Abstract
This study aimed to prospectively assess changes in muscle thickness (MT) and the cross-sectional area (CSA) of the rectus femoris (RF) muscle in a cohort of older adults, using musculoskeletal ultrasound at admission and at a 2-week follow-up during hospitalization in a post-acute care unit. Differences in frailty status and correlations of MT-RF and CSA-RF with current sarcopenia diagnostic criteria were also studied. Forty adults aged 79.5 (SD 9.5) years (57.5% women) participated, including 14 with frailty and 26 with pre-frailty. In the first week follow-up, men had a significant increase in MT (0.9 mm [95%CI 0.3 to 1.4], p = 0.003) and CSA (0.4 cm2 [95%CI 0.1 to 0.6], p = 0.007). During the second week, men continued to have a significant increase in MT (0.7 mm [95%CI 0.0 to 1.4], p = 0.036) and CSA (0.6 cm2 [95%CI 0.01 to 1.2], p = 0.048). Patients with frailty had lower values of MT-RF and CSA-RF at admission and during the hospitalization period. A moderate-to-good correlation of MT-RF and CSA with handgrip strength, fat-free mass and gait speed was observed. Musculoskeletal ultrasound was able to detect MT-RF and CSA-RF changes in older adults admitted to a post-acute care unit.
1. Introduction
Sarcopenia and frailty are strong predictors of morbidity, disability and death in older adults [1] Sarcopenia is a muscle and nutritional disease characterized by a decline in muscle strength and mass, rooted in adverse muscle changes that accrue throughout a lifetime [2,3]. Frailty syndrome is a clinical condition characterized by low muscle mass and strength [1], and an excessive vulnerability of the individual to endogenous and exogenous stressors [4,5]. Although both conditions are associated with extended hospitalization, even short periods of bed rest due to acute processes can lead to muscle loss and functional decline in older adults [6,7,8,9]. However, this muscle loss and functional decline can be reversed when early identification and tailored therapeutic approaches are applied in the early stages [1,10,11]. Sarcopenia may coexist with other age-related diseases that share some phenotypically overlapping features in frail older people and should be adequately assessed and treated [12]. Herein lies the importance of including an assessment of muscle mass and function in the comprehensive geriatric assessment as the basis for planning therapeutic interventions of geriatric medicine and for research purposes [13,14].
Computed tomography and magnetic resonance imaging (MRI) are the gold standard for muscle assessment [15,16], and dual X-ray densitometry (DXA) and electrical bioimpedance analysis (BIA) are the methods most commonly used in clinical practice. However, these technologies require certain levels of technical expertise and are not always available in clinical settings. Ultrasound has emerged as a reliable and valid tool to assess muscle quality and quantity in older populations [17,18], showing stronger correlations when compared with MRI [19], and is suitable for bedside use as part of the comprehensive geriatric assessment [20,21]. The Special Interest Group on Sarcopenia of the European Union Geriatric Medicine Society (EuGMS) has launched an evidence-based initiative aimed at promoting muscle mass assessment to detect sarcopenia through ultrasound (SARCUS) in older people [20,21].
Previous studies have shown good reliability in the ultrasound evaluation of muscles in the lower [22,23] and upper [24,25] extremities. Ultrasound measurements of rectus femoris, as a cross-sectional area (CSA) or muscle thickness (MT), are reliable, simple and robust tools to detect low muscle mass [26,27] and accurately predict sarcopenia in older patients [28,29], with high sensitivity and specificity [30]. CSA and MT are linearly related to maximal voluntary contraction strength in patients with chronic respiratory issues and are also associated with a high to very high prognostic risk in diabetic kidney disease [31]. These measurements assess muscle wasting in hemodialysis patients with protein energy wasting [32] and in patients with spinal cord injury [33]. They also predict adverse outcomes in patients discharged from a surgical intensive care unit [34], and can discriminate between sarcopenia and non-sarcopenia states [34,35]. However, evidence about the longitudinal changes in MT and CSA of the rectus femoris, assessed by ultrasound, remains unavailable in older hospitalized patients. These patients are particularly vulnerable to loss of muscle mass and function during acute diseases as a result of protein undernutrition, inflammatory (pro-catabolic) status or lack of physical activity [36,37].
Based on these considerations, we hypothesized that musculoskeletal ultrasound imaging of the rectus femoris is helpful to detect changes in muscle mass in older adults hospitalized in a geriatric post-acute care ward following a hospital stay for acute illness. The main objective of this study was to use musculoskeletal ultrasound to prospectively assess changes in MT and CSA of the rectus femoris muscle in older patients admitted to a post-acute care unit during a 2-week follow-up. The secondary objectives were to compare MT and CSA in patients with frailty and pre-frailty, and to study the correlations of MT and CSA of the rectus femoris muscle with current sarcopenia diagnostic criteria.
2. Materials and Methods
2.1. Study Design and Setting
The prospective cohort study followed STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines [38]. The study was conducted in the post-acute care unit of a university hospital in Barcelona (Catalonia, Spain) from October 2019 to March 2021.
2.2. Eligibility Criteria
Inclusion criteria: (1) men and women aged 65 years or older; (2) admitted to a post-acute care unit after hospitalization due to acute illness lasting at least 2 weeks; and (3) cognitive status that permitted understanding study procedures and interventions (Mini-Mental State Examination ≥ 21/30) [39]. Patients with pre-existing conditions that may compromise muscle assessment were excluded (i.e., paresis of the lower limbs due to any neurological disorder, systemic connective tissue disorders, hypo- or hyperthyroidism, lower limb edema at thigh level, systemic atrophies primarily affecting the central nervous system, end-stage renal disease and end-of-life diseases in palliative care).
2.3. Study Variables
2.3.1. Muscle Thickness and Cross-Sectional Area
The MT and CSA of the rectus femoris of the dominant or uninjured leg were measured using B-mode on the MyLab™ Seven (Esaote, Genoa, Italy) (Figure 1). All measurements were done at maximal muscle bulk and performed by the same experienced investigator, following the SARCUS protocol for evidence-based muscle assessment though ultrasound [20,21]. The measurement protocol is described in Table 1. Anatomic landmarks and measuring points are shown in Figure 2. All measures were conducted at admission to the post-acute care unit (baseline) and every seven days until discharge, up to a 2-week follow-up.
2.3.2. Muscle Strength
Handgrip strength (Kg) was assessed with the Jamar Plus digital dynamometer, following the Southampton protocol [40]. Patients performed a maximum voluntary isometric contraction of finger flexor muscles. The highest value of three reproducible maneuvers (<10% variability between values) was used for analysis. Values < 27 Kg for men and < 16 Kg for woman were considered as decreased [2].
2.3.3. Muscle Mass
Fat-free mass (Kg) was measured by BIA (Bodystat 1500, Bodystat Ltd., Isle of Man, British Isles, UK). The investigator in charge of assessments performed the BIA immediately after the ultrasound assessment, according to current recommendations [20,41] in a comfortable area with no metallic objects; no meals or drinks 3 h before measurements; no exhausting exercise 12 h before measurements; and no alcohol or caffeine consumption 24 h before measurements [42]. Throughout the examinations, all subjects held their arms and legs in the abduction position; in obese subjects, a towel or pillow was placed between the thighs to avoid skin contact [41]. The impedance values were obtained at a frequency of 50 kHz. Values < 80% of the reference values for the European population [43] were considered as as decreased.

Table 1.
The protocol of Muscle Thickness and Cross-Sectional area of rectus femoris measure using musculoskeletal ultrasound.
Table 1.
The protocol of Muscle Thickness and Cross-Sectional area of rectus femoris measure using musculoskeletal ultrasound.
| Description | |
|---|---|
| Muscle to assess | Rectus femoris of quadriceps |
| Patient position | Patient lying supine, hips and knees in a neutral position |
| Patient condition | The patient had to maintain the same position for at least 30 min before the assessment, measuring the muscle in a relaxed state and before any functional testing. |
| Ultrasound and probe characteristics | B-mode ultrasound, 12 MHz, with a 5 cm-linear transducer probe. |
| Probe position | The probe in neutral/perpendicular to the skin, with a generous amount of transmission gel, maintaining the minimal pressure possible between the transducer and the skin. |
| Anatomical landmarks | Proximal landmark: greater trochanter. Distal landmark: proximal border of the patella. |
| Measuring point | The middle point of distance between anatomical landmarks, marking this point with a demographic pencil. |
| Measurements procedures | With the transducer probe in the measuring point. The measure of Muscle Thickness:
|
| Final value | The investigator repeated each measurement three times and used the mean value. |
2.3.4. Physical Performance
Physical performance was estimated by gait speed assessed in a 4 m walking test, where values ≤ 0.8 m/s were considered as low gait speed. Participants were instructed to stand with both feet touching the starting line and to begin walking with usual aids (canes or walkers) at their usual pace after a verbal command [44]. Gait speed was considered 0 m/s in bedridden patients unable to stand or in those unable to walk safely.
2.3.5. Sarcopenia Risk
The validated Spanish version of the Strength, Assistance in walking, Rise from chair, Climb stairs, and Falls (SARC-F) questionnaire was administered to assess sarcopenia risk at admission in the post-acute care unit. SARC-F scores range from 0–10 (0 = best to 10 = worst) where a score ≥ 4 is indicative of sarcopenia risk [45,46].
2.3.6. Sarcopenia
Sarcopenia was diagnosed at baseline according to the revised European consensus on definition and diagnosis (EWGSOP2) (2): low muscle mass and low muscle strength. These criteria were applied in all participants, independently of the score obtained in the SARC-F questionnaire.
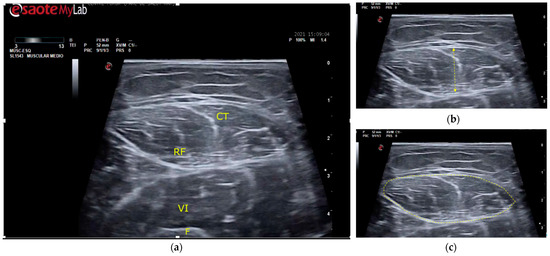
Figure 1.
Ultrasound of the rectus femoris muscle for MT and CSA measurement points. (a) Guiding structures are shown for proper recognition. RF, rectus femoris muscle; CT, central tendon of RF; VI, vastus intermedius muscle; F, femur. (b) MT (muscle thickness) was determined as the distance between the superior and inferior aponeurosis of the RF. (c) CSA (cross-sectional area) was measured by delimiting the cross-sectional area of the RF.
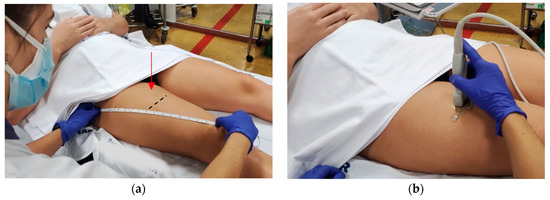
Figure 2.
Measuring points of thickness and cross-sectional area of the rectus femoris muscle according to the SARCopenia through UltraSound (SARCUS) protocol [20,21]. (a) MT and CSA of RF were measured at the dominant thigh at 50% (red arrow) of the distance between the greater trochanter and the proximal edge of the patella, with the patient’s legs in neutral position. The black line indicates where the images should be captured.; (b) A 5 cm width, 12 MHz linear transducer was placed perpendicular to the muscle. Abbreviations: CSA: Cross-sectional area, MT: muscle thickness, RF: Rectus femoris muscle.
2.3.7. Frailty Status
Frailty was assessed by the FRAIL scale at baseline in the post-acute care unit. The FRAIL questionnaire includes 5 components: Fatigue, Resistance, Ambulation, Illness and Loss of weight [47]. Scores range from 0–5 points (0 = best to 5 = worst) and indicate frailty (3–5), pre-frailty (1–2), and robust (0) health status [48].
2.3.8. Nutritional Risk
The Mini-Nutritional Assessment Short Form (MNA-SF) [32] was used to assess baseline malnutrition risk at admission to the post-acute care unit. Scores < 11 indicate risk of malnutrition [49,50].
2.3.9. Laboratory Values
Nutritional values were screened through albumin and prealbumin, and inflammation through C-reactive protein (CRP); vitamin D levels were also evaluated. A blood sample was collected on the day of admission to the unit.
2.3.10. Pharmacological Treatment
Non-steroidal anti-inflammatory drugs and drugs relevant to muscle mass, such as glucocorticoids, allopurinol, statins, insulin, and angiotensin II receptor blockers [51,52,53], were recorded upon admission to post-acute care.
2.3.11. Demographic and Clinical Characteristics
Other collected variables included age (years), sex (male/female), body mass index (Kg/m2) and fat mass (Kg). The Charlson Comorbidity Index was used to characterize the population. This international list of comorbidities has a maximum of 36 points, where a score ≥ 2 is an independent risk factor for 1-year mortality risk [54,55]. Falls in the last year were also recorded, as well as previous fragility fractures, defined as a pathological fracture resulting either from minimal trauma or no identifiable trauma at all [56,57]. The diagnosis associated with the functional deterioration that motivated admission to the post-acute care unit was recorded.
2.4. Study Procedures
Eligible participants were assessed by the researcher in charge of baseline and weekly assessments. Patients in post-acute care underwent an individualized rehabilitation program according to their functional needs (one hour each day, five days a week during hospitalization, usually a two-week stay). The programs generally focused on improving mobility, starting with the maintenance of joint range, postures, transfers, standing, and walking, followed by progressive muscle strength and endurance exercises.
2.5. Sample Size Calculation
Accepting an alpha risk of 0.05 and a beta risk of 0.2 in a two-sided test, 40 subjects were considered as necessary to recognize as statistically significant a change of muscle thickness ≥ 2.6 mm. The standard deviation was assumed to be 5.4. A drop-out rate of 15% was anticipated. The sample size was estimated by using the GRANMO calculator https://www.imim.es/ofertadeserveis/software-public/granmo/, accessed on 1 September 2019.
2.6. Statistics
Descriptive analysis was used to determine the clinical and demographic characteristics, using mean and standard deviation for quantitative variables, and absolute values and percentages for categorical variables. The normal data distribution of each variable was estimated with the Kolmogorov-Smirnov test. Chi-square or Fisher tests were used for univariate analysis of categorical variables. Changes in outcomes variables, adjusted by baseline values, were assessed by one-way analysis of variance (ANOVA), and multiple comparison post-test with Dunnett’s test. Intraclass correlation coefficients were calculated to test reliability. Differences in muscle thickness and the muscle cross-sectional area of rectus femoris among pre-frail and frail patients were evaluated with two-way ANOVA. The relationship between the primary and other variables was determined by Pearson correlation coefficient (r), where r ≤ 0.25 indicates absence or minimal relationship; 0.25 to 0.50, low to fair; 0.50 to 0.75, moderate to good; and ≥0.75, strong relationship [58]. The significance level was set at p ≤ 0.05. Graphics were processed with GraphPad Prism v.9.3.1 (GraphPad Software LLC, San Diego, CA, USA). Data analysis was performed using the IBM SPSS Statistics v.28 (SPSS Inc., Chicago, IL, USA) software package.
3. Results
From a total of 40 participants (aged 79.5 (SD 9.5) years; 57.5% women), 37 completed the baseline and first-week follow-up, 22 completed the 2-week follow-up, and 5 participants completed a 3-week follow-up. Three patients (7.5%) dropped out during the first week of follow-up due to severe deterioration of their health status. Patients had a length of stay in the post-acute care unit of 19.8 days (SD 7.8). Nineteen patients (47.5%) were admitted to the post-acute care unit for rehabilitation after a fracture, four (10.0%) to recover functional deterioration due to infection and seventeen (42.5%) for other causes of functional decline, such as acute illness, exacerbation of preexisting chronic diseases and scheduled surgeries. All participants had frailty (n = 14; 35%; women n = 10) or pre-frailty (n = 26; 65%; women n = 13), and 31 (77.5%) were at risk of sarcopenia. Twenty participants (50%) had low albumin levels, 24 (60%) had low prealbumin, 30 (75%) had low vitamin D levels and 37 (93%) showed high levels of CRP. Baseline demographic and clinical characteristics are displayed in Table 2.

Table 2.
Baseline demographic and clinical characteristics of study participants at admission to the post-acute care unit.
The test-retest reliability of MT and CSA of the rectus femoris using ultrasound was found to be excellent with an intraclass correlation coefficient of 0.970 and 0.968, respectively. Table 3 shows changes in MT and CSA of the rectus femoris, adjusted by their baseline values. In the first week follow-up, men had a significant increase in MT (0.9 mm [95%CI 0.3 to 1.4], p = 0.003] and CSA (0.4 cm2 [95%CI 0.1 to 0.6], p = 0.007). At the end of the second week, men continued to have a significant increase in MT (0.7 mm [95%CI 0.1 to 1.4], p = 0.036) and CSA (0.6 mm [95%CI 0.01 to 1.2], p = 0.048). Although MT and CSA in women showed an upwards trend throughout the follow-up, these changes were not statistically significant.
Figure 3 shows muscle changes according to frailty status during hospitalization in the post-acute care unit. Frail patients had significantly lower values of both MT and CSA at baseline and during hospitalization: MT mean difference 1.33 mm (95%CI 0.02 to 2.65, p = 0.047) and CSA 0.3 cm2 (0.29 to 1.47, p = 0.004). In the analysis by sex, women in the pre-frailty group had a significantly higher gain in MT (p = 0.037; 95%CI: −3.4 to −0.1) and CSA (p = 0.034; 95%CI: 0.1 to 1.7) of the rectus femoris muscle, compared to women with frailty.
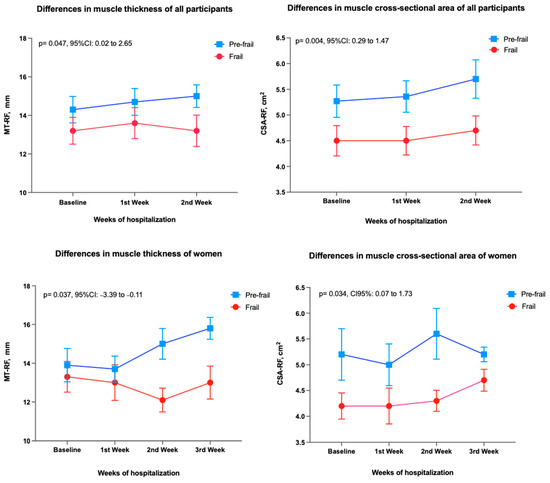
Figure 3.
Differences in muscle thickness and muscle cross-sectional area of rectus femoris among groups (Pre-frailty vs. Frailty) evaluated with two-way analysis of variance. The mean values and the standard error of the mean are shown. Abbreviations: CSA: Cross-sectional area, MT: muscle thickness, RF: Rectus femoris muscle, CI: Confidence Interval.

Table 3.
Changes in muscle thickness and cross-sectional area of the rectus femoris muscle assessed through ultrasound during hospitalization in the post-acute care unit according to sex distribution (n = 40).
Table 3.
Changes in muscle thickness and cross-sectional area of the rectus femoris muscle assessed through ultrasound during hospitalization in the post-acute care unit according to sex distribution (n = 40).
| Baseline | 1st Week Follow-Up | 2nd Week Follow-Up | |||
|---|---|---|---|---|---|
| Mean (SD) | Mean Difference | p | Mean Difference | p | |
| (95%CI) | (95%CI) | ||||
| Muscle thickness, mm | |||||
| Women | 13.2 (2.9) | 0.2 (−0.8 to 1.2) | 0.932 | 0.5 (−1.4 to 2.3) | 0.847 |
| Men | 14.9 (3.4) | 0.9 (0.3 to 1.4) | 0.003 | 0.7 (0.1 to 1.4) | 0.036 |
| Cross-sectional area, cm2 | |||||
| Women | 4.7 (1.5) | −0.1 (−0.5 to 0.3) | 0.926 | 0.2 (−0.5 to 0.9) | 0.752 |
| Men | 5.3 (1.3) | 0.4 (0.1 to 0.6) | 0.007 | 0.6 (0.01 to 1.2) | 0.048 |
Table 4 shows the Pearson correlation coefficients of MT and CSA of the rectus femoris muscle assessed through ultrasound: handgrip strength, fat-free mass and gait speed. A moderate to good correlation of CSA with handgrip strength and fat-free mass was observed at baseline and 1-week follow-up. A low to fair relationship was found between MT, handgrip strength and fat-free mass at baseline and 1-week follow-up (Figure 4). These correlations remained unchanged in the analysis by sex. No relationship was found between MT and gait speed; a low to fair relationship was observed between CSA and gait speed, only at the 1-week follow-up.

Table 4.
Pearson correlation coefficients of muscle thickness and cross-sectional area of the rectus femoris assessed by ultrasound with current sarcopenia diagnostic criteria during hospitalization in the post-acute care unit (n = 40).
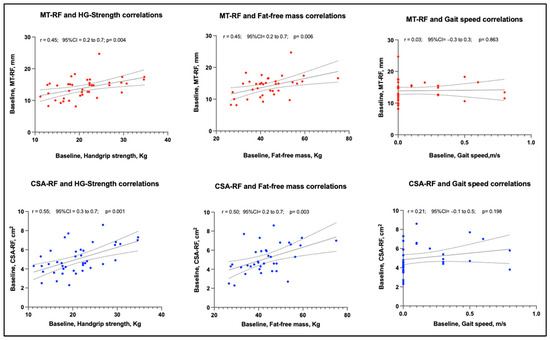
Figure 4.
The relationship of baseline muscle thickness and cross-sectional area of rectus femoris with muscle strength (handgrip), muscle mass (fat-free mass) and physical performance (gait speed), assessed during hospitalization in a post-acute care unit. Abbreviations: CSA: Cross-sectional area, HG: Handgrip; MT: Muscle thickness, RF: Rectus femoris muscle. r: the Pearson coefficient, CI: Confidence Interval.
4. Discussion
This prospective cohort study was aimed to quantify the changes in muscle size estimated with MT and CSA of the rectus femoris muscle, assessed by ultrasound during a 3-week follow-up in patients admitted to a post-acute care unit. The SARCUS protocol for an evidence-based muscle assessment through ultrasound was applied, and the results highlight the need for muscle health assessment during comprehensive geriatric evaluations in patients hospitalized for post-acute care.
Previous studies have reported the adverse effects of hospitalizations on muscle mass and function in older adults [6,11,59]. However, evidence has also shown that early tailored interventions, such as rehabilitation programs, can improve clinical adverse outcomes [59] and shorten hospitalization stays [60]. To the authors’ knowledge, this is the first study to report changes in MT and the CSA of the rectus femoris muscle in an older population hospitalized in a post-acute care unit after acute illness applying the SARCUS protocol.
The MT value, followed by CSA, are considered the simplest and most reproducible ultrasound parameters for muscle mass assessment [61]. Values for rectus femoris MT of 16 mm in women and 20 mm in men have been suggested as cut-off points in the diagnosis of sarcopenia [62]. Although there is no definite consensus on the use of these values, upon admission to post-acute care, our patients presented with a mean MT of 13.2 (SD 2.9) mm in women and 14.9 (SD 3.4) mm in men. By the proposed definition, all would qualify for a sarcopenia diagnosis. Even though the loss of muscle mass during the acute disease could not be quantified in our participants, a significant increase in MT and CSA of the rectus femoris was observed in men after the first week in the post-acute unit, with further changes after the second week. Women showed an upwards trend in MT and CSA throughout the follow-up, but these changes did not reach statistical significance.
In our study, there no were baseline differences in age, comorbidity or frailty status between men and women. A study comparing changes in muscle strength and MT in response to exercise in young adults suggests that MT response in women may have a different time course, with a delay in muscle strength recovery compared to men [63]. However, no clear explanation has been established for these sex-related differences and other factors also may have contributed to the results, such as sex hormones, inflammatory response, sedentary lifestyle and dietary habits, among others [64].
To the authors’ knowledge, this is the first study using musculoskeletal ultrasound as a bedside tool to measure muscle mass as part of the comprehensive geriatric assessment in post-acute care. Musculoskeletal ultrasound was able to detect changes in the 3-week follow-up, and even to differentiate the evolution of muscle changes in frail and pre-frail inpatients. These findings are of important clinical relevance for patients at risk of frailty, which have proven to benefit from interventions that help to improve their muscle mass [65]. Therefore, our study highlights the need for adequate identification of frailty and sarcopenia in this population and supports the importance of including muscle mass monitoring within the comprehensive geriatric assessment in hospitalized older adults [66] to target interventions (e.g., exercise) that enable attenuating or preventing muscle wasting [67].
Several study limitations should be acknowledged. First, the relatively limited sample size, especially for men at the 3-week follow-up, hindered a deeper analysis of MT and CSA changes in the rectus femoris. Second, the limited information about the number of bed rest days in the acute care unit prior to admission in the post-acute care unit precluded analysis of functional impairment before referral to the post-acute unit. Given that MT and the CSA of rectus femoris muscle parameters are highly sensitive to bed rest, those patients who had more days of bed rest during acute hospitalization may have presented with higher initial values than the rest of the patients, affecting the evolution of the MT and CSA changes. Finally, a selection bias must be acknowledged, given the absence of “robust” patients in the sample. These limitations should be addressed in further prospective multicentric studies with larger sample sizes. The study highlights the need to re-think diagnostic strategies in sarcopenia and implement updated evidence-based diagnostic tools as part of a comprehensive geriatric assessment in clinical practice, in an effort to improve the quality of care and remain at the forefront of best practices.
5. Conclusions
Musculoskeletal ultrasound was able to detect MT and CSA improvement in men during a 2-week follow-up in a post-acute care unit. Frail patients show lower values of these parameters during the hospitalization when compared to pre-frail patients. MT and CSA measured by ultrasound were correlated with handgrip strength and fat-free mass in this sample at baseline and at one-week follow-up.
Author Contributions
Conceptualization: D.M.-V., E.M. and D.S.-R.; Data curation: E.M. and D.M.-V.; Formal analysis: D.M.-V., E.M. and D.S.-R.; Investigation: S.P., F.M.C., E.M.-R., A.M.-P., E.D.J., L.C. and D.M.-V.; Methodology: E.M. and D.S.-R.; Supervision and Project administration: E.M. and D.S.-R.; Resources: E.M., D.M.-V., S.P., F.M.C., E.M.-R., A.M.-P., E.D.J., L.C., M.T.S., Y.C.P. and D.S.-R.; Software: D.M.-V. and D.S.-R.; Validation: D.M.-V., E.M. and D.S.-R.; Visualization: E.D.J., L.C., F.M.C., E.M.-R., S.P., M.T.S., Y.C.P. and A.M.-P.; Writing—original draft: E.M., D.M.-V. and D.S.-R. Writing—review and editing: S.P., F.M.C., E.M.-R., A.M.-P., E.D.J., L.C., D.M.-V., M.T.S., Y.C.P., E.M. and D.S.-R. This manuscript is part of Delky Meza-Valderrama’s PhD project entitled “Sarcopenia: Re-thinking strategies in the diagnostic and therapeutic approach” in the PhD program in Biomedicine, Department of Experimental and Health Sciences, Universitat Pompeu Fabra–Doctoral School, Barcelona, Catalonia, Spain. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
International research ethics guidelines were followed, including Good Clinical Practice (GCP) and the Helsinki Declaration (1964) and its further amendments (Fortaleza, 2013). The study protocol was reviewed and approved by the local Ethics Committee of the Hospital del Mar Research Institute, Barcelona, Catalonia, Spain (reference number 015/6288/I). Data were collected and treated in compliance with Spain’s confidentiality law concerning personal data (Ley Orgánica 15/1999, 13 December, Protección de Datos de Carácter Personal) and the European Union Regulation 2016/679, 27 April.
Informed Consent Statement
Detailed and understandable oral and written information was provided to patients and family members, and informed consent was obtained from all participants involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nascimento, C.M.; Ingles, M.; Salvador-Pascual, A.; Cominetti, M.R.; Gomez-Cabrera, M.C.; Viña, J. Sarcopenia, frailty and their prevention by exercise. Free Radic. Biol. Med. 2019, 132, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Proietti, M.; Cesari, M. Frailty: What Is It? Adv. Exp. Med. Biol. 2020, 1216, 1–7. [Google Scholar] [CrossRef]
- Tabue-Teguo, M.; Simo, N.; Gonzalez-Colaço Harmand, M.; Cesari, M.; Avila-Funes, J.A.; Féart, C.; Amiéva, H.; Dartigues, J.F. Frailty in elderly: A brief review. Gériatrie Et Psychol. Neuropsychiatr. Du Vieil. 2017, 15, 127–137. [Google Scholar] [CrossRef]
- Tanner, R.E.; Brunker, L.B.; Agergaard, J.; Barrows, K.M.; Briggs, R.A.; Kwon, O.S.; Young, L.M.; Hopkins, P.N.; Volpi, E.; Marcus, R.L.; et al. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J. Physiol. 2015, 593, 4259–4273. [Google Scholar] [CrossRef]
- Witteveen, E.; Wieske, L.; Verhamme, C.; Schultz, M.J.; van Schaik, I.N.; Horn, J. Muscle and nerve inflammation in intensive care unit-acquired weakness: A systematic translational review. J. Neurol. Sci. 2014, 345, 15–25. [Google Scholar] [CrossRef]
- Kortebein, P.; Ferrando, A.; Lombeida, J.; Wolfe, R.; Evans, W.J. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007, 297, 1772–1774. [Google Scholar] [CrossRef]
- Alley, D.E.; Koster, A.; Mackey, D.; Cawthon, P.; Ferrucci, L.; Simonsick, E.M.; Yu, B.; Hardy, S.; Goodpaster, B.; Sarkisian, C.; et al. Hospitalization and change in body composition and strength in a population-based cohort of older persons. J. Am. Geriatr. Soc. 2010, 58, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- English, K.L.; Paddon-Jones, D. Protecting muscle mass and function in older adults during bed rest. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 34–39. [Google Scholar] [CrossRef]
- Reidy, P.T.; Lindsay, C.C.; McKenzie, A.I.; Fry, C.S.; Supiano, M.A.; Marcus, R.L.; LaStayo, P.C.; Drummond, M.J. Aging-related effects of bed rest followed by eccentric exercise rehabilitation on skeletal muscle macrophages and insulin sensitivity. Exp. Gerontol. 2018, 107, 37–49. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Ferrillo, M.; Lippi, L.; Agostini, F.; de Sire, R.; Ferrara, P.E.; Raguso, G.; Riso, S.; Roccuzzo, A.; Ronconi, G.; et al. Sarcopenic Dysphagia, Malnutrition, and Oral Frailty in Elderly: A Comprehensive Review. Nutrients 2022, 14, 982. [Google Scholar] [CrossRef]
- Lee, H.; Lee, E.; Jang, I.Y. Frailty and Comprehensive Geriatric Assessment. J. Korean Med. Sci. 2020, 35, e16. [Google Scholar] [CrossRef] [PubMed]
- Schippinger, W. Comprehensive geriatric assessment. Wien. Med. Wochenschr. 2022, 172, 122–125. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; McCloskey, E.; Bruyere, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo de Carvalho, I.; Amuthavalli Thiyagarajan, J.; Bautmans, I.; Bertiere, M.C.; et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Nijholt, W.; Scafoglieri, A.; Jager-Wittenaar, H.; Hobbelen, J.S.M.; van der Schans, C.P. The reliability and validity of ultrasound to quantify muscles in older adults: A systematic review. J. Cachexia Sarcopenia Muscle 2017, 8, 702–712. [Google Scholar] [CrossRef]
- Van den Broeck, J.; Buzzatti, L.; Jager-Wittenaar, H.; Perkisas, S.; Scafoglieri, A. The validity of ultrasound-derived equation models to predict whole-body muscle mass: A systematic review. Clin. Nutr. ESPEN 2021, 46, 133–141. [Google Scholar] [CrossRef]
- Sponbeck, J.K.; Frandsen, C.R.; Ridge, S.T.; Swanson, D.A.; Swanson, D.C.; Johnson, A.W. Leg muscle cross-sectional area measured by ultrasound is highly correlated with MRI. J. Foot Ankle Res. 2021, 14, 5. [Google Scholar] [CrossRef]
- Perkisas, S.; Bastijns, S.; Baudry, S.; Bauer, J.; Beaudart, C.; Beckwée, D.; Cruz-Jentoft, A.; Gasowski, J.; Hobbelen, H.; Jager-Wittenaar, H.; et al. Application of ultrasound for muscle assessment in sarcopenia: 2020 SARCUS update. Eur. Geriatr. Med. 2021, 12, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Perkisas, S.; Baudry, S.; Bauer, J.; Beckwée, D.; De Cock, A.-M.; Hobbelen, H.; Jager-Wittenaar, H.; Kasiukiewicz, A.; Landi, F.; Marco, E.; et al. Application of ultrasound for muscle assessment in sarcopenia: Towards standardized measurements. Eur. Geriatr. Med. 2018, 9, 739–757. [Google Scholar] [CrossRef] [PubMed]
- Leigheb, M.; de Sire, A.; Colangelo, M.; Zagaria, D.; Grassi, F.A.; Rena, O.; Conte, P.; Neri, P.; Carriero, A.; Sacchetti, G.M.; et al. Sarcopenia Diagnosis: Reliability of the Ultrasound Assessment of the Tibialis Anterior Muscle as an Alternative Evaluation Tool. Diagnostics 2021, 11, 2158. [Google Scholar] [CrossRef] [PubMed]
- Hammond, K.; Mampilly, J.; Laghi, F.A.; Goyal, A.; Collins, E.G.; McBurney, C.; Jubran, A.; Tobin, M.J. Validity and reliability of rectus femoris ultrasound measurements: Comparison of curved-array and linear-array transducers. J. Rehabil. Res. Dev. 2014, 51, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Meza-Valderrama, D.; Sánchez-Rodríguez, D.; Perkisas, S.; Duran, X.; Bastijns, S.; Dávalos-Yerovi, V.; Da Costa, E.; Marco, E. The feasibility and reliability of measuring forearm muscle thickness by ultrasound in a geriatric inpatient setting: A cross-sectional pilot study. BMC Geriatr. 2022, 22, 137. [Google Scholar] [CrossRef]
- Pirri, C.; Pirri, N.; Porzionato, A.; Boscolo-Berto, R.; De Caro, R.; Stecco, C. Inter- and Intra-Rater Reliability of Ultrasound Measurements of Superficial and Deep Fasciae Thickness in Upper Limb. Diagnostics 2022, 12, 2195. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Ikezoe, T.; Taniguchi, M.; Yamada, Y.; Sawano, S.; Minani, S.; Asai, T.; Kimura, M.; Ichihashi, N. Cut-off Values for Lower Limb Muscle Thickness to Detect Low Muscle Mass for Sarcopenia in Older Adults. Clin. Interv. Aging 2021, 16, 1215–1222. [Google Scholar] [CrossRef]
- Berger, J.; Bunout, D.; Barrera, G.; de la Maza, M.P.; Henriquez, S.; Leiva, L.; Hirsch, S. Rectus femoris (RF) ultrasound for the assessment of muscle mass in older people. Arch. Gerontol. Geriatr. 2015, 61, 33–38. [Google Scholar] [CrossRef]
- Ozturk, Y.; Koca, M.; Burkuk, S.; Unsal, P.; Dikmeer, A.; Oytun, M.G.; Bas, A.O.; Kahyaoglu, Z.; Deniz, O.; Coteli, S.; et al. The role of muscle ultrasound to predict sarcopenia. Nutrition 2022, 101, 111692. [Google Scholar] [CrossRef]
- Wilkinson, T.J.; Gore, E.F.; Vadaszy, N.; Nixon, D.G.D.; Watson, E.L.; Smith, A.C. Utility of Ultrasound as a Valid and Accurate Diagnostic Tool for Sarcopenia: Sex-Specific Cutoff Values in Chronic Kidney Disease. J. Ultrasound Med. 2021, 40, 457–467. [Google Scholar] [CrossRef]
- Barotsis, N.; Galata, A.; Hadjiconstanti, A.; Panayiotakis, G. The ultrasonographic measurement of muscle thickness in sarcopenia. A prediction study. Eur. J. Phys. Rehabil. Med. 2020, 56, 427–437. [Google Scholar] [CrossRef]
- Lin, X.; Chen, Z.; Huang, H.; Zhong, J.; Xu, L. Diabetic kidney disease progression is associated with decreased lower-limb muscle mass and increased visceral fat area in T2DM patients. Front. Endocrinol. (Lausanne) 2022, 13, 1002118. [Google Scholar] [CrossRef]
- Sahathevan, S.; Khor, B.H.; Singh, B.K.S.; Sabatino, A.; Fiaccadori, E.; Daud, Z.A.M.; Ali, M.S.; Narayanan, S.S.; Tallman, D.; Chinna, K.; et al. Association of Ultrasound-Derived Metrics of the Quadriceps Muscle with Protein Energy Wasting in Hemodialysis Patients: A Multicenter Cross-Sectional Study. Nutrients 2020, 12, 3597. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.R.J.; Kong, K.H. Ultrasound Measurements of Rectus Femoris and Locomotor Outcomes in Patients with Spinal Cord Injury. Life 2022, 12, 1073. [Google Scholar] [CrossRef]
- Mueller, N.; Murthy, S.; Tainter, C.R.; Lee, J.; Riddell, K.; Fintelmann, F.J.; Grabitz, S.D.; Timm, F.P.; Levi, B.; Kurth, T.; et al. Can Sarcopenia Quantified by Ultrasound of the Rectus Femoris Muscle Predict Adverse Outcome of Surgical Intensive Care Unit Patients as well as Frailty? A Prospective, Observational Cohort Study. Ann. Surg. 2016, 264, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Rustani, K.; Kundisova, L.; Capecchi, P.L.; Nante, N.; Bicchi, M. Ultrasound measurement of rectus femoris muscle thickness as a quick screening test for sarcopenia assessment. Arch. Gerontol. Geriatr. 2019, 83, 151–154. [Google Scholar] [CrossRef] [PubMed]
- McKendry, J.; Thomas, A.C.Q.; Phillips, S.M. Muscle Mass Loss in the Older Critically Ill Population: Potential Therapeutic Strategies. Nutr. Clin. Pract. 2020, 35, 607–616. [Google Scholar] [CrossRef]
- Dirks, M.L.; Wall, B.T.; van de Valk, B.; Holloway, T.M.; Holloway, G.P.; Chabowski, A.; Goossens, G.H.; van Loon, L.J. One Week of Bed Rest Leads to Substantial Muscle Atrophy and Induces Whole-Body Insulin Resistance in the Absence of Skeletal Muscle Lipid Accumulation. Diabetes 2016, 65, 2862–2875. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Initiative, S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Crum, R.M.; Anthony, J.C.; Bassett, S.S.; Folstein, M.F. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993, 269, 2386–2391. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Lukaski, H.C. Requirements for clinical use of bioelectrical impedance analysis (BIA). Ann. N. Y. Acad. Sci. 1999, 873, 72–76. [Google Scholar] [CrossRef]
- Větrovská, R.; Vilikus, Z.; Klaschka, J.; Stránská, Z.; Svačina, Š.; Svobodová, Š.; Matoulek, M. Does impedance measure a functional state of the body fat? Physiol. Res. 2014, 63, S309–S320. [Google Scholar] [CrossRef]
- Schutz, Y.; Kyle, U.U.; Pichard, C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18-98 y. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Wang, Y.C. Four-Meter Gait Speed: Normative Values and Reliability Determined for Adults Participating in the NIH Toolbox Study. Arch. Phys. Med. Rehabil. 2019, 100, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, D.; Marco, E.; Dávalos-Yerovi, V.; López-Escobar, J.; Messaggi-Sartor, M.; Barrera, C.; Ronquillo-Moreno, N.; Vázquez-Ibar, O.; Calle, A.; Inzitari, M.; et al. Translation and Validation of the Spanish Version of the SARC-F Questionnaire to Assess Sarcopenia in Older People. J. Nutr. Health Aging 2019, 23, 518–524. [Google Scholar] [CrossRef]
- Abellan van Kan, G.; Rolland, Y.M.; Morley, J.E.; Vellas, B. Frailty: Toward a clinical definition. J. Am. Med. Dir. Assoc. 2008, 9, 71–72. [Google Scholar] [CrossRef]
- Woo, J.; Yu, R.; Wong, M.; Yeung, F.; Wong, M.; Lum, C. Frailty Screening in the Community Using the FRAIL Scale. J. Am. Med. Dir. Assoc. 2015, 16, 412–419. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef]
- Liu, H.; Jiao, J.; Zhu, M.; Wen, X.; Jin, J.; Wang, H.; Lv, D.; Zhao, S.; Sun, X.; Wu, X.; et al. Nutritional Status According to the Short-Form Mini Nutritional Assessment (MNA-SF) and Clinical Characteristics as Predictors of Length of Stay, Mortality, and Readmissions Among Older Inpatients in China: A National Study. Front. Nutr. 2022, 9, 815578. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.I.; Priego, T.; López-Calderón, A. Hormones and Muscle Atrophy. In Muscle Atrophy; Xiao, J., Ed.; Springer: Singapore, 2018; pp. 207–233. [Google Scholar] [CrossRef]
- Ferrando, B.; Olaso-Gonzalez, G.; Sebastia, V.; Viosca, E.; Gomez-Cabrera, M.C.; Viña, J. Alopurinol y su papel en el tratamiento de la sarcopenia. Rev. Española De Geriatría Y Gerontol. 2014, 49, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Sartiani, L.; Spinelli, V.; Laurino, A.; Blescia, S.; Raimondi, L.; Cerbai, E.; Mugelli, A. Pharmacological perspectives in sarcopenia: A potential role for renin-angiotensin system blockers? Clin. Cases Miner. Bone Metab. 2015, 12, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P.; Josse, R.G. 2002 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. Cmaj 2002, 167, S1–S34. [Google Scholar]
- Borgström, F.; Karlsson, L.; Ortsäter, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; Javaid, M.K.; et al. Fragility fractures in Europe: Burden, management and opportunities. Arch. Osteoporos. 2020, 15, 59. [Google Scholar] [CrossRef]
- Portney, L.G. Foundations of Clinical Research: Applications to Evidence-Based Practice, 4th ed.; McGraw Hill: Philadelphia, PA, USA, 2020; Available online: https://fadavispt.mhmedical.com/book.aspx?bookID=2885 (accessed on 10 October 2022).
- Liu, K.; Shibata, J.; Fukuchi, K.; Takahashi, K.; Sonoo, T.; Ogura, T.; Goto, T. Optimal timing of introducing mobilization therapy for ICU patients with sepsis. J. Intensive Care 2022, 10, 22. [Google Scholar] [CrossRef]
- Fisher, S.R.; Kuo, Y.-f.; Graham, J.E.; Ottenbacher, K.J.; Ostir, G.V. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch. Intern. Med. 2010, 170, 1942–1943. [Google Scholar] [CrossRef]
- Ticinesi, A.; Meschi, T.; Narici, M.V.; Lauretani, F.; Maggio, M. Muscle Ultrasound and Sarcopenia in Older Individuals: A Clinical Perspective. J. Am. Med. Dir. Assoc. 2017, 18, 290–300. [Google Scholar] [CrossRef]
- Minetto, M.A.; Caresio, C.; Menapace, T.; Hajdarevic, A.; Marchini, A.; Molinari, F.; Maffiuletti, N.A. Ultrasound-Based Detection of Low Muscle Mass for Diagnosis of Sarcopenia in Older Adults. PMR 2016, 8, 453–462. [Google Scholar] [CrossRef]
- Flores, D.F.; Gentil, P.; Brown, L.E.; Pinto, R.S.; Carregaro, R.L.; Bottaro, M. Dissociated time course of recovery between genders after resistance exercise. J. Strength Cond Res. 2011, 25, 3039–3044. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Asai, H.; Toyama, H.; Kunita, K.; Yaguchi, C.; Kiyota, N.; Tomita, H.; Jacobs, J.V. Changes in muscle thickness of gastrocnemius and soleus associated with age and sex. Aging Clin. Exp. Res. 2010, 22, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.D.; Chen, H.C.; Huang, S.W.; Liou, T.H. The Role of Muscle Mass Gain Following Protein Supplementation Plus Exercise Therapy in Older Adults with Sarcopenia and Frailty Risks: A Systematic Review and Meta-Regression Analysis of Randomized Trials. Nutrients 2019, 11, 1713. [Google Scholar] [CrossRef]
- Pilotto, A.; Cella, A.; Daragjati, J.; Veronese, N.; Musacchio, C.; Mello, A.M.; Logroscino, G.; Padovani, A.; Prete, C.; Panza, F. Three Decades of Comprehensive Geriatric Assessment: Evidence Coming From Different Healthcare Settings and Specific Clinical Conditions. J. Am. Med. Dir. Assoc. 2017, 18, 192.e1. [Google Scholar] [CrossRef] [PubMed]
- Distefano, G.; Goodpaster, B.H. Effects of Exercise and Aging on Skeletal Muscle. Cold Spring Harb. Perspect. Med. 2018, 8, a029785. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).