Type II Heterojunction Formed between {010} or {012} Facets Dominated Bismuth Vanadium Oxide and Carbon Nitride to Enhance the Photocatalytic Degradation of Tetracycline
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of the g-C3N4
2.3. Preparations of {012}BiVO4 and {012}BiVO4/g-C3N4
2.4. Preparation of {010}BiVO4 and {010}BiVO4/g-C3N4
2.5. Structure and Characterization of Materials
2.6. Photocatalytic Activity Experiment
2.7. Photoelectrochemical Tests
2.8. Photocatalytic Trapping Agent Experiment
3. Results and Discussion
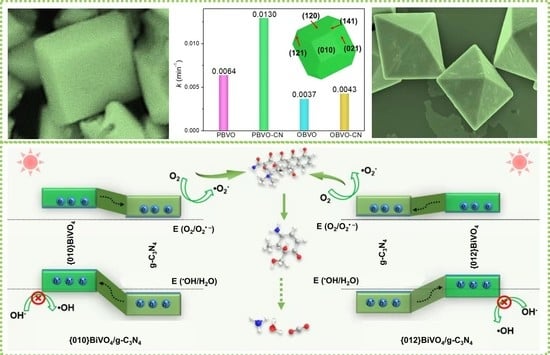
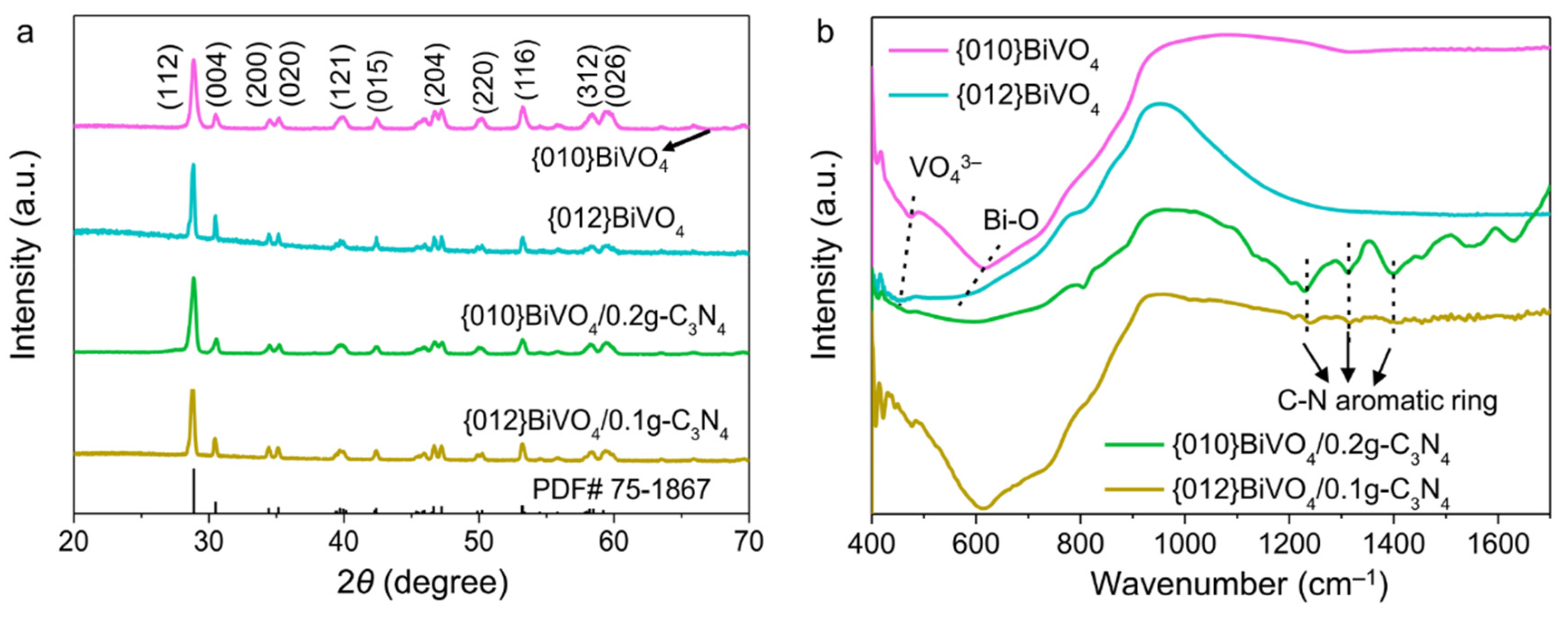
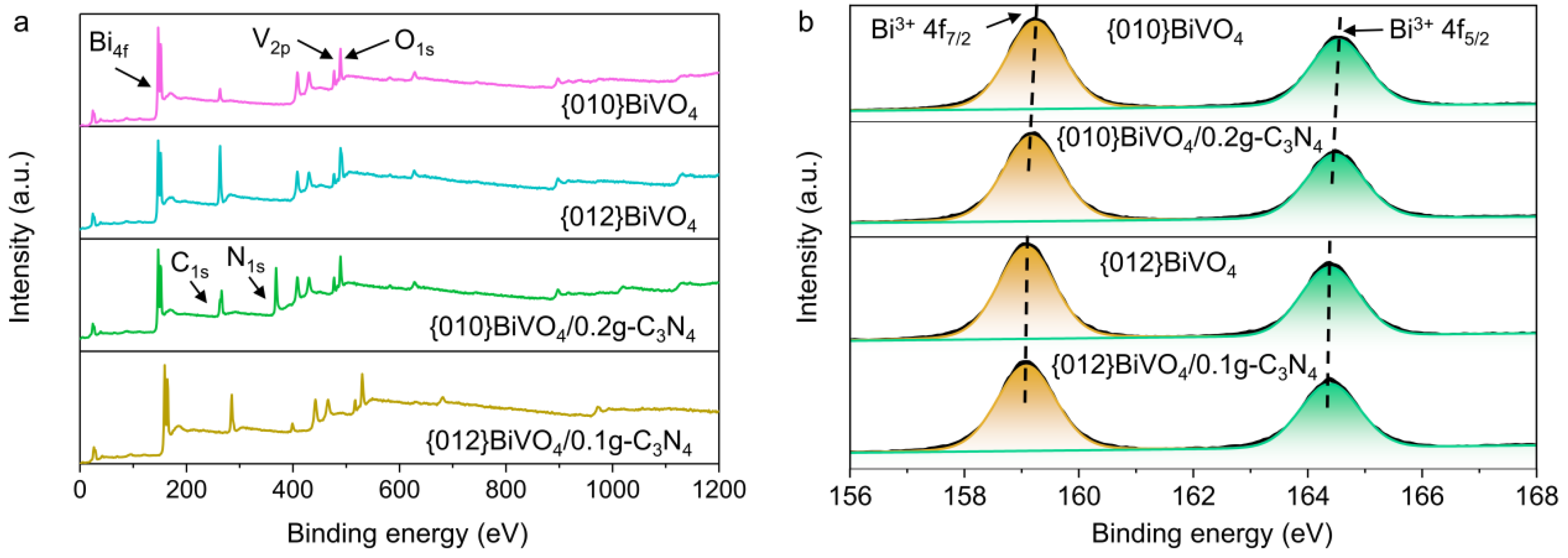
3.1. Characteristics
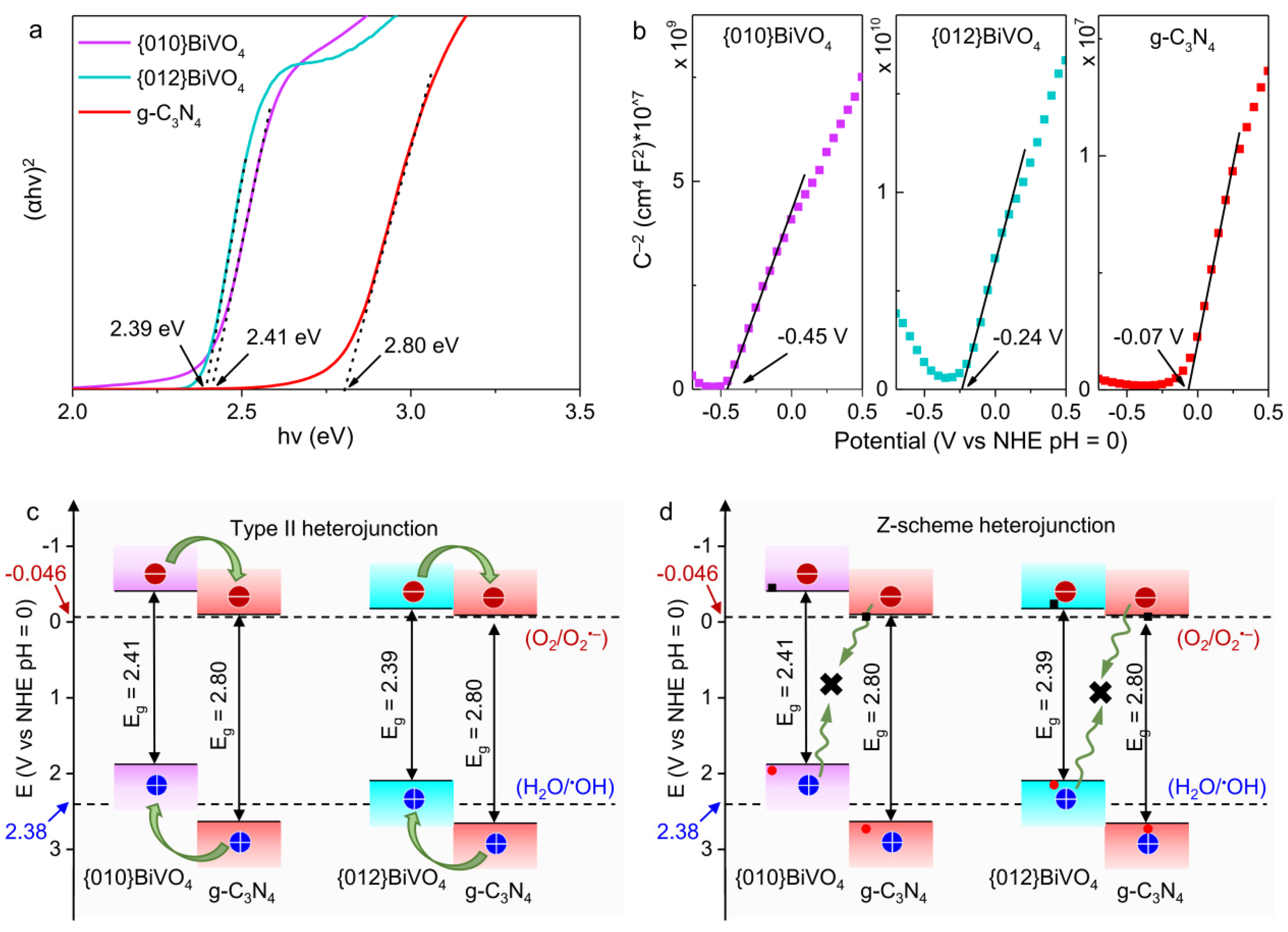
3.2. Construction of Energy Band Structure
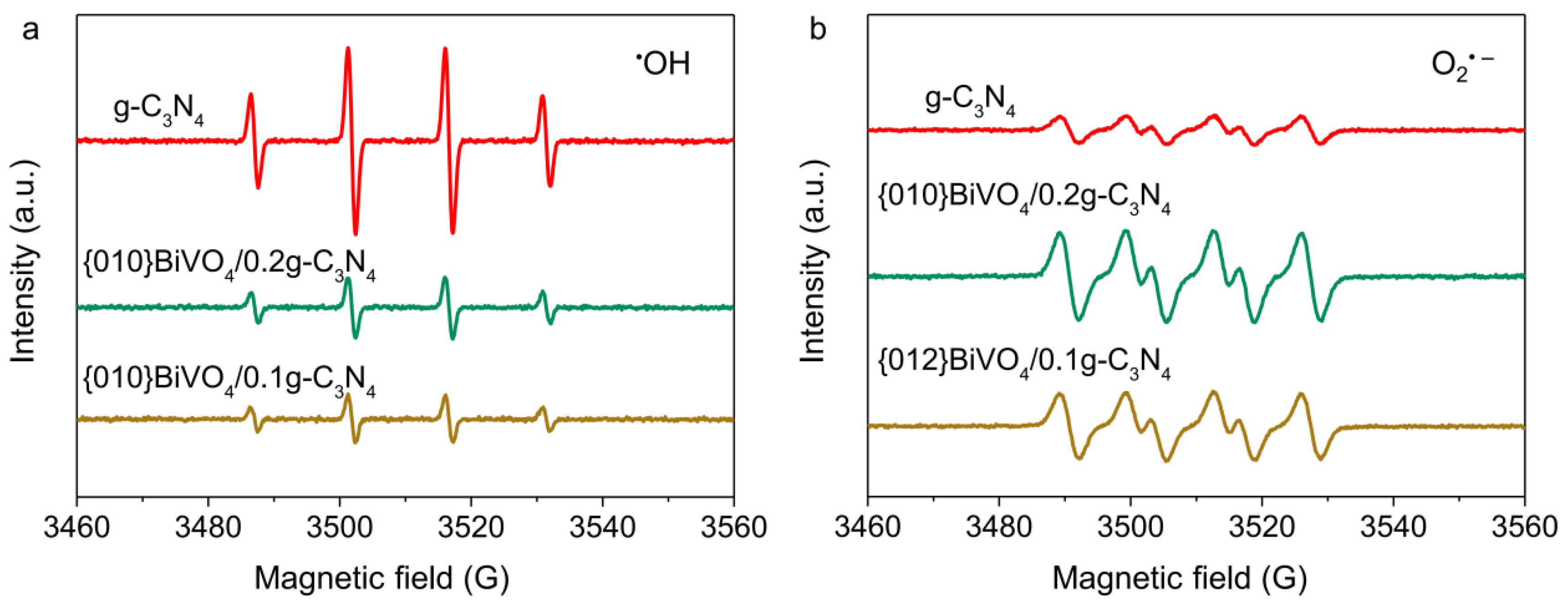
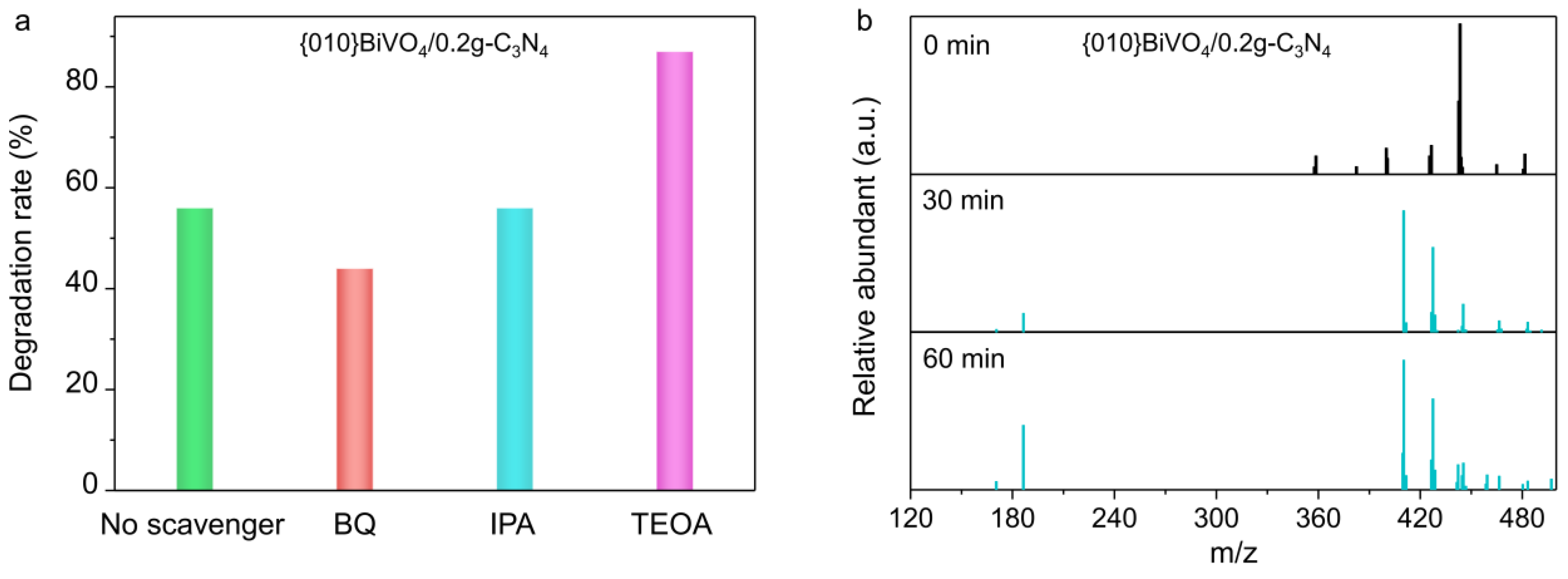
3.3. Radical Species Analyses
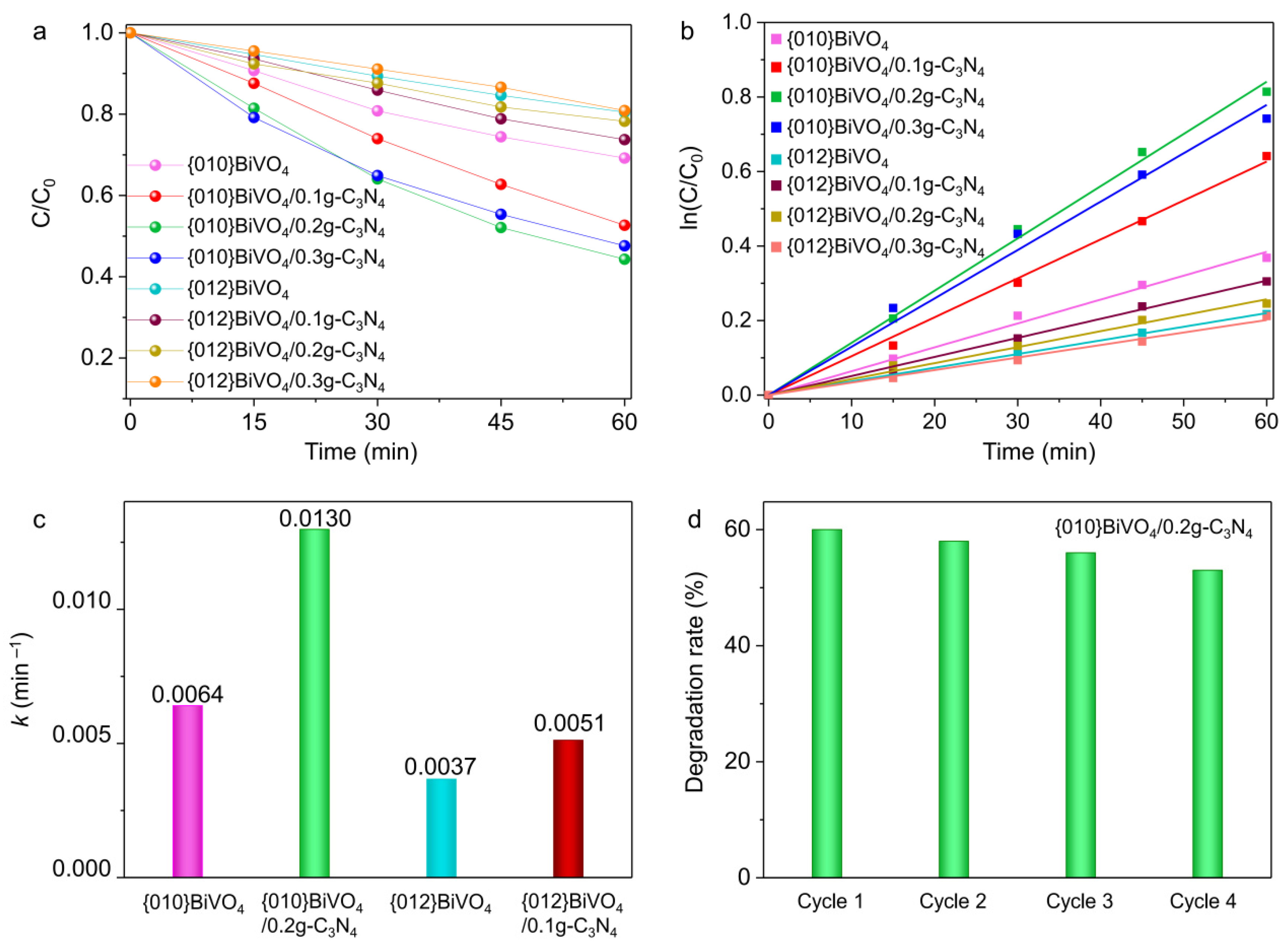
3.4. Photocatalytic Performances
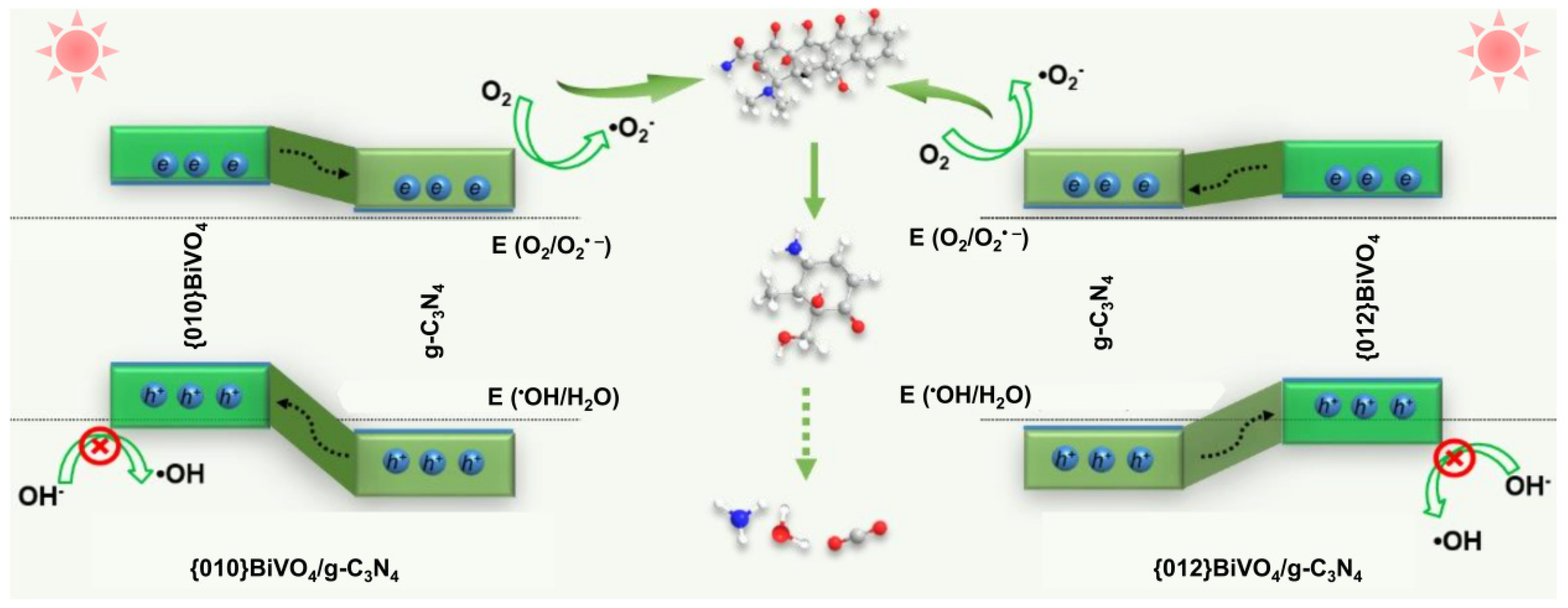
3.5. Photocatalytic Degradation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gobel, A.; McArdell, C.S.; Joss, A.; Siegrist, H.; Giger, W. Fate of sulfonamides, macrolides, and trimethoprim in different wastewater treatment technologies. Sci. Total Environ. 2007, 372, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Q.; Xiong, G.; Ding, F.; He, Y.; Ren, B.; You, L.; Fan, X.; Hardacre, C.; Sun, Y. Bakelite-type anionic microporous organic polymers with high capacity for selective adsorption of cationic dyes from water. Chem. Eng. J. 2019, 366, 404–414. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Miao, X.S.; Bishay, F.; Chen, M.; Metcalfe, C.D. Occurrence of antimicrobials in the final effluents of wastewater treatment plants in Canada. Environ. Sci. Technol. 2004, 38, 3533–3541. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.; Paulo, M.; Silva, L.J.G.; Seifrtová, M.; Lino, C.M.; Solich, P. Tetracycline antibiotics in hospital and municipal wastewaters: A pilot study in Portugal. Anal. Bioanal. Chem. 2010, 396, 2929–2936. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef]
- Watkinson, A.J.; Murby, E.J.; Kolpin, D.W.; Costanzo, S.D. The occurrence of antibiotics in an urban watershed: From wastewater to drinking water. Sci. Total Environ. 2009, 407, 2711–2723. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V. Semiconductor photocatalysis-past, present, and future outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Photocatalytic CO2 reduction by CdS promoted with a zeolitic imidazolate framework. Appl. Catal. B Environ. 2015, 162, 494–500. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, W.; An, W.; Liu, L.; Liang, Y.; Zhu, Y. Combination of photoelectrocatalysis and adsorption for removal of bisphenol A over TiO2-graphene hydrogel with 3D network structure. Appl. Catal. B Environ. 2018, 221, 36–46. [Google Scholar] [CrossRef]
- Wan, C.; Zhou, L.; Xu, S.; Jin, B.; Ge, X.; Qian, X.; Xu, L.; Chen, F.; Zhan, X.; Yang, Y.; et al. Defect engineered mesoporous graphitic carbon nitride modified with AgPd nanoparticles for enhanced photocatalytic hydrogen evolution from formic acid. Chem. Eng. J. 2022, 429, 132388. [Google Scholar]
- Wan, C.; Zhou, L.; Sun, L.; Xu, L.; Cheng, D.; Chen, F.; Zhan, X.; Yang, Y. Boosting visible-light-driven hydrogen evolution from for mic acid over AgPd/2D g-C3N4 nanosheets Mott-Schottky photocatalyst. Chem. Eng. J. 2020, 396, 125229. [Google Scholar] [CrossRef]
- Wang, M.; Ioccozia, J.; Sun, L.; Lin, C.; Lin, Z. Inorganic-modified semiconductor TiO2 nanotube arrays for photocatalysis. Energy Environ. Sci. 2014, 7, 2182–2202. [Google Scholar] [CrossRef]
- Reddy, I.N.; Reddy, C.V.; Shim, J.; Akkinepally, B.; Cho, M.; Yoo, K.; Kim, D. Excellent visible-light driven photocatalyst of (Al, Ni) Co-doped ZnO structures for organic dye degradation. Catal. Today 2020, 340, 277–285. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J.; Zhang, J.; Niu, J.; Zhao, J.; Wei, Y.; Yao, B. Photocatalytic degradation of ciprofloxacin using Zn-doped Cu2O particles: Analysis of degradation pathways and intermediates. Chem. Eng. J. 2019, 374, 316–327. [Google Scholar] [CrossRef]
- Li, H.; Sun, Y.; Cai, B.; Gan, S.; Han, D.; Niu, L.; Wu, T. Hierarchically Z-scheme photocatalyst of Ag@AgCl decorated on BiVO4 (040) with enhancing photoelectrochemical and photocatalytic performance. Appl. Catal. B Environ. 2015, 170–171, 206–214. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based heterostructured photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Wei, R.; Tang, N.; Jiang, L.; Yang, J.; Guo, J.; Yuan, X.; Liang, J.; Zhu, Y.; Wu, Z.; Li, H. Bimetallic nanoparticles meet polymeric carbon nitride: Fabrications, catalytic applications and perspectives. Coord. Chem. Rev. 2022, 462, 214500. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Matsumura, M. Crystal faces of rutile and anatase TiO2 particles and their roles in photocatalytic reactions. New J. Chem. 2002, 26, 1167–1170. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, G.; Liu, T.; Su, Y.; Ren, H.; Zhang, X.; Xia, A.; Lv, L.; Liu, Y. Photocatalytic properties of the g-C3N4/{010} facets BiVO4 interface Z-Scheme photocatalysts induced by BiVO4 surface heterojunction. Appl. Catal. B Environ. 2018, 234, 37–49. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Li, X.; Zeng, G.; Wang, D.; Niu, C.; Zhao, J.; An, H.; Xie, T.; Deng, Y. Hierarchical assembly of graphene-bridged Ag3PO4/Ag/BiVO4 (040) Z-scheme photocatalyst: An efficient, sustainable and heterogeneous catalyst with enhanced visible-light photoactivity towards tetracycline degradation under visible light irradiation. Appl. Catal. B Environ. 2017, 200, 330–342. [Google Scholar] [CrossRef]
- Chen, F.; Wu, C.; Wang, J.; François-Xavier, C.P.; Wintgens, T. Highly efficient Z-scheme structured visible-light photocatalyst constructed by selective doping of Ag@AgBr and Co3O4 separately on {010} and {110} facets of BiVO4: Pre-separation channel and hole-sink effects. Appl. Catal. B Environ. 2019, 250, 31–41. [Google Scholar] [CrossRef]
- Lai, C.; Zhang, M.; Li, B.; Huang, D.; Zeng, G.; Qin, L.; Liu, X.; Yi, H.; Cheng, M.; Li, L.; et al. Fabrication of CuS/BiVO4 (0 4 0) binary heterojunction photocatalysts with enhanced photocatalytic activity for Ciprofloxacin degradation and mechanism insight. Chem. Eng. J. 2019, 358, 891–902. [Google Scholar] [CrossRef]
- Wang, M.; Guo, P.; Zhang, Y.; Liu, T.; Li, S.; Xie, Y.; Wang, Y.; Zhu, T. Eu doped g-C3N4 nanosheet coated on flower-like BiVO4 powders with enhanced visible light photocatalytic for tetracycline degradation. Appl. Surf. Sci. 2018, 453, 11–22. [Google Scholar] [CrossRef]
- Li, C.; Che, H.; Liu, C.; Che, G.; Charpentier, P.A.; Xu, W.Z.; Wang, X.; Liu, L. Facile fabrication of g-C3N4 QDs/BiVO4 Z-scheme heterojunction towards enhancing photodegradation activity under visible light. J. Taiwan Inst. Chem. Eng. 2019, 95, 669–681. [Google Scholar] [CrossRef]
- Li, Z.; Jin, C.; Wang, M.; Kang, J.; Wu, Z.; Yang, D.; Zhu, T. Novel rugby-like g-C3N4/BiVO4 core/shell Z-scheme composites prepared via low-temperature hydrothermal method for enhanced photocatalytic performance. Sep. Purif. Technol. 2020, 232, 115937. [Google Scholar] [CrossRef]
- Le Minh Tri, N.; Cam, N.T.D.; Pham, H.D.; Van Thuan, D.; Pham, T.D.; Nguyen, V.T.; Trung, N.T.; Tung, M.H.T.; Phuong, T.T.T.; Nguyen, T.T.P.; et al. Development of g-C3N4/BiVO4 binary component heterojunction as an advanced visible light-responded photocatalyst for polluted antibiotics degradation. Top. Catal. 2020, 63, 1206–1214. [Google Scholar] [CrossRef]
- Martinez, J.L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 2009, 157, 2893–2902. [Google Scholar] [CrossRef]
- LaPara, T.M.; Madson, M.; Borchardt, S.; Lang, K.S.; Johnson, T.J. Multiple discharges of treated municipal wastewater have a small effect on the quantities of numerous antibiotic resistance determinants in the Upper Mississippi river. Environ. Sci. Technol. 2015, 49, 11509–11515. [Google Scholar] [CrossRef]
- Carlesi Jara, C.; Fino, D.; Specchia, V.; Saracco, G.; Spinelli, P. Electrochemical removal of antibiotics from wastewaters. Appl. Catal. B Environ. 2007, 70, 479–487. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Lu, Z.; Wang, G.; Li, P.; Gao, Y.; Huang, X.; Huang, W.; Uji, H.; Lu, G. Synthesis of 42-faceted bismuth vanadate microcrystals for enhanced photocatalytic activity. J. Colloid Interface Sci. 2019, 542, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Praus, P.; Lang, J.; Martaus, A.; Svoboda, L.; Matějka, V.; Kormunda, M.; Šihor, M.; Reli, M.; Kočí, K. Composites of BiVO4 and g-C3N4: Synthesis, properties and photocatalytic decomposition of azo dye AO7 and nitrous oxide. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1219–1234. [Google Scholar] [CrossRef]
- Yu, Y.M.; Liu, K.; Zhang, Y.Y.; Xing, X.; Li, H. Synthesis of g-C3N4/BiVO4 heterojunction composites for photocatalytic degradation of nonylphenol ethoxylate. Sep. Purif. Technol. 2020, 250, 8. [Google Scholar]
- Zhu, J.Y.; Zhu, Y.Y.; Chen, Z.; Wu, S.J.; Fang, X.J.; Yao, Y. Efficient photoelectrochemical performance of gamma irradiated g-C3N4 and its g-C3N4@BiVO4 heterojunction for Solar water splitting. J. Phys. Chem. C 2019, 123, 9013–9026. [Google Scholar]
- Xiao, F.; Xu, J.; Cao, L.; Jiang, S.; Zhang, Q.; Wang, L. In situ hydrothermal fabrication of visible light-driven g-C3N4/SrTiO3 composite for photocatalytic degradation of TC. Environ. Sci. Pollut. Res. Int. 2020, 27, 5788–5796. [Google Scholar] [CrossRef]
- Xu, X.; Zou, Q.; Yuan, Y.; Ji, F.; Fan, Z.; Zhou, B. Preparation of BiVO4-graphene nanocomposites and their photocatalytic activity. J. Nanomater. 2014, 2014, 77. [Google Scholar] [CrossRef]
- Qiao, F.; Wang, J.; Ai, S.; Li, L. As a new peroxidase mimetics: The synthesis of selenium doped graphitic carbon nitride nanosheets and applications on colorimetric detection of H2O2 and xanthine. Sens. Actuators B Chem. 2015, 216, 418–427. [Google Scholar] [CrossRef]
- Zhao, X.; Fan, Y.; Zhang, W.; Zhang, X.; Han, D.; Niu, L.; Ivaska, A. Nanoengineering Construction of Cu2O Nanowire Arrays Encapsulated with g-C3N4 as 3D Spatial Reticulation All-Solid-State Direct Z-Scheme Photocatalysts for Photocatalytic Reduction of Carbon Dioxide. ACS Catal. 2020, 10, 6367–6376. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, X.; Chen, L. BiVO4/BiO0.67F1.66 heterojunction enhanced charge carrier separation to boost photocatalytic activity. J. Nanopart. Res. 2019, 21, 61. [Google Scholar] [CrossRef]
- Wang, G.L.; Zhang, W.Q.; Li, J.Y.; Dong, X.L.; Zhang, X.F. Carbon quantum dots decorated BiVO4 quantum tube with enhanced photocatalytic performance for efficient degradation of organic pollutants under visible and near-infrared light. J. Mater. Sci. 2019, 54, 6488–6499. [Google Scholar] [CrossRef]
- Kuang, P.Y.; Su, Y.Z.; Chen, G.F.; Luo, Z.S.; Xing, Y.; Li, N.; Liu, Z.Q. g-C3N4 decorated ZnO nanorod arrays for enhanced photoelectrocatalytic performance. Appl. Surf. Sci. 2015, 358, 296–303. [Google Scholar] [CrossRef]
- Xu, J.J.; Feng, B.B.; Wang, Y.; Qi, Y.D.; Niu, J.F.; Chen, M.D. BiOCl decorated NaNbO3 nanocubes: A novel p-n heterojunction photocatalyst with Improved activity for ofloxacin degradation. Front. Chem. 2015, 6, 393. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Svenum, I.H.; Ronning, M.; Yang, J. Core-Shell Nanostructures of graphene-wrapped CdS nanoparticles and TiO2 (CdS@G@TiO2): The role of graphene in enhanced photocatalytic H2 generation. Catalysts 2020, 10, 358. [Google Scholar] [CrossRef]
- Akple, M.S.; Ishigaki, T.; Madhusudan, P. Bio-inspired honeycomb-like graphitic carbon nitride for enhanced visible light photocatalytic CO2 reduction activity. Environ. Sci. Pollut. Res. 2020, 27, 22604–22618. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Jiang, J.; Li, H.; Zhang, L. New insight into daylight photocatalysis of AgBr@Ag: Synergistic effect between semiconductor photocatalysis and plasmonic photocatalysis. Chem. Eur. J. 2012, 18, 6360–6369. [Google Scholar] [CrossRef]
- Li, S.; Xue, B.; Wang, C.; Jiang, W.; Hu, S.; Liu, Y.; Wang, H.; Liu, J. Facile fabrication of flower-like BiOI/BiOCOOH p–n hetero junctions for highly efficient visible-light-driven photocatalytic removal of harmful antibiotics. Nanomaterials 2019, 9, 1571. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, F.; Zhang, H.; Zhang, J.; Han, L. Photocatalytic degradation of 4-chlorophenol over Ag/MFe2O4 (M = Co, Zn, Cu, and Ni) prepared by a modified chemical co-precipitation method: A comparative study. RSC Adv. 2015, 5, 55499–55512. [Google Scholar] [CrossRef]
- Fu, H.; Yang, L.; Hu, D.; Yu, C.; Ling, Y.; Xie, Y.; Li, S.; Zhao, J. Titanium dioxide nano-heterostructure with nanoparticles decorat ing nanowires for high-performance photocatalysis. Int. J. Hydrogen Energy 2018, 43, 10359–10367. [Google Scholar] [CrossRef]
- Nagaraju, P.; Puttaiah, S.H.; Wantala, K.; Shahmoradi, B. Preparation of modified ZnO nanoparticles for photocatalytic degradation of chlorobenzene. Appl. Water Sci. 2020, 10, 137. [Google Scholar] [CrossRef]
- Ganeshbabu, M.; Kannan, N.; Venkatesh, P.S.; Paulraj, G.; Jeganathan, K.; MubarakAli, D. Synthesis and characterization of BiVO4 nanoparticles for environmental applications. RSC Adv. 2020, 10, 18315–18322. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xie, X.; Li, J.; Han, D.; Ma, Y.; Fan, Y.; Han, D.; Niu, L. Type II Heterojunction Formed between {010} or {012} Facets Dominated Bismuth Vanadium Oxide and Carbon Nitride to Enhance the Photocatalytic Degradation of Tetracycline. Int. J. Environ. Res. Public Health 2022, 19, 14770. https://doi.org/10.3390/ijerph192214770
Zhang X, Xie X, Li J, Han D, Ma Y, Fan Y, Han D, Niu L. Type II Heterojunction Formed between {010} or {012} Facets Dominated Bismuth Vanadium Oxide and Carbon Nitride to Enhance the Photocatalytic Degradation of Tetracycline. International Journal of Environmental Research and Public Health. 2022; 19(22):14770. https://doi.org/10.3390/ijerph192214770
Chicago/Turabian StyleZhang, Xiaojing, Xianglun Xie, Jianan Li, Dongfang Han, Yingming Ma, Yingying Fan, Dongxue Han, and Li Niu. 2022. "Type II Heterojunction Formed between {010} or {012} Facets Dominated Bismuth Vanadium Oxide and Carbon Nitride to Enhance the Photocatalytic Degradation of Tetracycline" International Journal of Environmental Research and Public Health 19, no. 22: 14770. https://doi.org/10.3390/ijerph192214770
APA StyleZhang, X., Xie, X., Li, J., Han, D., Ma, Y., Fan, Y., Han, D., & Niu, L. (2022). Type II Heterojunction Formed between {010} or {012} Facets Dominated Bismuth Vanadium Oxide and Carbon Nitride to Enhance the Photocatalytic Degradation of Tetracycline. International Journal of Environmental Research and Public Health, 19(22), 14770. https://doi.org/10.3390/ijerph192214770