Wildfire Smoke Exposure during Pregnancy: A Review of Potential Mechanisms of Placental Toxicity, Impact on Obstetric Outcomes, and Strategies to Reduce Exposure
Abstract
1. Introduction
1.1. The Complexity of Wildfire Smoke Composition
1.2. Wildfire Smoke, PM2.5, and Impact on Perinatal Outcomes
1.2.1. Preterm Birth
1.2.2. Fetal Growth
| Citation | Type of Exposure | Time | Location | Study Design | Study Population | Sample Size (n) | Description of Exposure | Primary Outcome(s) | Secondary Outcome(s) |
|---|---|---|---|---|---|---|---|---|---|
| Abdo (2019) [29] | Wildfire smoke | 2007–2015 | Colorado, USA | Retrospective cohort | Singleton births with estimated gestational age between 30 and 42 weeks | 446,961 | Wildfire smoke PM2.5 and non-smoke PM2.5 linked by maternal residence ZIP code; method combined NOAA’s satellite imagery-based HMS to determine daily smoke plume extent with spatial interpolation of ground-based PM2.5 monitor values from US EPA AQS | PTB, BW | NICU admission, gestational diabetes, gestational hypertension, assisted ventilation at |
| Assibey-Mensah (2020) [39] | Wood smoke and traffic particle pollution | 2009–2013 | New York, USA | Retrospective cohort | Birth certificate data with gestation age estimated between 24 and 42 weeks | 20,596 | PM2.5, black carbon, Delta-C (wood smoke marker) concentrations, temperature, and relative humidity measured hourly, gestational month-specific concentrations of each at maternal residential address estimated using land-use regression model | Preeclampsia, any type (early- and late-onset) | |
| Breton (2011) [38] | Wildfire smoke | 2003–2004 | California, USA | Retrospective cohort | Pregnant women living in southern California | Not reported | Wildfire PM2.5 and ambient PM2.5 assigned exposures from maternal addresses geocoded for week of wildfire, exposure estimates derived in a GIS framework, MODIS satellite imaging used to obtain smoke information | BW | SGA, PTB |
| Heft-Neal (2022) [30] | Wildfire smoke | 2006–2012 | California, USA | Retrospective cohort | Singleton births in California with estimated gestation age between 23 and 41 weeks | 3,002,014 | Wildfire smoke plume extent assembled from NOAA’s satellite imagery-based HMS, high-resolution, temporally and spatially resolved gridded estimates of surface PM2.5 developed using machine learning algorithms to incorporate ground monitor data, chemical transport model predictions, and satellite observations; exposures linked to maternal address ZIP code | PTB | PTB subtype and severity (<28 weeks, <32 weeks) |
| Holstius (2012) [35] | Wildfire smoke | 2001–2005 | California, USA | Time-series | Birth records from the California Automated Vital Statistics System for infants delivered at term with BW between 1–6 kg | 886,034 | Temporally defined smoke exposure from MODIS satellite imagery, sensitivity analysis using maternal residence census tracts closer to monitors with average PM10 of <40 µg/m3 classified as low exposure, >40 µg/m3 classified as high exposure. | BW | |
| Jayachandran (2009) [40] | Wildfire air pollution | 1997 | Sumatra, Indonesia | Ecological | Birth cohorts conceived before or after wildfire events | 3751 | TOMS aerosol index | Fetal loss, infant mortality | |
| McCoy (2016) [37] | Wildfire smoke | 2002–2013 | Colorado, USA | Retrospective cohort | Live births in Colorado with maternal home address within 20 miles of a fire burn and smoke plume | 7398 | Proximity of self-reported maternal residence to wild-fire smoke using fine-scaled spatial dataset of plumes in GIS from satellite images of 28 wildfires in Colorado | BW, GA | |
| O’Donnell (2013) [34] | Wildfire event | 2009 | Victoria, Australia | Retrospective cohort | All births registered in Victoria | 287,688 | Proximity of maternal residence to Black Saturday wildfires | BW, PTB, changes to sex-ratio | |
| O’Donnell (2015) [32] | Wildfire event | 2000–2010 | Canberra, Australia | Retrospective cohort | All births registered in Canberra | 48,408 | Proximity of maternal residence to wildfire | BW, GA | |
| Prass (2012) [36] | Forest fire event | 2001–2006 | Porto Velho, Brazil | Cross- sectional | Singleton live births | 22,012 | Heat spots (all forest fires in Amazon region from 2001–2006) compared to time periods with lowest numbers of heat spots using NOAA satellite images | BW |
1.3. Anatomy of the Human Maternal-Fetal Interface and Particulate Matter Deposition
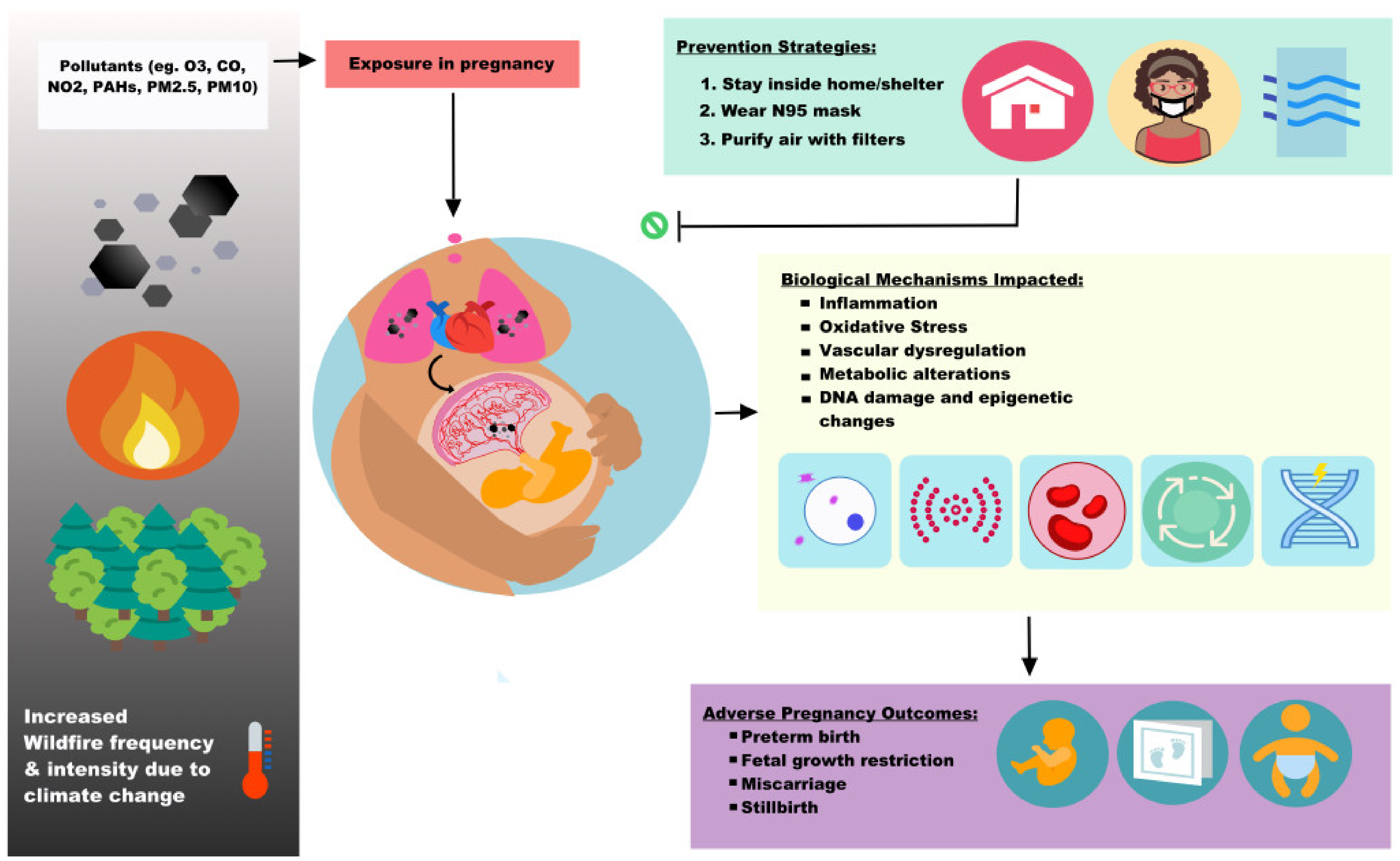
1.4. Biologic Mechanisms of Damage from Wildfire, Air Pollution and Inhaled Toxins
1.4.1. Inflammation
1.4.2. Oxidative Stress
1.4.3. Endocrine Dysfunction
1.4.4. Hemodynamic and Vascular Function
1.4.5. Coagulation
1.4.6. Epigenetic Alterations
1.4.7. Telomere Length
2. Review of the Current Human, Animal and Placental Studies
2.1. Exposure Reduction Strategies and Implications for Research
2.1.1. Behavioral Modifications, Air Filters, and Masks
2.1.2. Personal Air Quality Monitors
2.1.3. Implications for Research
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CO | carbon monoxide |
| CTB | cytotrophoblasts |
| HEPA | high efficiency particulate air |
| NO2 | nitrogen dioxide |
| OS | oxidative stress |
| O3 | ozone |
| PM | particulate matter |
| PM2.5 | particulate matter < 2.5 µm |
| PM10 | particulate matter < 10 µm |
| PAH | polycyclic aromatic hydrocarbon |
| PTB | preterm birth |
| ROS | reactive oxygen species |
| STB | syncytiotrophoblasts |
| US | United States (US) |
| WS | wildfire smoke |
References
- 2021 Fire Season. Available online: https://www.fire.ca.gov/incidents/2021/ (accessed on 28 July 2022).
- Burke, M.; Driscoll, A.; Heft-Neal, S.; Xue, J.; Burney, J.; Wara, M. The Changing Risk and Burden of Wildfire in the United States. Proc. Natl. Acad. Sci. USA 2021, 118, e2011048118. [Google Scholar] [CrossRef] [PubMed]
- Safety during a Wildfire|Wildfires. Available online: https://www.cdc.gov/disasters/wildfires/duringfire.html (accessed on 31 July 2022).
- Confalonieri, U.; Menne, B.; Akhtar, R.; Ebi, K.L.; Hauengue, M.; Kovats, R.S.; Revich, B.; Woodward, A. Human Health. In Climate Change 2007: Impacts, Adaptation and Vulnerability; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Langmann, B.; Duncan, B.; Textor, C.; Trentmann, J.; van der Werf, G.R. Vegetation Fire Emissions and Their Impact on Air Pollution and Climate. Atmos. Environ. 2009, 43, 107–116. [Google Scholar] [CrossRef]
- Wu, J.; M Winer, A.; J Delfino, R. Exposure Assessment of Particulate Matter Air Pollution before, during, and after the 2003 Southern California Wildfires. Atmos. Environ. 2006, 40, 3333–3348. [Google Scholar] [CrossRef]
- Fisk, W.J.; Chan, W.R. Health Benefits and Costs of Filtration Interventions That Reduce Indoor Exposure to PM2.5 during Wildfires. Indoor Air 2017, 27, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Delfino, R.J.; Brummel, S.; Wu, J.; Stern, H.; Ostro, B.; Lipsett, M.; Winer, A.; Street, D.H.; Zhang, L.; Tjoa, T.; et al. The Relationship of Respiratory and Cardiovascular Hospital Admissions to the Southern California Wildfires of 2003. Occup. Environ. Med. 2009, 66, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Amjad, S.; Chojecki, D.; Osornio-Vargas, A.; Ospina, M.B. Wildfire Exposure during Pregnancy and the Risk of Adverse Birth Outcomes: A Systematic Review. Environ. Int. 2021, 156, 106644. [Google Scholar] [CrossRef]
- Lamichhane, D.K.; Leem, J.-H.; Lee, J.-Y.; Kim, H.-C. A Meta-Analysis of Exposure to Particulate Matter and Adverse Birth Outcomes. Environ. Health Toxicol. 2015, 30, e2015011. [Google Scholar] [CrossRef]
- Carré, J.; Gatimel, N.; Moreau, J.; Parinaud, J.; Léandri, R. Does Air Pollution Play a Role in Infertility?: A Systematic Review. Environ. Health 2017, 16, 82. [Google Scholar] [CrossRef]
- Wegesser, T.C.; Pinkerton, K.E.; Last, J.A. California Wildfires of 2008: Coarse and Fine Particulate Matter Toxicity. Environ. Health Perspect. 2009, 117, 893–897. [Google Scholar] [CrossRef]
- Huxley-Reicher, B.; Folger, M.; Casale, M. Trouble in the Air: Millions of Americans Breathed Polluted Air in 2020. Environment Washington Research & Policy Center and WashPRIG Foundation. Available online: https://publicinterestnetwork.org/wp-content/uploads/2021/10/WA-Trouble-in-the-Air-Web.pdf (accessed on 1 October 2022).
- Liu, J.C.; Pereira, G.; Uhl, S.A.; Bravo, M.A.; Bell, M.L. A Systematic Review of the Physical Health Impacts from Non-Occupational Exposure to Wildfire Smoke. Environ. Res. 2015, 136, 120–132. [Google Scholar] [CrossRef]
- Liu, J.C.; Mickley, L.J.; Sulprizio, M.P.; Dominici, F.; Yue, X.; Ebisu, K.; Anderson, G.B.; Khan, R.F.A.; Bravo, M.A.; Bell, M.L. Particulate Air Pollution from Wildfires in the Western US under Climate Change. Clim. Change 2016, 138, 655–666. [Google Scholar] [CrossRef]
- Bell, M.L.; Dominici, F.; Ebisu, K.; Zeger, S.L.; Samet, J.M. Spatial and Temporal Variation in PM2.5 Chemical Composition in the United States for Health Effects Studies. Environ. Health Perspect. 2007, 115, 989–995. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Exposure and Health Effects of Mixtures of Air Pollutants. Available online: https://19january2017snapshot.epa.gov/air-research/exposure-and-health-effects-mixtures-air-pollutants (accessed on 12 March 2022).
- United States Environmental Protection Agency. America’s Children and the Environment. Health | Neurodevelopmental Disorders. Available online: https://www.epa.gov/sites/default/files/2019-07/documents/ace3-neurodevelopmental-updates_0.pdf (accessed on 12 March 2022).
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Samet, J.M.; Dominici, F. Hospital Admissions and Chemical Composition of Fine Particle Air Pollution. Am. J. Respir. Crit. Care Med. 2009, 179, 1115–1120. [Google Scholar] [CrossRef]
- Liu, J.C.; Peng, R.D. The Impact of Wildfire Smoke on Compositions of Fine Particulate Matter by Ecoregion in the Western US. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 765–776. [Google Scholar] [CrossRef]
- Voulgarakis, A.; Field, R.D. Fire Influences on Atmospheric Composition, Air Quality and Climate. Curr. Pollut. Rep. 2015, 1, 70–81. [Google Scholar] [CrossRef]
- California Air Resources Board. New Analysis Shows Spikes of Metal Contaminants, Including Lead, in 2018 Camp Fire Wildfire Smoke|California Air Resources Board. Available online: https://ww2.arb.ca.gov/news/new-analysis-shows-spikes-metal-contaminants-including-lead-2018-camp-fire-wildfire-smoke (accessed on 12 March 2022).
- California Department of Forestry and Fire Protection. Top 20 Deadliest California Wildfires. Available online: https://www.fire.ca.gov/media/lbfd0m2f/top20_deadliest.pdf (accessed on 29 August 2022).
- Nriagu, J.O. Global Inventory of Natural and Anthropogenic Emissions of Trace Metals to the Atmosphere. Nature 1979, 279, 409–411. [Google Scholar] [CrossRef]
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; Ghissassi, F.E.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. The Carcinogenicity of Outdoor Air Pollution. Lancet Oncol. 2013, 14, 1262–1263. [Google Scholar] [CrossRef]
- Black, C.; Gerriets, J.E.; Fontaine, J.H.; Harper, R.W.; Kenyon, N.J.; Tablin, F.; Schelegle, E.S.; Miller, L.A. Early Life Wildfire Smoke Exposure Is Associated with Immune Dysregulation and Lung Function Decrements in Adolescence. Am. J. Respir. Cell Mol. Biol. 2017, 56, 657–666. [Google Scholar] [CrossRef]
- Makkonen, U.; Hellén, H.; Anttila, P.; Ferm, M. Size Distribution and Chemical Composition of Airborne Particles in South-Eastern Finland during Different Seasons and Wildfire Episodes in 2006. Sci. Total Environ. 2010, 408, 644–651. [Google Scholar] [CrossRef]
- Ward, R. Neonatal Complications Following Preterm Birth. BJOG Int. J. Obstet. Gynaecol. 2003, 110, 8–16. [Google Scholar] [CrossRef]
- Abdo, M.; Ward, I.; O’Dell, K.; Ford, B.; Pierce, J.; Fischer, E.; Crooks, J. Impact of Wildfire Smoke on Adverse Pregnancy Outcomes in Colorado, 2007–2015. Ijerph 2019, 16, 3720. [Google Scholar] [CrossRef] [PubMed]
- Heft-Neal, S.; Driscoll, A.; Yang, W.; Shaw, G.; Burke, M. Associations between Wildfire Smoke Exposure during Pregnancy and Risk of Preterm Birth in California. Environ. Res. 2022, 203, 111872. [Google Scholar] [CrossRef] [PubMed]
- Belbasis, L.; Savvidou, M.D.; Kanu, C.; Evangelou, E.; Tzoulaki, I. Birth Weight in Relation to Health and Disease in Later Life: An Umbrella Review of Systematic Reviews and Meta-Analyses. BMC Med. 2016, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.H.; Behie, A.M. Effects of Wildfire Disaster Exposure on Male Birth Weight in an Australian Population. EMPH 2015, 2015, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, Å.; Laivuori, H.; Loft, A.; Oldereid, N.B.; Pinborg, A.; Petzold, M.; Romundstad, L.B.; Söderström-Anttila, V.; Bergh, C. The Association Between High Birth Weight and Long-Term Outcomes—Implications for Assisted Reproductive Technologies: A Systematic Review and Meta-Analysis. Front. Pediatr. 2021, 9, 675775. [Google Scholar] [CrossRef]
- O’Donnell, M.H.; Behie, A.M. Effects of Bushfire Stress on Birth Outcomes: A Cohort Study of the 2009 Victorian Black Saturday Bushfires. Int. J. Disaster Risk Reduct. 2013, 5, 98–106. [Google Scholar] [CrossRef]
- Holstius, D.M.; Reid, C.E.; Jesdale, B.M.; Morello-Frosch, R. Birth Weight Following Pregnancy during the 2003 Southern California Wildfires. Environ. Health Perspect. 2012, 120, 1340–1345. [Google Scholar] [CrossRef]
- Prass, T.S.; Lopes, S.R.C.; Dórea, J.G.; Marques, R.C.; Brandão, K.G. Amazon Forest Fires Between 2001 and 2006 and Birth Weight in Porto Velho. Bull. Environ. Contam. Toxicol. 2012, 89, 1–7. [Google Scholar] [CrossRef]
- Mccoy, S.J.; Zhao, X. Wildfire and Infant Health: A Geospatial Approach to Estimating the Health Impacts of Wildfire Smoke Exposure. Appl. Econ. Lett. 2021, 28, 32–37. [Google Scholar] [CrossRef]
- Breton, C.; Park, C.; Wu, J. Effect of Prenatal Exposure to Wildfire-Generated PM2.5 on Birth Weight. Epidemiology 2011, 22, S66. [Google Scholar] [CrossRef]
- Assibey-Mensah, V.; Glantz, J.C.; Hopke, P.K.; Jusko, T.A.; Thevenet-Morrison, K.; Chalupa, D.; Rich, D.Q. Wintertime Wood Smoke, Traffic Particle Pollution, and Preeclampsia. Hypertension 2020, 75, 851–858. [Google Scholar] [CrossRef]
- Jayachandran, S. Air Quality and Early-Life Mortality Evidence from Indonesia’s Wildfires. J. Hum. Resour. 2009, 44, 916–954. [Google Scholar] [CrossRef]
- Magzamen, S.; Gan, R.W.; Liu, J.; O’Dell, K.; Ford, B.; Berg, K.; Bol, K.; Wilson, A.; Fischer, E.V.; Pierce, J.R. Differential Cardiopulmonary Health Impacts of Local and Long-Range Transport of Wildfire Smoke. GeoHealth 2021, 5, e2020GH000330. [Google Scholar] [CrossRef]
- Gundacker, C.; Ellinger, I. The Unique Applicability of the Human Placenta to the Adverse Outcome Pathway (AOP) Concept: The Placenta Provides Fundamental Insights into Human Organ Functions at Multiple Levels of Biological Organization. Reprod. Toxicol. 2020, 96, 273–281. [Google Scholar] [CrossRef]
- Tang, Z.; Tadesse, S.; Norwitz, E.; Mor, G.; Abrahams, V.M.; Guller, S. Isolation of Hofbauer Cells from Human Term Placentas with High Yield and Purity. Am. J. Reprod. Immunol. 2011, 66, 336–348. [Google Scholar] [CrossRef]
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and Function of the Normal Human Placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef]
- Fisher, S.J. Why Is Placentation Abnormal in Preeclampsia? Am. J. Obstet. Gynecol. 2015, 213, S115–S122. [Google Scholar] [CrossRef]
- Jauniaux, E.; Jurkovic, D. Placenta Accreta: Pathogenesis of a 20th Century Iatrogenic Uterine Disease. Placenta 2012, 33, 244–251. [Google Scholar] [CrossRef]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm Labor: One Syndrome, Many Causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef]
- Maltepe, E.; Fisher, S.J. Placenta: The Forgotten Organ. Annu. Rev. Cell Dev. Biol. 2015, 31, 523–552. [Google Scholar] [CrossRef]
- Bové, H.; Bongaerts, E.; Slenders, E.; Bijnens, E.M.; Saenen, N.D.; Gyselaers, W.; Van Eyken, P.; Plusquin, M.; Roeffaers, M.B.J.; Ameloot, M.; et al. Ambient Black Carbon Particles Reach the Fetal Side of Human Placenta. Nat. Commun. 2019, 10, 3866. [Google Scholar] [CrossRef]
- Bongaerts, E.; Bové, H.; Lambrichts, I.; Saenen, N.D.; Gyselaers, W.; Plusquin, M.; Roeffaers, M.B.J.; Ameloot, M.; Nawrot, T.S. Reply to ‘Fetal Side’ of the Placenta: Anatomical Mis-Annotation of Carbon Particle ‘Transfer’ across the Human Placenta. Nat. Commun. 2021, 12, 7050. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, L.; Lindgren, R.; Nääv, Å.; Krais, A.M.; Strandberg, B.; Lundh, T.; Boman, C.; Isaxon, C.; Hansson, S.R.; Malmqvist, E. Exposure to Wood Smoke Particles Leads to Inflammation, Disrupted Proliferation and Damage to Cellular Structures in a Human First Trimester Trophoblast Cell Line. Environ. Pollut. 2020, 264, 114790. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, C.A.; Raaschou-Nielsen, O.; Loft, S.; Møller, P.; Vermeulen, R.; Palli, D.; Chadeau-Hyam, M.; Xun, W.W.; Vineis, P. Biomarkers of Ambient Air Pollution and Lung Cancer: A Systematic Review. Occup. Environ. Med. 2012, 69, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, N.; Vlaanderen, J.; Chadeau-Hyam, M.; Beelen, R.; Modig, L.; Palli, D.; Bergdahl, I.A.; Vineis, P.; Hoek, G.; Kyrtopoulos, S.A.; et al. Inflammatory Markers in Relation to Long-Term Air Pollution. Environ. Int. 2015, 81, 1–7. [Google Scholar] [CrossRef]
- Pettit, A.P.; Brooks, A.; Laumbach, R.; Fiedler, N.; Wang, Q.; Strickland, P.O.; Madura, K.; Zhang, J.; Kipen, H.M. Alteration of Peripheral Blood Monocyte Gene Expression in Humans Following Diesel Exhaust Inhalation. Inhal. Toxicol. 2012, 24, 172–181. [Google Scholar] [CrossRef]
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The Health Effects of Ambient PM2.5 and Potential Mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74. [Google Scholar] [CrossRef]
- Kannan, S.; Misra, D.P.; Dvonch, J.T.; Krishnakumar, A. Exposures to Airborne Particulate Matter and Adverse Perinatal Outcomes: A Biologically Plausible Mechanistic Framework for Exploring Potential Effect Modification by Nutrition. Environ. Health Perspect. 2006, 114, 1636–1642. [Google Scholar] [CrossRef]
- Saenen, N.D.; Plusquin, M.; Bijnens, E.; Janssen, B.G.; Gyselaers, W.; Cox, B.; Fierens, F.; Molenberghs, G.; Penders, J.; Vrijens, K.; et al. In Utero Fine Particle Air Pollution and Placental Expression of Genes in the Brain-Derived Neurotrophic Factor Signaling Pathway: An Environage Birth Cohort Study. Environ. Health Perspect. 2015, 123, 834–840. [Google Scholar] [CrossRef]
- Kemp, M.W. Preterm Birth, Intrauterine Infection, and Fetal Inflammation. Front. Immunol. 2014, 5, 574. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and Causes of Preterm Birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Cappelletti, M.; Della Bella, S.; Ferrazzi, E.; Mavilio, D.; Divanovic, S. Inflammation and Preterm Birth. J. Leukoc. Biol. 2016, 99, 67–78. [Google Scholar] [CrossRef]
- Palm, M.; Axelsson, O.; Wernroth, L.; Larsson, A.; Basu, S. Involvement of Inflammation in Normal Pregnancy. Acta Obs. Gynecol. Scand. 2013, 92, 601–605. [Google Scholar] [CrossRef]
- Wei, S.-Q.; Fraser, W.; Luo, Z.-C. Inflammatory Cytokines and Spontaneous Preterm Birth in Asymptomatic Women: A Systematic Review. Obstet. Gynecol. 2010, 116, 393–401. [Google Scholar] [CrossRef]
- Ferguson, K.K.; McElrath, T.F.; Chen, Y.-H.; Mukherjee, B.; Meeker, J.D. Longitudinal Profiling of Inflammatory Cytokines and C-Reactive Protein during Uncomplicated and Preterm Pregnancy. Am. J. Reprod. Immunol. 2014, 72, 326–336. [Google Scholar] [CrossRef]
- Holmes, V.A.; Wallace, J.M.W.; Gilmore, W.S.; McFaul, P.; Alexander, H.D. Plasma Levels of the Immunomodulatory Cytokine Interleukin-10 during Normal Human Pregnancy: A Longitudinal Study. Cytokine 2003, 21, 265–269. [Google Scholar] [CrossRef]
- Wu, C.-M.; Adetona, A.; Song, C.; Naeher, L.; Adetona, O. Measuring Acute Pulmonary Responses to Occupational Wildland Fire Smoke Exposure Using Exhaled Breath Condensate. Arch. Environ. Occup. Health 2020, 75, 65–69. [Google Scholar] [CrossRef]
- Adetona, A.M.; Adetona, O.; Gogal, R.M.; Diaz-Sanchez, D.; Rathbun, S.L.; Naeher, L.P. Impact of Work Task-Related Acute Occupational Smoke Exposures on Select Proinflammatory Immune Parameters in Wildland Firefighters. J. Occup. Environ. Med. 2017, 59, 679–690. [Google Scholar] [CrossRef]
- Swiston, J.R.; Davidson, W.; Attridge, S.; Li, G.T.; Brauer, M.; van Eeden, S.F. Wood Smoke Exposure Induces a Pulmonary and Systemic Inflammatory Response in Firefighters. Eur. Respir. J. 2008, 32, 129–138. [Google Scholar] [CrossRef]
- Basilio, E.; Gaw, S.L.; Padula, A.; Buarpung, S.; Robinson, J.F. Association between Fetal Hofbauer Cells and Air Quality Index in Pregnancies Exposed to Wildfire Smoke. Am. J. Obstet. Gynecol. 2022, 226, S27–S28. [Google Scholar] [CrossRef]
- Buxton, M.A.; Meraz-Cruz, N.; Sanchez, B.N.; Gronlund, C.J.; Foxman, B.; Vadillo-Ortega, F.; O’Neill, M.S. Air Pollution and Inflammation: Findings from Concurrent Repeated Measures of Systemic and Reproductive Tract Cytokines during Term Pregnancy in Mexico City. Sci. Total Environ. 2019, 681, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Dailey, L.A.; Soukup, J.M.; Grambow, S.C.; Devlin, R.B.; Huang, Y.-C.T. Seasonal Variations in Air Pollution Particle-Induced Inflammatory Mediator Release and Oxidative Stress. Environ. Health Perspect. 2005, 113, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Diaz-Sanchez, D.; Ng, D.; Hiura, T.; Saxon, A. Enhancement of Allergic Inflammation by the Interaction between Diesel Exhaust Particles and the Immune System. J. Allergy Clin. Immunol. 1998, 102, 539–554. [Google Scholar] [CrossRef]
- Detmar, J.; Jurisicova, A. Embryonic Resorption and Polycyclic Aromatic Hydrocarbons: Putative Immune-Mediated Mechanisms. Syst. Biol. Reprod. Med. 2010, 56, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Hejl, A.M.; Adetona, O.; Diaz-Sanchez, D.; Carter, J.D.; Commodore, A.A.; Rathbun, S.L.; Naeher, L.P. Inflammatory Effects of Woodsmoke Exposure Among Wildland Firefighters Working at Prescribed Burns at the Savannah River Site, SC. J. Occup. Environ. Hyg. 2013, 10, 173–180. [Google Scholar] [CrossRef]
- Capitanio, J.P.; Del Rosso, L.A.; Gee, N.; Lasley, B.L. Adverse Biobehavioral Effects in Infants Resulting from Pregnant Rhesus Macaques’ Exposure to Wildfire Smoke. Nat. Commun. 2022, 13, 1774. [Google Scholar] [CrossRef]
- Willson, B.E.; Gee, N.A.; Willits, N.H.; Li, L.; Zhang, Q.; Pinkerton, K.E.; Lasley, B.L. Effects of the 2018 Camp Fire on Birth Outcomes in Non-Human Primates: Case-Control Study. Reprod. Toxicol. 2021, 105, 128–135. [Google Scholar] [CrossRef]
- Brown, A.P.; Cai, L.; Laufer, B.I.; Miller, L.A.; LaSalle, J.M.; Ji, H. Long-Term Effects of Wildfire Smoke Exposure during Early Life on the Nasal Epigenome in Rhesus Macaques. Environ. Int. 2022, 158, 106993. [Google Scholar] [CrossRef]
- Williams, K.M.; Franzi, L.M.; Last, J.A. Cell-Specific Oxidative Stress and Cytotoxicity after Wildfire Coarse Particulate Matter Instillation into Mouse Lung. Toxicol. Appl. Pharm. 2013, 266, 48–55. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The Effects of Oxidative Stress on Female Reproduction: A Review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Adetona, O.; Zhang, J.; Hall, D.B.; Wang, J.-S.; Vena, J.E.; Naeher, L.P. Occupational Exposure to Woodsmoke and Oxidative Stress in Wildland Firefighters. Sci. Total Environ. 2013, 449, 269–275. [Google Scholar] [CrossRef]
- Risom, L.; Møller, P.; Loft, S. Oxidative Stress-Induced DNA Damage by Particulate Air Pollution. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 592, 119–137. [Google Scholar] [CrossRef]
- Sørensen, M.; Autrup, H.; Hertel, O.; Wallin, H.; Knudsen, L.E.; Loft, S. Personal Exposure to PM2.5 and Biomarkers of DNA Damage. Cancer Epidemiol. Biomark. Prev. 2003, 12, 191–196. [Google Scholar]
- Topinka, J.; Binková, B.; Mracková, G.; Stávková, Z.; Benes, I.; Dejmek, J.; Lenícek, J.; Srám, R.J. DNA Adducts in Human Placenta as Related to Air Pollution and to GSTM1 Genotype. Mutat. Res. 1997, 390, 59–68. [Google Scholar] [CrossRef]
- Perera, F.P.; Whyatt, R.M.; Jedrychowski, W.; Rauh, V.; Manchester, D.; Santella, R.M.; Ottman, R. Recent Developments in Molecular Epidemiology: A Study of the Effects of Environmental Polycyclic Aromatic Hydrocarbons on Birth Outcomes in Poland. Am. J. Epidemiol. 1998, 147, 309–314. [Google Scholar] [CrossRef]
- Perera, F.P.; Jedrychowski, W.; Rauh, V.; Whyatt, R.M. Molecular Epidemiologic Research on the Effects of Environmental Pollutants on the Fetus. Environ. Health Perspect. 1999, 107, 451–460. [Google Scholar] [CrossRef]
- Massion, P.P.; Sequist, L.V.; Pao, W. 51—Biology of Lung Cancer. In Murray and Nadel’s Textbook of Respiratory Medicine, 6th ed.; Broaddus, V.C., Mason, R.J., Ernst, J.D., King, T.E., Lazarus, S.C., Murray, J.F., Nadel, J.A., Slutsky, A.S., Gotway, M.B., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2016; pp. 912–926.e6. [Google Scholar] [CrossRef]
- Ferguson, K.K.; McElrath, T.F.; Pace, G.G.; Weller, D.; Zeng, L.; Pennathur, S.; Cantonwine, D.E.; Meeker, J.D. Urinary Polycyclic Aromatic Hydrocarbon Metabolite Associations with Biomarkers of Inflammation, Angiogenesis, and Oxidative Stress in Pregnant Women. Environ. Sci. Technol. 2017, 51, 4652–4660. [Google Scholar] [CrossRef]
- Hartwig, A.; Asmuss, M.; Ehleben, I.; Herzer, U.; Kostelac, D.; Pelzer, A.; Schwerdtle, T.; Bürkle, A. Interference by Toxic Metal Ions with DNA Repair Processes and Cell Cycle Control: Molecular Mechanisms. Environ. Health Perspect. 2002, 110, 797–799. [Google Scholar] [CrossRef]
- Arita, Y.; Kirk, M.; Gupta, N.; Antony, R.; Park, H.-J.; Stecker, M.M.; Peltier, M.R. Effect of 2,6-Xylidine (DMA) on Secretion of Biomarkers for Inflammation and Neurodevelopment by the Placenta. J. Reprod. Immunol. 2022, 149, 103458. [Google Scholar] [CrossRef]
- Iodice, S.; Hoxha, M.; Ferrari, L.; Carbone, I.F.; Anceschi, C.; Miragoli, M.; Pesatori, A.C.; Persico, N.; Bollati, V. Particulate Air Pollution, Blood Mitochondrial DNA Copy Number, and Telomere Length in Mothers in the First Trimester of Pregnancy: Effects on Fetal Growth. Oxidative Med. Cell. Longev. 2018, 2018, e5162905. [Google Scholar] [CrossRef] [PubMed]
- Saenen, N.D.; Vrijens, K.; Janssen, B.G.; Madhloum, N.; Peusens, M.; Gyselaers, W.; Vanpoucke, C.; Lefebvre, W.; Roels, H.A.; Nawrot, T.S. Placental Nitrosative Stress and Exposure to Ambient Air Pollution During Gestation: A Population Study. Am. J. Epidemiol. 2016, 184, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Bandookwala, M.; Sengupta, P. 3-Nitrotyrosine: A Versatile Oxidative Stress Biomarker for Major Neurodegenerative Diseases. Int. J. Neurosci. 2020, 130, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-I.; Kim, H.-B.; Kim, H.-C.; Lee, S.-Y.; Kang, M.-J.; Cho, H.-J.; Yoon, J.; Jung, S.; Lee, E.; Yang, H.-J.; et al. Particulate Matter at Third Trimester and Respiratory Infection in Infants, Modified by GSTM1. Pediatr. Pulmonol. 2020, 55, 245–253. [Google Scholar] [CrossRef]
- Luyten, L.J.; Saenen, N.D.; Janssen, B.G.; Vrijens, K.; Plusquin, M.; Roels, H.A.; Debacq-Chainiaux, F.; Nawrot, T.S. Air Pollution and the Fetal Origin of Disease: A Systematic Review of the Molecular Signatures of Air Pollution Exposure in Human Placenta. Environ. Res. 2018, 166, 310–323. [Google Scholar] [CrossRef]
- Kimm, S.Y.S. Fetal Origins of Adult Disease: The Barker Hypothesis Revisited—2004. Curr. Opin. Endocrinol. Diabetes Obes. 2004, 11, 192–196. [Google Scholar] [CrossRef]
- Wang, T.; Han, Y.; Li, H.; Wang, Y.; Xue, T.; Chen, X.; Chen, W.; Fan, Y.; Qiu, X.; Gong, J.; et al. Changes in Bioactive Lipid Mediators in Response to Short-Term Exposure to Ambient Air Particulate Matter: A Targeted Lipidomic Analysis of Oxylipin Signaling Pathways. Environ. Int. 2021, 147, 106314. [Google Scholar] [CrossRef]
- Martens, D.S.; Gouveia, S.; Madhloum, N.; Janssen, B.G.; Plusquin, M.; Vanpoucke, C.; Lefebvre, W.; Forsberg, B.; Nording, M.; Nawrot, T.S. Neonatal Cord Blood Oxylipins and Exposure to Particulate Matter in the Early-Life Environment: An Environage Birth Cohort Study. Environ. Health Perspect. 2017, 125, 691–698. [Google Scholar] [CrossRef]
- Yin, F.; Lawal, A.; Ricks, J.; Fox, J.R.; Larson, T.; Navab, M.; Fogelman, A.M.; Rosenfeld, M.E.; Araujo, J.A. Diesel Exhaust Induces Systemic Lipid Peroxidation and Development of Dysfunctional Pro-Oxidant and pro-Inflammatory High-Density Lipoprotein. Arter. Thromb. Vasc. Biol. 2013, 33, 1153–1161. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, X.; Geng, X.; Yue, H.; Li, G.; Sang, N. Maternal PM2.5 Exposure and Abnormal Placental Nutrient Transport. Ecotoxicol. Environ. Saf. 2021, 207, 111281. [Google Scholar] [CrossRef]
- Janssen, B.G.; Saenen, N.D.; Roels, H.A.; Madhloum, N.; Gyselaers, W.; Lefebvre, W.; Penders, J.; Vanpoucke, C.; Vrijens, K.; Nawrot, T.S. Fetal Thyroid Function, Birth Weight, and in Utero Exposure to Fine Particle Air Pollution: A Birth Cohort Study. Environ. Health Perspect. 2017, 125, 699–705. [Google Scholar] [CrossRef]
- Cathey, A.L.; Watkins, D.J.; Rosario, Z.Y.; Vélez Vega, C.M.; Loch-Caruso, R.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Polycyclic Aromatic Hydrocarbon Exposure Results in Altered CRH, Reproductive, and Thyroid Hormone Concentrations during Human Pregnancy. Sci. Total Environ. 2020, 749, 141581. [Google Scholar] [CrossRef]
- Wang, S.L.; Lucier, G.W.; Everson, R.B.; Sunahara, G.I.; Shiverick, K.T. Smoking-Related Alterations in Epidermal Growth Factor and Insulin Receptors in Human Placenta. Mol. Pharm. 1988, 34, 265–271. [Google Scholar]
- Guyda, H.J. Metabolic Effects of Growth Factors and Polycyclic Aromatic Hydrocarbons on Cultured Human Placental Cells of Early and Late Gestation. J. Clin. Endocrinol. Metab. 1991, 72, 718–723. [Google Scholar] [CrossRef]
- Dejmek, J.; Solanský, I.; Benes, I.; Lenícek, J.; Srám, R.J. The Impact of Polycyclic Aromatic Hydrocarbons and Fine Particles on Pregnancy Outcome. Environ. Health Perspect. 2000, 108, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Boeldt, D.S.; Bird, I.M. Vascular Adaptation in Pregnancy and Endothelial Dysfunction in Preeclampsia. J. Endocrinol. 2017, 232, R27–R44. [Google Scholar] [CrossRef]
- Brook, R.D.; Brook, J.R.; Urch, B.; Vincent, R.; Rajagopalan, S.; Silverman, F. Inhalation of Fine Particulate Air Pollution and Ozone Causes Acute Arterial Vasoconstriction in Healthy Adults. Circulation 2002, 105, 1534–1536. [Google Scholar] [CrossRef]
- Miller, C.N.; Dye, J.A.; Henriquez, A.R.; Stewart, E.J.; Lavrich, K.S.; Carswell, G.K.; Ren, H.; Freeborn, D.L.; Snow, S.J.; Schladweiler, M.C.; et al. Ozone-Induced Fetal Growth Restriction in Rats Is Associated with Sexually Dimorphic Placental and Fetal Metabolic Adaptation. Mol. Metab. 2020, 42, 101094. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial Dysfunction: A Marker of Atherosclerotic Risk. Arter. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Virdis, A.; Schiffrin, E.L. Vascular Inflammation: A Role in Vascular Disease in Hypertension? Curr. Opin. Nephrol. Hypertens. 2003, 12, 181–187. [Google Scholar] [CrossRef]
- Bekkar, B.; Pacheco, S.; Basu, R.; DeNicola, N. Association of Air Pollution and Heat Exposure With Preterm Birth, Low Birth Weight, and Stillbirth in the US: A Systematic Review. JAMA Netw. Open 2020, 3, e208243. [Google Scholar] [CrossRef] [PubMed]
- Valentino, S.A.; Tarrade, A.; Aioun, J.; Mourier, E.; Richard, C.; Dahirel, M.; Rousseau-Ralliard, D.; Fournier, N.; Aubrière, M.-C.; Lallemand, M.-S.; et al. Maternal Exposure to Diluted Diesel Engine Exhaust Alters Placental Function and Induces Intergenerational Effects in Rabbits. Part. Fibre Toxicol. 2015, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, J.A.; Barua, R.S. The Pathophysiology of Cigarette Smoking and Cardiovascular Disease: An Update. J. Am. Coll. Cardiol. 2004, 43, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Goerre, S.; Staehli, C.; Shaw, S.; Lüscher, T.F. Effect of Cigarette Smoking and Nicotine on Plasma Endothelin-1 Levels. J. Cardiovasc. Pharm. 1995, 26, S236–S238. [Google Scholar] [CrossRef]
- Otsuka, R.; Watanabe, H.; Hirata, K.; Tokai, K.; Muro, T.; Yoshiyama, M.; Takeuchi, K.; Yoshikawa, J. Acute Effects of Passive Smoking on the Coronary Circulation in Healthy Young Adults. JAMA 2001, 286, 436–441. [Google Scholar] [CrossRef]
- Bainbridge, S.A.; Belkacemi, L.; Dickinson, M.; Graham, C.H.; Smith, G.N. Carbon Monoxide Inhibits Hypoxia/Reoxygenation-Induced Apoptosis and Secondary Necrosis in Syncytiotrophoblast. Am. J. Pathol. 2006, 169, 774–783. [Google Scholar] [CrossRef][Green Version]
- Greer, I.A.; Aharon, A.; Brenner, B.; Gris, J.-C. Coagulation and Placenta-Mediated Complications. Rambam Maimonides Med. J. 2014, 5, e0034. [Google Scholar] [CrossRef]
- Salafia, C.M.; Pezzullo, J.C.; López-Zeno, J.A.; Simmens, S.; Minior, V.K.; Vintzileos, A.M. Placental Pathologic Features of Preterm Preeclampsia. Am. J. Obs. Gynecol. 1995, 173, 1097–1105. [Google Scholar] [CrossRef]
- Tan, A.W.K.; Li, R.H.L.; Ueda, Y.; Stern, J.A.; Hussain, M.; Haginoya, S.; Sharpe, A.N.; Gunther-Harrington, C.T.; Epstein, S.E.; Nguyen, N. Platelet Priming and Activation in Naturally Occurring Thermal Burn Injuries and Wildfire Smoke Exposure Is Associated With Intracardiac Thrombosis and Spontaneous Echocardiographic Contrast in Feline Survivors. Front. Vet. Sci. 2022, 9, 946. [Google Scholar] [CrossRef]
- Sharpe, A.N.; Gunther-Harrington, C.T.; Epstein, S.E.; Li, R.H.L.; Stern, J.A. Cats with Thermal Burn Injuries from California Wildfires Show Echocardiographic Evidence of Myocardial Thickening and Intracardiac Thrombi. Sci. Rep. 2020, 10, 2648. [Google Scholar] [CrossRef]
- Pekkanen, J.; Brunner, E.J.; Anderson, H.R.; Tiittanen, P.; Atkinson, R.W. Daily Concentrations of Air Pollution and Plasma Fibrinogen in London. Occup. Environ. Med. 2000, 57, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Döring, A.; Wichmann, H.-E.; Koenig, W. Increased Plasma Viscosity during an Air Pollution Episode: A Link to Mortality? Lancet 1997, 349, 1582–1587. [Google Scholar] [CrossRef]
- Prescott, G.J.; Lee, R.J.; Cohen, G.R.; Elton, R.A.; Lee, A.J.; Fowkes, F.G.R.; Agius, R.M. Investigation of Factors Which Might Indicate Susceptibility to Particulate Air Pollution. Occup. Environ. Med. 2000, 57, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Seaton, A.; Soutar, A.; Crawford, V.; Elton, R.; McNerlan, S.; Cherrie, J.; Watt, M.; Agius, R.; Stout, R. Particulate Air Pollution and the Blood. Thorax 1999, 54, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; MacNee, W. Potential Mechanisms of Adverse Pulmonary and Cardiovascular Effects of Particulate Air Pollution (PM10). Int. J. Hyg. Environ. Health 2001, 203, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.H.; Nilsson, T.K.; Johnson, O. Von Willebrand Factor in Plasma: A Novel Risk Factor for Recurrent Myocardial Infarction and Death. Br. Heart J. 1991, 66, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Riediker, M.; Cascio, W.E.; Griggs, T.R.; Herbst, M.C.; Bromberg, P.A.; Neas, L.; Williams, R.W.; Devlin, R.B. Particulate Matter Exposure in Cars Is Associated with Cardiovascular Effects in Healthy Young Men. Am. J. Respir. Crit. Care Med. 2004, 169, 934–940. [Google Scholar] [CrossRef]
- Mercelina-Rournans, P.E.A.M.; Ubachs, J.M.H.; van Wersch, J.W.J. Coagulation and Fibrinolysis in Smoking and Nonsmoking Pregnant Women. BJOG Int. J. Obstet. Gynaecol. 1996, 103, 789–794. [Google Scholar] [CrossRef]
- Wilson, D.W.; Aung, H.H.; Lame, M.W.; Plummer, L.; Pinkerton, K.E.; Ham, W.; Kleeman, M.; Norris, J.W.; Tablin, F. Exposure of Mice to Concentrated Ambient Particulate Matter Results in Platelet and Systemic Cytokine Activation. Inhal. Toxicol. 2010, 22, 267–276. [Google Scholar] [CrossRef]
- Nelissen, E.C.M.; van Montfoort, A.P.A.; Dumoulin, J.C.M.; Evers, J.L.H. Epigenetics and the Placenta. Hum. Reprod. Update 2011, 17, 397–417. [Google Scholar] [CrossRef]
- Zhang, B.; Kim, M.Y.; Elliot, G.; Zhou, Y.; Zhao, G.; Li, D.; Lowdon, R.F.; Gormley, M.; Kapidzic, M.; Robinson, J.F.; et al. Human Placental Cytotrophoblast Epigenome Dynamics over Gestation and Alterations in Placental Disease. Dev. Cell. 2021, 56, 1238–1252.e5. [Google Scholar] [CrossRef]
- Apicella, C.; Ruano, C.S.M.; Méhats, C.; Miralles, F.; Vaiman, D. The Role of Epigenetics in Placental Development and the Etiology of Preeclampsia. Int. J. Mol. Sci. 2019, 20, 2837. [Google Scholar] [CrossRef]
- You, J.S.; Jones, P.A. Cancer Genetics and Epigenetics: Two Sides of the Same Coin? Cancer Cell 2012, 22, 9–20. [Google Scholar] [CrossRef]
- Feinberg, A.P.; Tycko, B. The History of Cancer Epigenetics. Nat. Rev. Cancer 2004, 4, 143–153. [Google Scholar] [CrossRef]
- Prunicki, M.; Miller, S.; Hopkins, A.; Poulin, M.; Movassagh, H.; Yan, L.; Nadeau, K.C. Wildfire Smoke Exposure Is Associated with Decreased Methylation of the PDL2 Gene. J. Immunol. 2020, 204, 146.17. [Google Scholar]
- Prunicki, M.; Stell, L.; Dinakarpandian, D.; de Planell-Saguer, M.; Lucas, R.W.; Hammond, S.K.; Balmes, J.R.; Zhou, X.; Paglino, T.; Sabatti, C.; et al. Exposure to NO2, CO, and PM2.5 Is Linked to Regional DNA Methylation Differences in Asthma. Clin. Epigenet. 2018, 10, 2. [Google Scholar] [CrossRef]
- Bind, M.-A.C.; Coull, B.A.; Peters, A.; Baccarelli, A.A.; Tarantini, L.; Cantone, L.; Vokonas, P.S.; Koutrakis, P.; Schwartz, J.D. Beyond the Mean: Quantile Regression to Explore the Association of Air Pollution with Gene-Specific Methylation in the Normative Aging Study. Environ. Health Perspect. 2015, 123, 759–765. [Google Scholar] [CrossRef]
- Bind, M.-A.; Lepeule, J.; Zanobetti, A.; Gasparrini, A.; Baccarelli, A.A.; Coull, B.A.; Tarantini, L.; Vokonas, P.S.; Koutrakis, P.; Schwartz, J. Air Pollution and Gene-Specific Methylation in the Normative Aging Study: Association, Effect Modification, and Mediation Analysis. Epigenetics 2014, 9, 448–458. [Google Scholar] [CrossRef]
- Lepeule, J.; Bind, M.-A.C.; Baccarelli, A.A.; Koutrakis, P.; Tarantini, L.; Litonjua, A.; Sparrow, D.; Vokonas, P.; Schwartz, J.D. Epigenetic Influences on Associations between Air Pollutants and Lung Function in Elderly Men: The Normative Aging Study. Environ. Health Perspect. 2014, 122, 566–572. [Google Scholar] [CrossRef]
- Somineni, H.K.; Zhang, X.; Biagini Myers, J.M.; Kovacic, M.B.; Ulm, A.; Jurcak, N.; Ryan, P.H.; Khurana Hershey, G.K.; Ji, H. Ten-Eleven Translocation 1 (TET1) Methylation Is Associated with Childhood Asthma and Traffic-Related Air Pollution. J. Allergy Clin. Immunol. 2016, 137, 797–805.e5. [Google Scholar] [CrossRef]
- Liu, X.; Ye, Y.; Chen, Y.; Li, X.; Feng, B.; Cao, G.; Xiao, J.; Zeng, W.; Li, X.; Sun, J.; et al. Effects of Prenatal Exposure to Air Particulate Matter on the Risk of Preterm Birth and Roles of Maternal and Cord Blood LINE-1 Methylation: A Birth Cohort Study in Guangzhou, China. Environ. Int. 2019, 133, 105177. [Google Scholar] [CrossRef] [PubMed]
- Tantoh, D.M.; Lee, K.-J.; Nfor, O.N.; Liaw, Y.-C.; Lin, C.; Chu, H.-W.; Chen, P.-H.; Hsu, S.-Y.; Liu, W.-H.; Ho, C.-C.; et al. Methylation at Cg05575921 of a Smoking-Related Gene (AHRR) in Non-Smoking Taiwanese Adults Residing in Areas with Different PM2.5 Concentrations. Clin. Epigenet. 2019, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Ivorra, C.; Fraga, M.F.; Bayón, G.F.; Fernández, A.F.; Garcia-Vicent, C.; Chaves, F.J.; Redon, J.; Lurbe, E. DNA Methylation Patterns in Newborns Exposed to Tobacco in Utero. J. Transl. Med. 2015, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Colla, S.; Liesa, M.; Moslehi, J.; Müller, F.L.; Guo, M.; Cooper, M.; Kotton, D.; Fabian, A.J.; Walkey, C.; et al. Telomere Dysfunction Induces Metabolic and Mitochondrial Compromise. Nature 2011, 470, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Janssen, B.G.; Gyselaers, W.; Byun, H.-M.; Roels, H.A.; Cuypers, A.; Baccarelli, A.A.; Nawrot, T.S. Placental Mitochondrial DNA and CYP1A1 Gene Methylation as Molecular Signatures for Tobacco Smoke Exposure in Pregnant Women and the Relevance for Birth Weight. J. Transl. Med. 2017, 15, 5. [Google Scholar] [CrossRef]
- Huuskonen, P.; Storvik, M.; Reinisalo, M.; Honkakoski, P.; Rysä, J.; Hakkola, J.; Pasanen, M. Microarray Analysis of the Global Alterations in the Gene Expression in the Placentas From Cigarette-Smoking Mothers. Clin. Pharm. 2008, 83, 542–550. [Google Scholar] [CrossRef]
- Abraham, E.; Rousseaux, S.; Agier, L.; Giorgis-Allemand, L.; Tost, J.; Galineau, J.; Hulin, A.; Siroux, V.; Vaiman, D.; Charles, M.-A.; et al. Pregnancy Exposure to Atmospheric Pollution and Meteorological Conditions and Placental DNA Methylation. Environ. Int. 2018, 118, 334–347. [Google Scholar] [CrossRef]
- Cabalín, C.; Villalobos-Labra, R.; Toledo, F.; Sobrevia, L. Involvement of A2B Adenosine Receptors as Anti-Inflammatory in Gestational Diabesity. Mol. Asp. Med. 2019, 66, 31–39. [Google Scholar] [CrossRef]
- Wojcik, M.; Zieleniak, A.; Mac-Marcjanek, K.; Wozniak, L.A.; Cypryk, K. The Elevated Gene Expression Level of the A2B Adenosine Receptor Is Associated with Hyperglycemia in Women with Gestational Diabetes Mellitus: A2B AR Expression in Diabetic Pregnancy. Diabetes Metab. Res. Rev. 2014, 30, 42–53. [Google Scholar] [CrossRef]
- Entringer, S.; Epel, E.S.; Kumsta, R.; Lin, J.; Hellhammer, D.H.; Blackburn, E.H.; Wüst, S.; Wadhwa, P.D. Stress Exposure in Intrauterine Life Is Associated with Shorter Telomere Length in Young Adulthood. Proc. Natl. Acad. Sci. USA 2011, 108, E513–E518. [Google Scholar] [CrossRef]
- Salihu, H.M.; Pradhan, A.; King, L.; Paothong, A.; Nwoga, C.; Marty, P.J.; Whiteman, V. Impact of Intrauterine Tobacco Exposure on Fetal Telomere Length. Am. J. Obstet. Gynecol. 2015, 212, e1–e205. [Google Scholar] [CrossRef]
- Mirzakhani, H.; De Vivo, I.; Leeder, J.S.; Gaedigk, R.; Vyhlidal, C.A.; Weiss, S.T.; Tantisira, K. Early Pregnancy Intrauterine Fetal Exposure to Maternal Smoking and Impact on Fetal Telomere Length. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 218, 27–32. [Google Scholar] [CrossRef]
- Hong, X.; Liu, C.; Chen, X.; Song, Y.; Wang, Q.; Wang, P.; Hu, D. Maternal Exposure to Airborne Particulate Matter Causes Postnatal Immunological Dysfunction in Mice Offspring. Toxicology 2013, 306, 59–67. [Google Scholar] [CrossRef]
- Lee, J.W.; Jaffar, Z.; Pinkerton, K.E.; Porter, V.; Postma, B.; Ferrini, M.; Holian, A.; Roberts, K.; Cho, Y.H. Alterations in DNA Methylation and Airway Hyperreactivity in Response to in Utero Exposure to Environmental Tobacco Smoke. Inhal. Toxicol. 2015, 27, 724–730. [Google Scholar] [CrossRef]
- Adebambo, O.A.; Shea, D.; Fry, R.C. Cadmium Disrupts Signaling of the Hypoxia-Inducible (HIF) and Transforming Growth Factor (TGF-β) Pathways in Placental JEG-3 Trophoblast Cells via Reactive Oxygen Species. Toxicol. Appl. Pharmacol. 2018, 342, 108–115. [Google Scholar] [CrossRef]
- Janssen, B.G.; Munters, E.; Pieters, N.; Smeets, K.; Cox, B.; Cuypers, A.; Fierens, F.; Penders, J.; Vangronsveld, J.; Gyselaers, W.; et al. Placental Mitochondrial DNA Content and Particulate Air Pollution during in Utero Life. Environ. Health Perspect. 2012, 120, 1346–1352. [Google Scholar] [CrossRef]
- Janssen, B.G.; Godderis, L.; Pieters, N.; Poels, K.; Kiciński, M.; Cuypers, A.; Fierens, F.; Penders, J.; Plusquin, M.; Gyselaers, W.; et al. Placental DNA Hypomethylation in Association with Particulate Air Pollution in Early Life. Part. Fibre Toxicol. 2013, 10, 22. [Google Scholar] [CrossRef]
- Janssen, B.G.; Byun, H.-M.; Gyselaers, W.; Lefebvre, W.; Baccarelli, A.A.; Nawrot, T.S. Placental Mitochondrial Methylation and Exposure to Airborne Particulate Matter in the Early Life Environment: An ENVIRONAGE Birth Cohort Study. Epigenetics 2015, 10, 536–544. [Google Scholar] [CrossRef]
- Lovinsky-Desir, S.; Lawrence, J.; Jung, K.H.; Rundle, A.G.; Hoepner, L.A.; Yan, B.; Perera, F.; Perzanowski, M.S.; Miller, R.L.; Chillrud, S.N. Assessment of Exposure to Air Pollution in Children: Determining Whether Wearing a Personal Monitor Affects Physical Activity. Environ. Res. 2018, 166, 340–343. [Google Scholar] [CrossRef]
- Allen, R.W.; Barn, P. Individual- and Household-Level Interventions to Reduce Air Pollution Exposures and Health Risks: A Review of the Recent Literature. Curr. Environ. Health Rep. 2020, 7, 424–440. [Google Scholar] [CrossRef]
- Reid, C.E.; Brauer, M.; Johnston, F.H.; Jerrett, M.; Balmes, J.R.; Elliott, C.T. Critical Review of Health Impacts of Wildfire Smoke Exposure. Environ. Health Perspect. 2016, 124, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Barn, P.K.; Elliott, C.T.; Allen, R.W.; Kosatsky, T.; Rideout, K.; Henderson, S.B. Portable Air Cleaners Should Be at the Forefront of the Public Health Response to Landscape Fire Smoke. Environ. Health 2016, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, D.A.; Autenrieth, D.A.; Hart, J.F.; Capoccia, S. Control of Wildfire-Sourced PM2.5 in an Office Setting Using a Commercially Available Portable Air Cleaner. J. Occup. Environ. Hyg. 2020, 17, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Kizer, K.W. Extreme Wildfires—A Growing Population Health and Planetary Problem. JAMA 2020, 324, 1605. [Google Scholar] [CrossRef] [PubMed]
- Mott, J.A. Wildland Forest Fire Smoke: Health Effects and Intervention Evaluation, Hoopa, California, 1999. West. J. Med. 2002, 176, 157–162. [Google Scholar] [CrossRef]
- Ha, S.; Nobles, C.; Kanner, J.; Sherman, S.; Cho, S.-H.; Perkins, N.; Williams, A.; Grobman, W.; Biggio, J.; Subramaniam, A.; et al. Air Pollution Exposure Monitoring among Pregnant Women with and without Asthma. Int. J. Environ. Res. Public Health 2020, 17, 4888. [Google Scholar] [CrossRef]
- Nethery, E.; Brauer, M.; Janssen, P. Time–Activity Patterns of Pregnant Women and Changes during the Course of Pregnancy. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 317–324. [Google Scholar] [CrossRef]
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A Resource for Assessing Exposure to Environmental Pollutants. J. Expo. Sci. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef]
- Barn, P.; Gombojav, E.; Ochir, C.; Boldbaatar, B.; Beejin, B.; Naidan, G.; Galsuren, J.; Legtseg, B.; Byambaa, T.; Hutcheon, J.A.; et al. The Effect of Portable HEPA Filter Air Cleaner Use during Pregnancy on Fetal Growth: The UGAAR Randomized Controlled Trial. Environ. Int. 2018, 121, 981–989. [Google Scholar] [CrossRef]
- Roeckner, J.T.; Krstić, N.; Sipe, B.H.; Običan, S.G. N95 Filtering Facepiece Respirator Use during Pregnancy: A Systematic Review. Am. J. Perinatol. 2020, 37, 995–1001. [Google Scholar] [CrossRef]
- Roberge, R.J.; Kim, J.-H.; Powell, J.B. N95 Respirator Use during Advanced Pregnancy. Am. J. Infect. Control 2014, 42, 1097–1100. [Google Scholar] [CrossRef]
- PurpleAir Community. About the Data Category—Data. Available online: https://community.purpleair.com/t/about-the-data-category/70 (accessed on 31 July 2022).
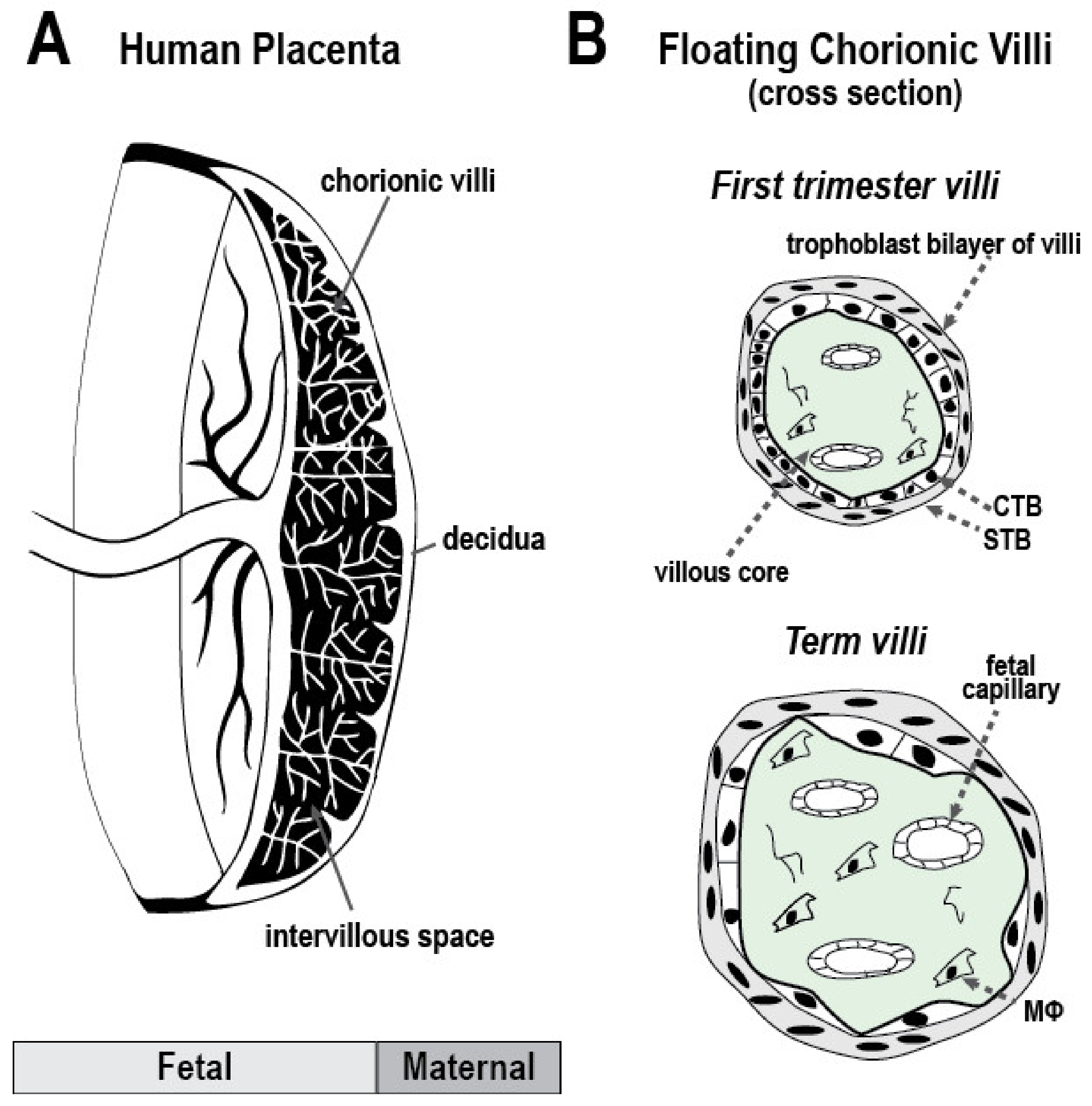
| Citation | Type of Exposure | Time | Location | Animal Model | Sample Source(s) | Sample Size (n) | Description of Exposure | Primary Outcome(s) |
|---|---|---|---|---|---|---|---|---|
| Black (2017) [26] | Wildfire smoke | 2008 | California, USA | Adult female rhesus macaque (Macaca mulatta) exposed in infancy to wildfire season in 2008 | Blood | 50 | Ozone and PM2.5 concentrations from air monitoring stations | PBMC in vitro challenge testing, cytokine protein assay 6 h after TLR ligand addition, IL-6 and IL-8 levels, Pulmonary function measures |
| Brown (2022) [76] | Wildfire smoke | 2008–2009 | California, USA | Adult female rhesus macaque (Macaca mulatta) exposed in their first three months of life to wildfire season in 2008 | Nasal epithelium, peripheral blood | 22 | Wildfire smoke PM2.5, ozone exposure during early life | Whole genome bisulfite sequencing to identify differentially methylated regions from nasal epithelium, RNA-sequencing on a subset of samples |
| Capitano (2022) [74] | Wildfire smoke | 2018 | California, USA | Infant female rhesus macaque (Macaca mulatta) | --- | 56 | Mean daily PM2.5 | BioBehavioral Assessment, CRP levels via high sensitivity assay, cortisol concentration via I125 radioimmunoassay |
| Detmar (2008) [72] | Polycyclic aromatic hydrocarbons | 2008 | Toronto, Canada | C57BI/6 female mice | Placenta, fetus | 4 | Subcutaneous injections of PAH over a 9-week period | Fetal growth, placental cell death rates, expression of antiapoptotic Xiap, proapoptotic Bax, levels of cleaved poly(ADP-ribose) polymerase-, and active caspase-3 |
| Hong (2013) [152] | Particulate matter | 2012 | Fujian, China | Mouse | Blood, spleen, thymus | 40 | Instillation of airborne PM solution into mouse lung | IL-4 and IFN-γ levels in plasma and spleen, splenic lymphocyte proliferation, GATA-3 and T-bet mRNA in spleen tested, histopathology of spleen and thymus. |
| Lee (2015) [153] | Environmental tobacco smoke | 2015 | California and Montana, USA | Mouse | Bronchial alveolar lavage fluid | 4 | Tobacco smoke (1.0 mg/m3) for 6 h/day | Global DNA methylation, cytokine measurements |
| Valentino (2016) [111] | Diesel engine exhaust | 2016 | France | New-Zealand white female rabbits (INRA1077 line) | Maternal lung, maternal and fetal plasma, placenta, fetus | 28 | Inhalation of diesel exhaust from 3rd to 27th day post-conception (20 days over 31 day gestation) | Ultrasound with biometry and Doppler monitoring, birth weight, TEM of lung, vascular and placental tissue to identify NPs |
| Miller (2020) [107] | Ozone | 2020 | North Carolina, USA | Long-Evans rat | Placenta, fetus | 8 | Gaseous ozone for 4 h in the mornings of gestation days 5 and 6 (during implantation) | DNA and RNA expression from placenta, hepatic gene expression, mitochondrial respiration, metabolic assessment |
| Zhu (2021) [99] | PM2.5 | 2021 | Taiguan, China | C57BL/6 mice | Placenta, fetus | 55 | Oropharyngeal aspiration of PM2.5 every other day starting on embryonic day 0.5 | Expression of proliferating cell nuclear antigen, mRNA of amino acids, long-chain polyunsaturated fatty acid, glucose, glycogen, triglycerides, and folate transporters |
| Citation | Type of Exposure | Time | Location | Study Population | Sample Source(s) | Sample Size (n) | Description of Exposure | Primary Outcome(s) |
|---|---|---|---|---|---|---|---|---|
| Abraham (2018) [146] | Air pollution | 2003–2006 | France | Singleton pregnancies enrolled before 24 weeks of gestation | Placenta samples collected at delivery | 688 | NO2 and PM10 hourly concentrations modelled using maternal home address, mean daily temperature and humidity from nearest stationary monitors | Genome-wide DNA placental methylation levels |
| Adebambo (2018) [154] | Cadmium treatment | 2018 | Longjiang River, China | JEG-3 choriocarcinoma cell line | Placental trophoblast cells | 6 | Environmental water samples from a cadmium spill site in China | Reactive oxygen species (ROS), expression of metallothionein (MT) isoforms, HIF1α, and TGFβ associated genes and proteins |
| Arita (2022) [89] | Dimethylaniline (DMA) | 2022 | New York, New Jersey, USA | Placental explants | Placental explant cultures from term placentas collected from elective cesarean sections | 12 | DMA instilled into placental explant well plates to final concentrations of 0–50 μM | IL-1β, TNF-α, IL-6, sgp130, IL-10, BDNF, HO-1, 8-IsoP, P4, T, E2 quantification using immunoassay reagents |
| Basilio (2021) [68] | Wildfire smoke | 2018–2019 | California | Mid gestation placenta | First and second trimester placenta from elective terminations of pregnancy | 12 | Average daily AQI levels during gestation | Fetal Hofbauer cells (CD68+) |
| Bové (2019) [49] | Air pollution | 2012–2016 | Belgium | ENVIRONAGE birth cohort, singleton births recruited on arrival for delivery | Term and preterm placenta collected within 10 min after birth | 10 | Ambient exposure to BC determined using maternal residential address using spatial and temporal integration from satellite images and pollution data from fixed monitoring stations | Detection of BC particles, BC load |
| Bainbridge (2006) [115] | Carbon monoxide | 2006 | Canada | Placental explants | Placental explant cultures from term placentas collected from elective cesarean sections | 13 | Carbon monoxide infused culture medium | Apoptotic Index using TUNEL assay, Immunohistochemical staining for p85 Fragment of PARP, morphology of villous tissue, villous tissue integrity |
| Erlandsson (2020) [51] | Wood smoke | 2020 | Sweden | First trimester trophoblast cell line HTR-8 | HTR-8 was derived by transfecting cells from chorionic villi explants from placentas of 6–12 week gestation | 6-well plates | Smoke burned on wood stove from logs of four different species (silver birch, quaking aspen, Norway spruce, and Scots pine) at a nominal burn rate and high burn rate, wood particles collected and extracted then aliquoted into well plates | hCG, progesterone and IL-6; cellular particle localization and uptake by TEM, cytotoxicity assay, PAH analysis, membrane integrity testing |
| Janssen (2012) [155] | Air pollution | 2012–2016 | Belgium | ENVIRONAGE birth cohort, singleton births recruited on arrival for delivery | Human, placenta and cord blood | 174 | PM10 incremental exposure | mtDNA content |
| Janssen (2013) [156] | Air pollution | 2012–2016 | Belgium | ENVIRONAGE birth cohort, singleton births recruited on arrival for delivery | Human, placenta | 240 | PM2.5 incremental exposure | Global DNA methylation |
| Janssen (2015) [157] | Air pollution | 2012–2016 | Belgium | ENVIRONAGE birth cohort, singleton births recruited on arrival for delivery | Human, placenta | 381 | PM2.5 incremental exposure | mtDNA methylation |
| Saenen (2015) [57] | Air pollution | 2012–2016 | Belgium | ENVIRONAGE birth cohort, singleton births recruited on arrival for delivery | Human, placenta | 90 | PM2.5 incremental exposure | Gene expression in BDNF and SYN1 pathways |
| Saenen (2016) [91] | Air pollution | 2012–2016 | Belgium | ENVIRONAGE birth cohort, singleton births recruited on arrival for delivery | Human, placenta | 336 | PM2.5 incremental exposure | 3-nitrotyrosine |
| Prevention of WS Exposure |
|---|
| (1) Stay indoors with a high-efficiency air filter * |
| (2) Seek shelter with a high-efficiency air filter * |
| (3) Use N95 respirator or P100 respirator |
| (4) Reduce outdoor exposure |
| (5) Reduce strenuous activities to reduce inhalation |
| (6) Evacuate safely and prepare an evacuation kit with food, water, and medications for 7–10 days, first aid supplies and important documents |
| (7) Do not consume any food, beverages, or medications that have been exposed to burn debris or ash; avoid using wood-burning stoves, fireplaces, gas, propane, or vacuum |
| (8) Protect pets by keeping them indoors. If you must evacuate without your pets, never tie them up |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basilio, E.; Chen, R.; Fernandez, A.C.; Padula, A.M.; Robinson, J.F.; Gaw, S.L. Wildfire Smoke Exposure during Pregnancy: A Review of Potential Mechanisms of Placental Toxicity, Impact on Obstetric Outcomes, and Strategies to Reduce Exposure. Int. J. Environ. Res. Public Health 2022, 19, 13727. https://doi.org/10.3390/ijerph192113727
Basilio E, Chen R, Fernandez AC, Padula AM, Robinson JF, Gaw SL. Wildfire Smoke Exposure during Pregnancy: A Review of Potential Mechanisms of Placental Toxicity, Impact on Obstetric Outcomes, and Strategies to Reduce Exposure. International Journal of Environmental Research and Public Health. 2022; 19(21):13727. https://doi.org/10.3390/ijerph192113727
Chicago/Turabian StyleBasilio, Emilia, Rebecca Chen, Anna Claire Fernandez, Amy M. Padula, Joshua F. Robinson, and Stephanie L. Gaw. 2022. "Wildfire Smoke Exposure during Pregnancy: A Review of Potential Mechanisms of Placental Toxicity, Impact on Obstetric Outcomes, and Strategies to Reduce Exposure" International Journal of Environmental Research and Public Health 19, no. 21: 13727. https://doi.org/10.3390/ijerph192113727
APA StyleBasilio, E., Chen, R., Fernandez, A. C., Padula, A. M., Robinson, J. F., & Gaw, S. L. (2022). Wildfire Smoke Exposure during Pregnancy: A Review of Potential Mechanisms of Placental Toxicity, Impact on Obstetric Outcomes, and Strategies to Reduce Exposure. International Journal of Environmental Research and Public Health, 19(21), 13727. https://doi.org/10.3390/ijerph192113727







