska, M.; Kwiatkowska, J.; et al. Effects of long-term administration of pantoprazole on bone mineral density in young male rats. Pharmacol. Rep. 2016, 68, 1060–1064. [Google Scholar] [CrossRef]
- Histing, T.; Stenger, D.; Scheuer, C.; Metzger, W.; Garcia, P.; Holstein, J.H.; Klein, M.; Pohlemann, T.; Menger, M.D. Pantoprazole, a proton pump inhibitor, delays fracture healing in mice. Calcif. Tissue Int. 2012, 90, 507–514. [Google Scholar] [CrossRef]
- Menger, M.M.; Bremer, P.; Scheuer, C.; Rollmann, M.F.; Braun, B.J.; Herath, S.C.; Orth, M.; Spater, T.; Pohlemann, T.; Menger, M.D.; et al. Pantoprazole impairs fracture healing in aged mice. Sci. Rep. 2020, 10, 22376. [Google Scholar] [CrossRef]
- Naseri, E.; Yenisehirli, A. Proton pump inhibitors omeprazole and lansoprazole induce relaxation of isolated human arteries. Eur. J. Pharmacol. 2006, 531, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, W.; Teucher, N.; Sossalla, S.; Kettlewell, S.; Werner, C.; Raddatz, D.; Elgner, A.; Tenderich, G.; Pieske, B.; Ramadori, G.; et al. Negative inotropy of the gastric proton pump inhibitor pantoprazole in myocardium from humans and rabbits: Evaluation of mechanisms. Circulation 2007, 116, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Ishihara, N.; Nagayama, D.; Saiki, A.; Tatsuno, I. 7-ketocholesterol induces apoptosis of MC3T3-E1 cells associated with reactive oxygen species generation, endoplasmic reticulum stress and caspase-3/7 dependent pathway. Mol. Genet. Metab. Rep. 2017, 10, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Aydin, C.; Sarac, B.; Koyuncu, A.; Yildirim, S.; Sen, M.; Sarioglu, Y. Relaxant efect of omeprazole and lansoprazole in guinea pig gallbladder muscle strips in vitro. J. Gastroenterol. 2003, 38, 765–771. [Google Scholar] [CrossRef]
- Yurtsever, A.S.; Pektas, M.; Ozkur, M.; Un, I.; Erenmemisoglu, A.; Buyukafsar, K. Proton pump inhibitors omeprazole, lansoprazole and pantoprazole induce relaxation in the rat lower oesophageal sphincter. J. Pharm. Pharmacol. 2011, 63, 1295–1300. [Google Scholar] [CrossRef]
- Kuntz, J.L.; Chrischilles, E.A.; Pendergast, J.F.; Herwaldt, L.A.; Polgreen, P.M. Incidence of and risk factors for community-associated Clostridium difficile infection: A nested case-control study. BMC Infect. Dis. 2011, 11, 194. [Google Scholar] [CrossRef]
- Thomson, R.D.; Lestina, L.S.; Bensen, S.P.; Toor, A.; Maheshwari, Y.; Ratcliffe, N.R. Lansoprazole- associated microscopic colitis: A case series. Am. J. Gastroenterol. 2002, 97, 2908–2913. [Google Scholar] [CrossRef]
- Hirschowitz, B.; Worthington, J.; Mohnen, J. Vitamin B12 deficiency in hypersecretors during long term acid suppression-with proton pump inhibitors. Aliment. Pharmacol. Ther. 2008, 27, 1110–1121. [Google Scholar] [CrossRef]
- Ray, S.; Delaney, M.; Muller, A.F. Proton pump inhibitors and acute interstitial nephritis. BMJ 2010, 341, c4412. [Google Scholar] [CrossRef]

| Study Cohorts | |||
|---|---|---|---|
| Variable | Other PPIs | Lansoprazole | p Value |
| (n = 2620) | (n = 655) | ||
| Mean age | 57.15 ± 11.32 | 57.73 ± 11.52 | 0.242 |
| Sex (No., %) | 1.000 | ||
| Women | 1020 (38.9%) | 255 (38.9%) | |
| Men | 1600 (61.1%) | 400 (61.1%) | |
| Comorbidities (No., %) | |||
| Cerebral vascular disease | 456 (17.4%) | 112 (17.1%) | 0.854 |
| Chronic liver disease | 532 (20.3%) | 149 (22.7%) | 0.168 |
| Chronic kidney disease | 152 (5.8%) | 38 (5.8%) | 1.000 |
| Hyperlipidemia | 462 (17.6%) | 99 (15.1%) | 0.126 |
| Hypertension | 1266 (48.3%) | 320 (48.9%) | 0.807 |
| Diabetes mellitus | 631 (24.1%) | 151 (23.1%) | 0.580 |
| Malignancy | 359 (13.7%) | 81 (12.4%) | 0.370 |
| Congestive heart failure | 89 (3.4%) | 27 (4.1%) | 0.369 |
| Variable | No. of Subjects | No. of Osteoporosis Cases | Crude HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|
| Overall | ||||
| Other PPIs | 2620 | 777 | 1.00 | 1.00 |
| Lansoprazole | 655 | 109 | 0.60 (0.49–0.74) | 0.56 (0.46–0.68) |
| Sex | ||||
| Men | ||||
| Other PPIs | 1600 | 323 | 1.00 | 1.00 |
| Lansoprazole | 400 | 47 | 0.66 (0.49–0.90) | 0.60 (0.44–0.82) |
| Women | ||||
| Other PPIs | 1020 | 454 | 1.00 | 1.00 |
| Lansoprazole | 255 | 62 | 0.54 (0.41–0.70) | 0.50 (0.38–0.66) |
| Age (years) | ||||
| 40–49 | ||||
| Other PPIs | 840 | 108 | 1.00 | 1.00 |
| Lansoprazole | 210 | 14 | 0.60 (0.34–1.05) | 0.56 (0.32–0.98) |
| 50–59 | ||||
| Other PPIs | 688 | 166 | 1.00 | 1.00 |
| Lansoprazole | 172 | 26 | 0.70 (0.47–1.07) | 0.66 (0.43–1.00) |
| 60–69 | ||||
| Other PPIs | 568 | 224 | 1.00 | 1.00 |
| Lansoprazole | 142 | 32 | 0.59 (0.41–0.86) | 0.52 (0.36–0.76) |
| 70–79 | ||||
| Other PPIs | 524 | 279 | 1.00 | 1.00 |
| Lansoprazole | 131 | 37 | 0.53 (0.37–0.74) | 0.50 (0.36–0.71) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, M.-H.; Chen, Y.-C.; Wu, W.-T.; Lin, M.-H.; Yang, Y.-J.; Hueng, D.-Y.; Lin, T.-K.; Chou, Y.-C.; Sun, C.-A. Clinical Use of Lansoprazole and the Risk of Osteoporosis: A Nationwide Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 15359. https://doi.org/10.3390/ijerph192215359
Chung M-H, Chen Y-C, Wu W-T, Lin M-H, Yang Y-J, Hueng D-Y, Lin T-K, Chou Y-C, Sun C-A. Clinical Use of Lansoprazole and the Risk of Osteoporosis: A Nationwide Cohort Study. International Journal of Environmental Research and Public Health. 2022; 19(22):15359. https://doi.org/10.3390/ijerph192215359
Chicago/Turabian StyleChung, Ming-Hsuan, Yong-Chen Chen, Wen-Tung Wu, Ming-Hsun Lin, Yun-Ju Yang, Dueng-Yuan Hueng, Tsung-Kun Lin, Yu-Ching Chou, and Chien-An Sun. 2022. "Clinical Use of Lansoprazole and the Risk of Osteoporosis: A Nationwide Cohort Study" International Journal of Environmental Research and Public Health 19, no. 22: 15359. https://doi.org/10.3390/ijerph192215359
APA StyleChung, M.-H., Chen, Y.-C., Wu, W.-T., Lin, M.-H., Yang, Y.-J., Hueng, D.-Y., Lin, T.-K., Chou, Y.-C., & Sun, C.-A. (2022). Clinical Use of Lansoprazole and the Risk of Osteoporosis: A Nationwide Cohort Study. International Journal of Environmental Research and Public Health, 19(22), 15359. https://doi.org/10.3390/ijerph192215359







