Diphasic Sheeting Device with Cyanex-301 for Dislodging Feature of Divalent Cadmium from Industrial Effluent
Abstract
:1. Introduction
2. Materials and Methods
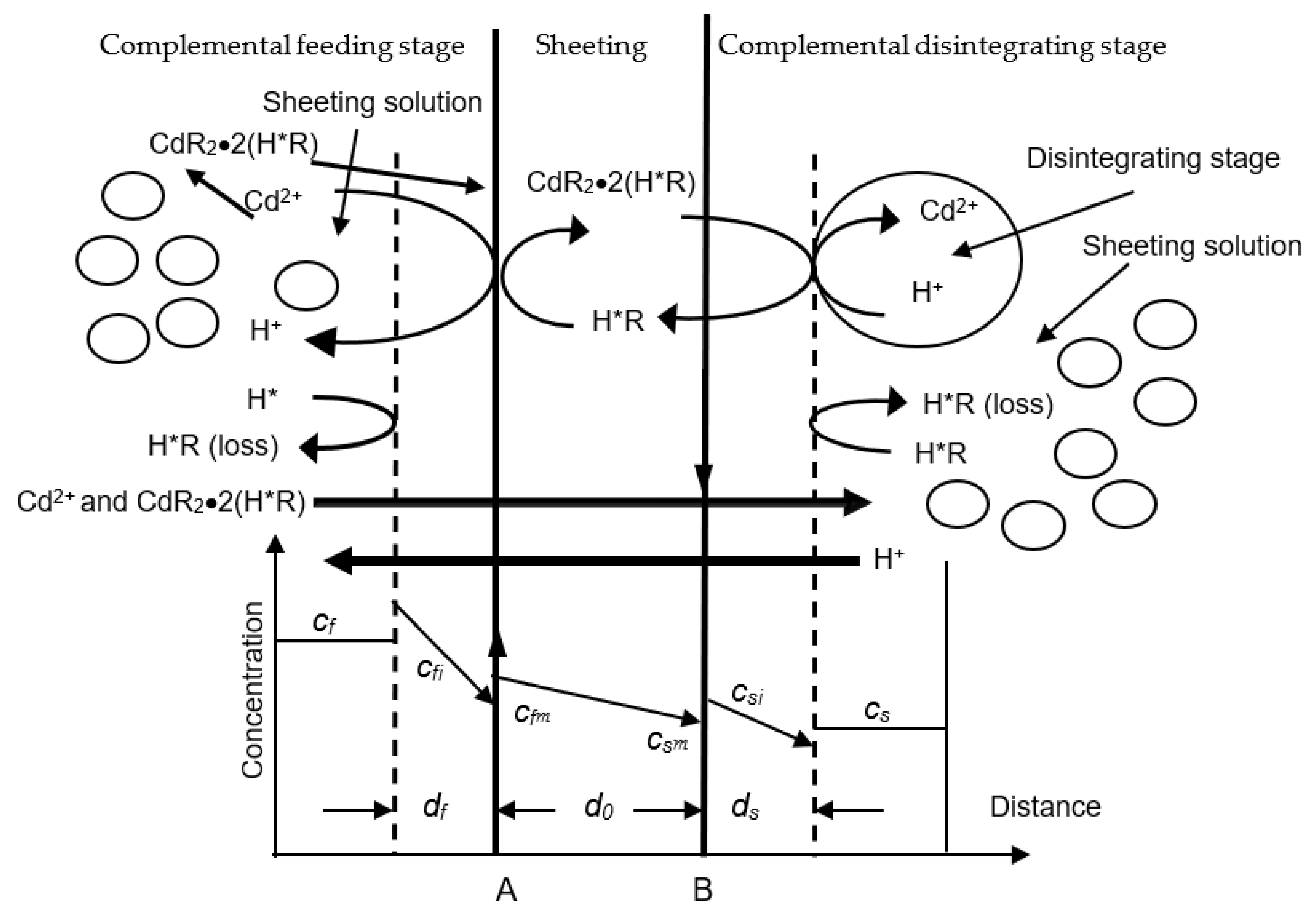
2.1. Theoretical Methods
- (1)
- The transferring of metallo-ions from the complemental feeding stage to the interfacial layer of feeding-sheeting stage.
- (2)
- On the feeding side interfacial layer of the DSD, dislodging of the divalent metallo-ions from liquid liquors including Cyanex-301 may be represented as the Equation (1) below.
- (3)
- The metallic complex has been transferred through the sheeting A–B.
- (4)
- At the other side of the interfacial layer of the sheeting, the metallic complex is dissolved sheeting liquor and the metallo-ions are resolved by disintegrating dissolvant. The chemical reaction can be expressed as the Equation (2) below.
- (1)
- The Cd(II) transfers in the organic medium only as the CdR2•2(H*R) complex.
- (2)
- There is no net flow on account of convection within the fluid sheeting.
- (3)
- The metallo-ions react only with Cyanex-301 at the sheeting interfacial layers.
- (4)
- The Cyanex-301 monomer and dimer are in equilibrium at all times throughout organic stage [12].
- (5)
2.2. Reagents and Instruments
2.3. Experimental Procedure
3. Results and Discussion
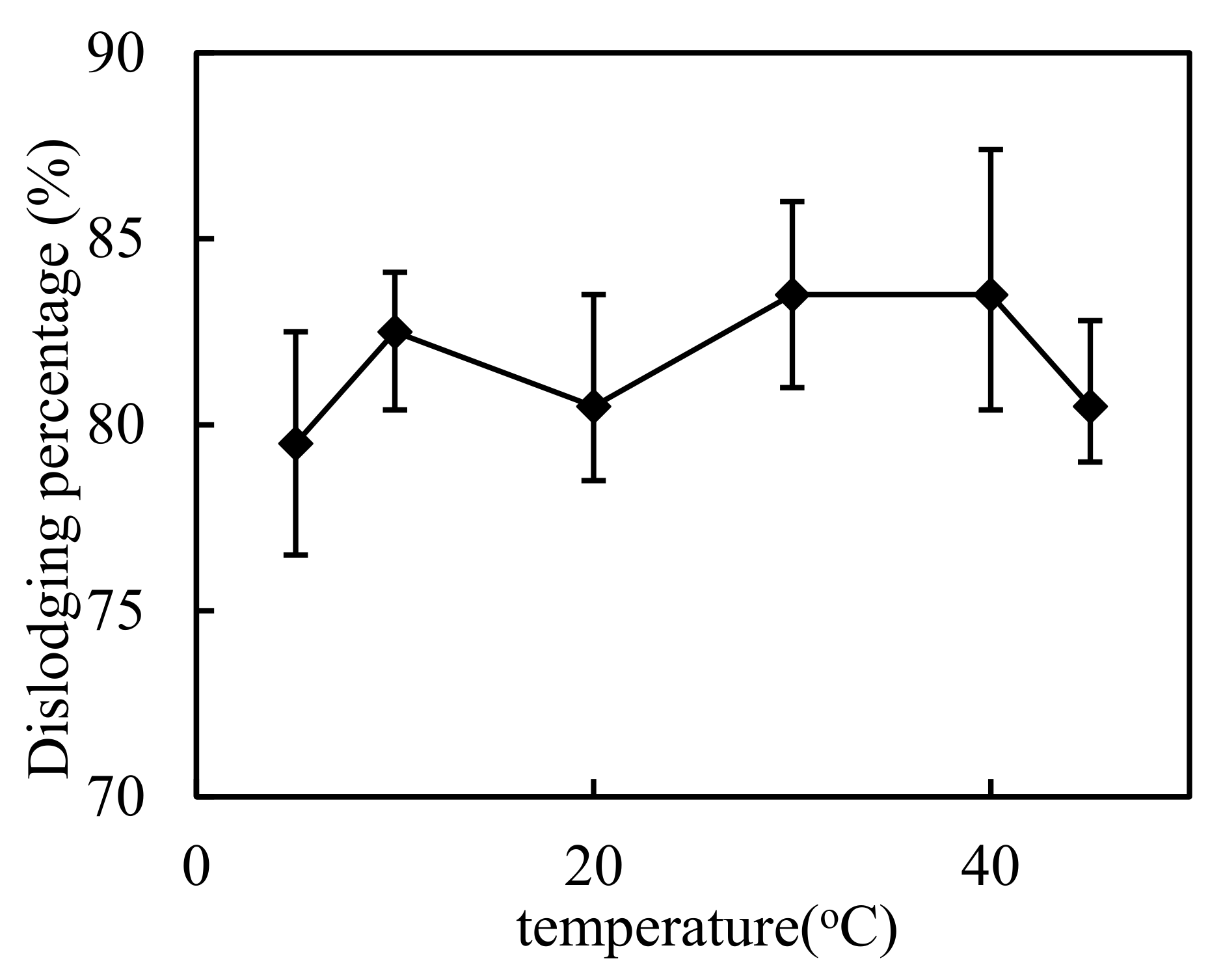
3.1. Impact of Temperature
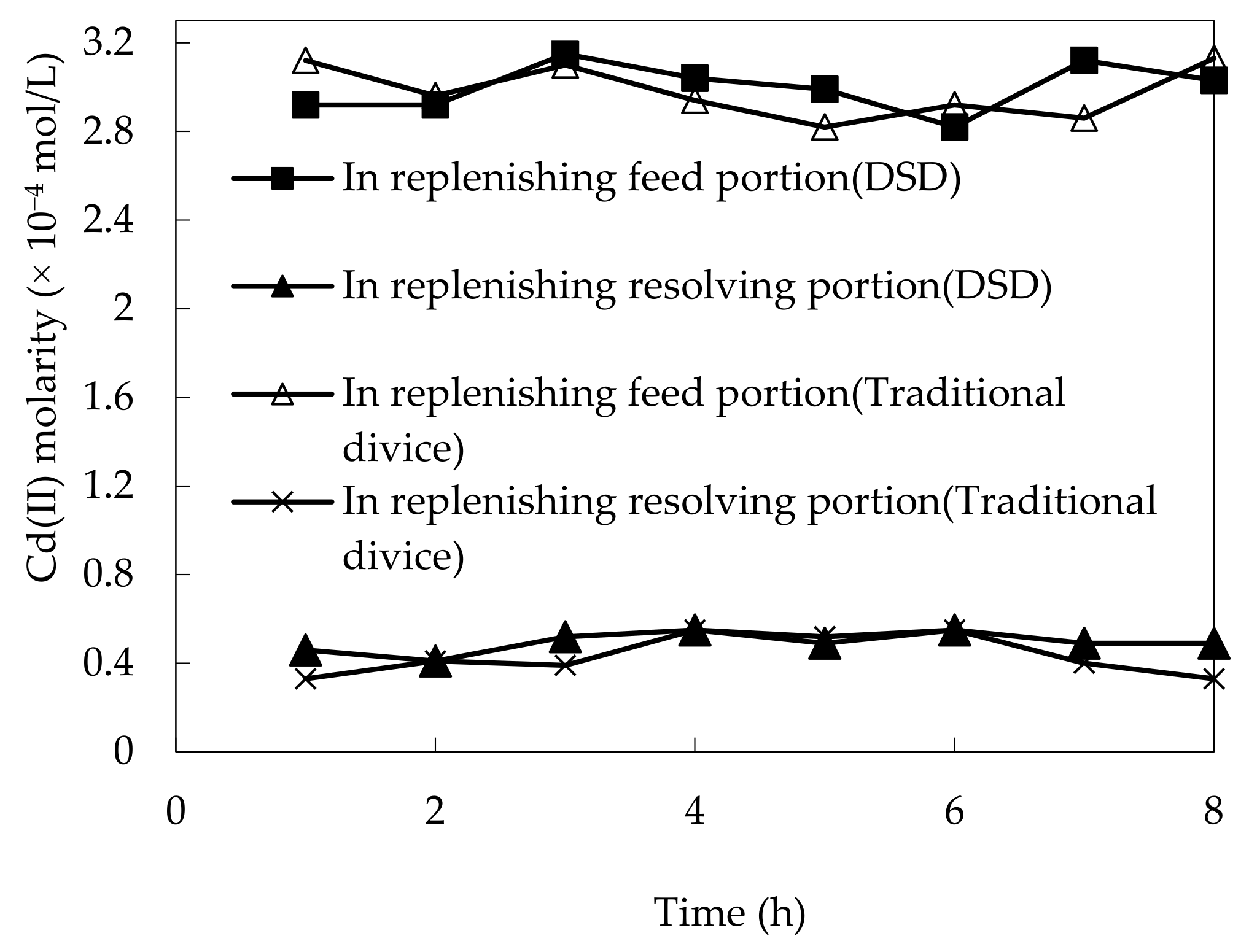
3.2. Constancy of DSD
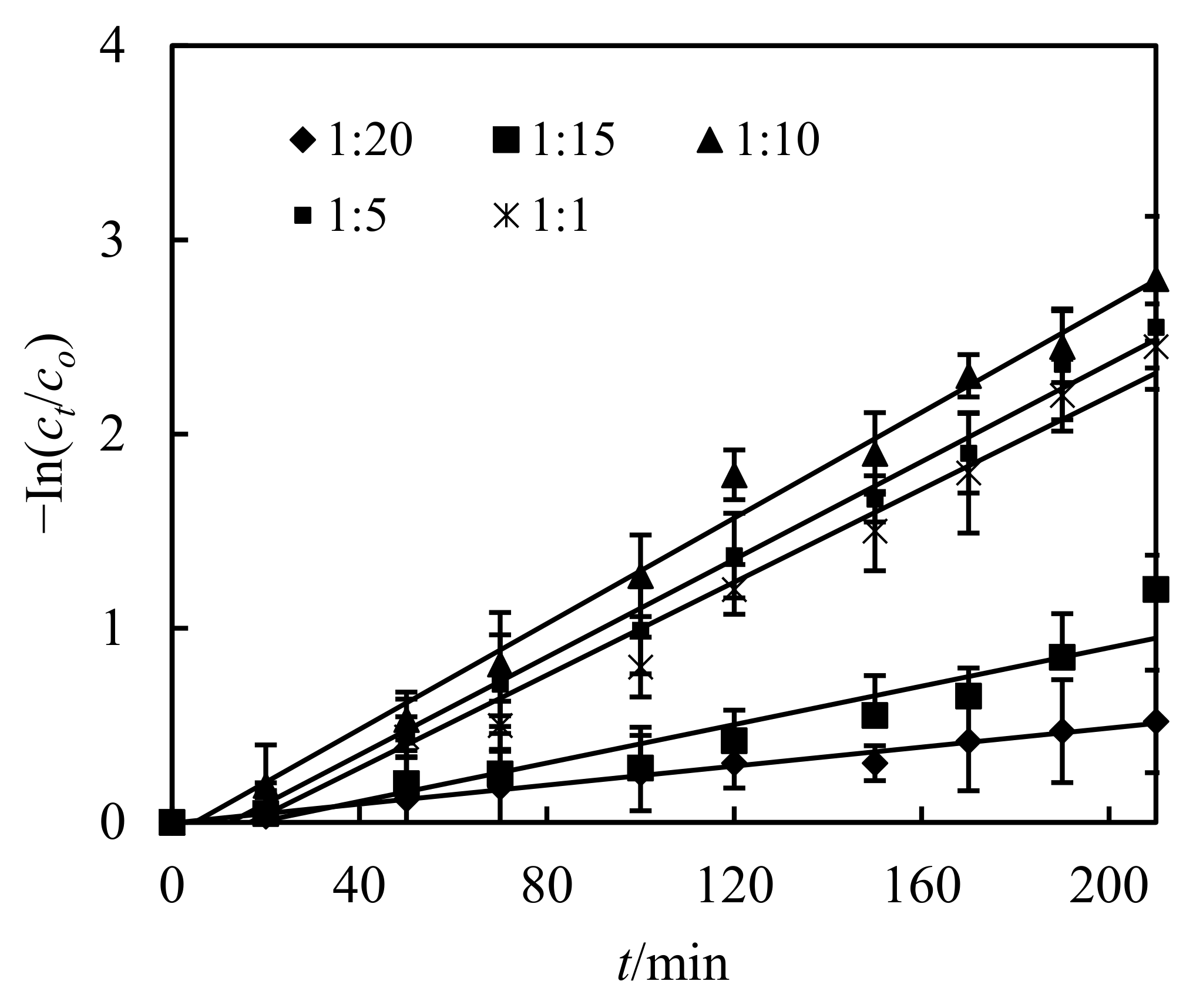
3.3. Impact of the Volumetric Ratio of Sheeting Liquor and Feeding Liquor(S/F)
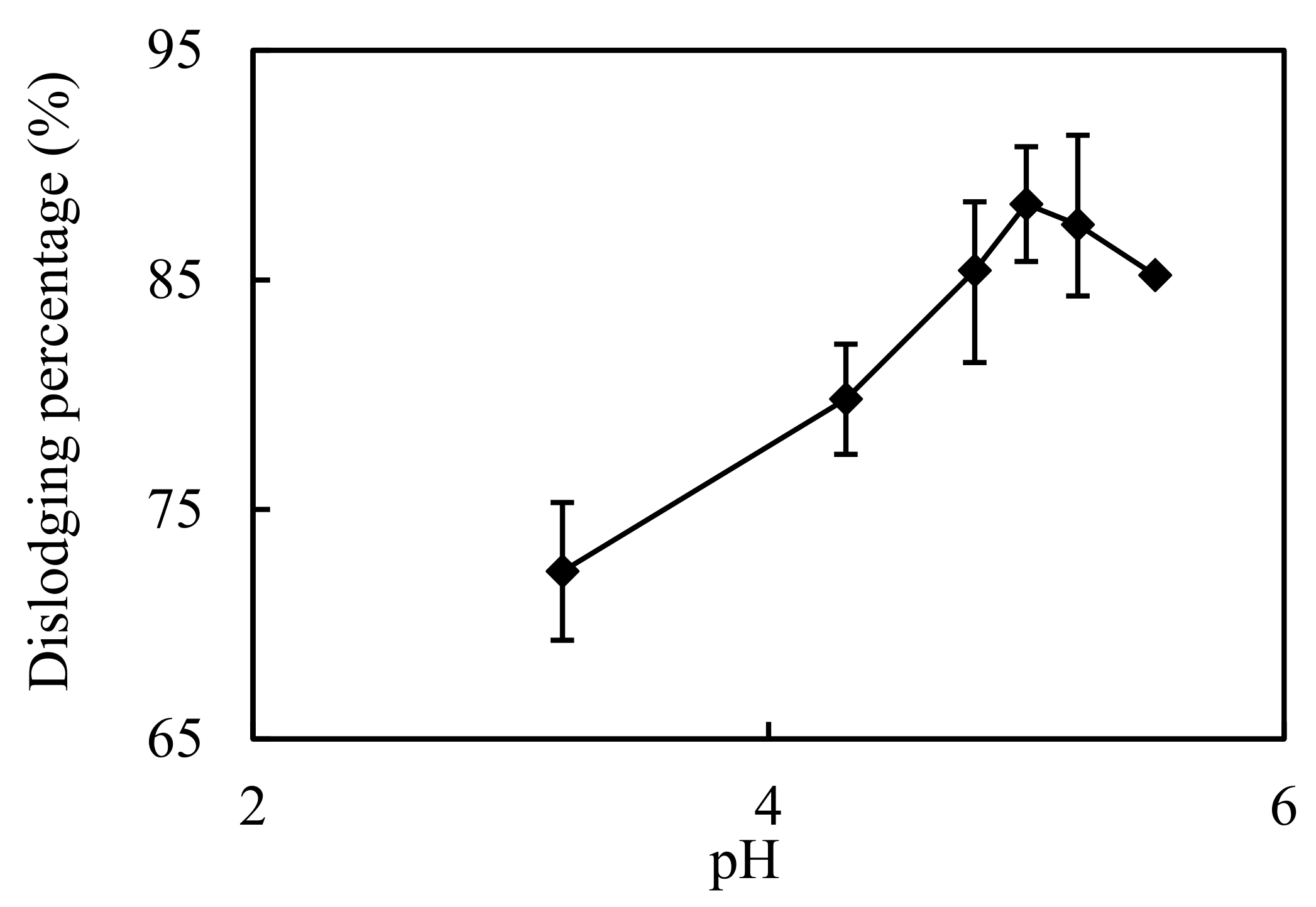
3.4. Impact of pH in the Complemental Feeding Stage
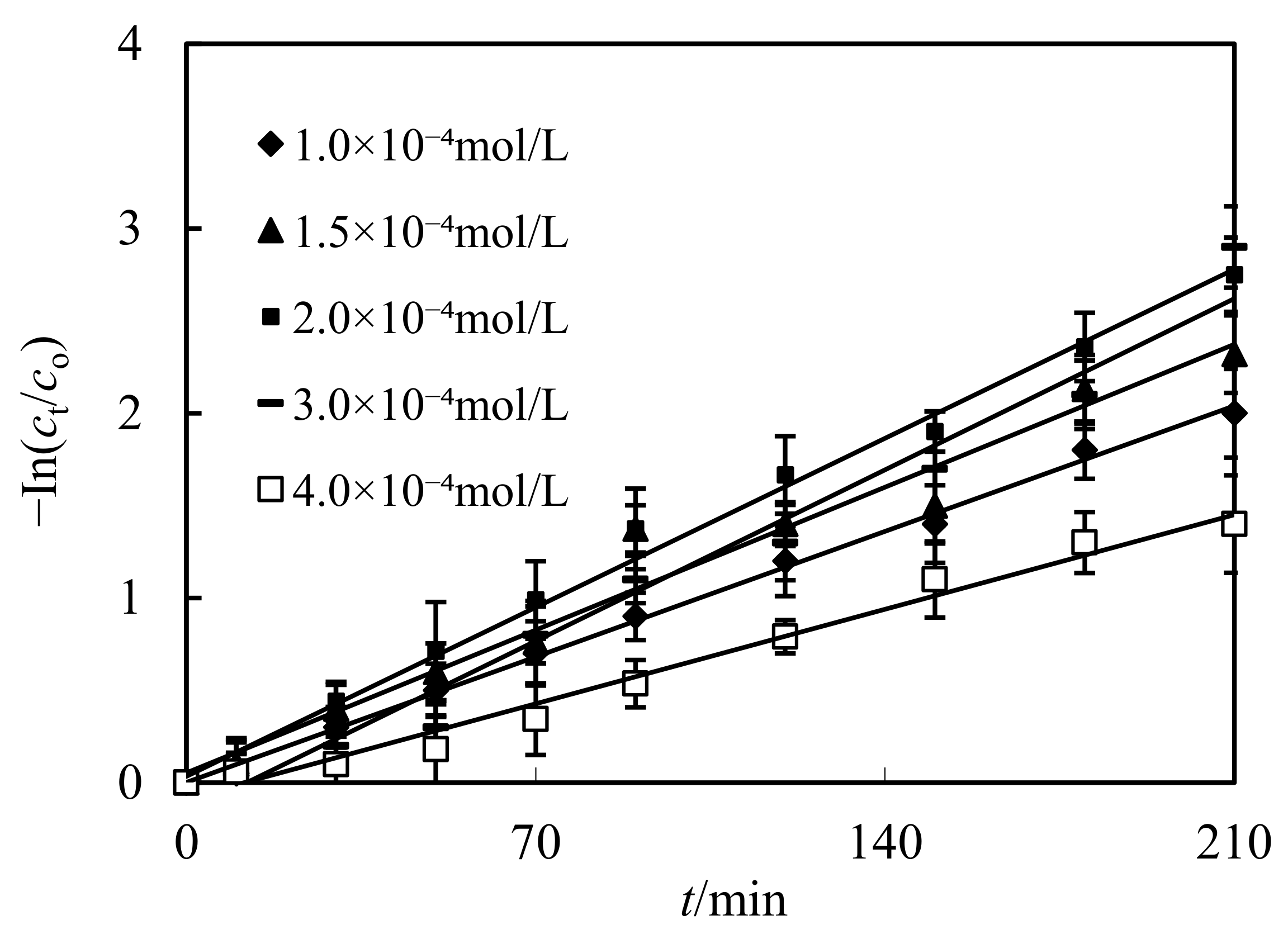
3.5. Impact of Initial Molarity of Cd(II) in the Complemental Disintegrating Stage
3.6. Impact of the Volumetric Ratio of Sheeting Liquor and Hydrochloric Acid Liquor(S/D)
3.7. Impact of the Molarity of Hydrochloric Acid in the Complemental Disintegrating Stage
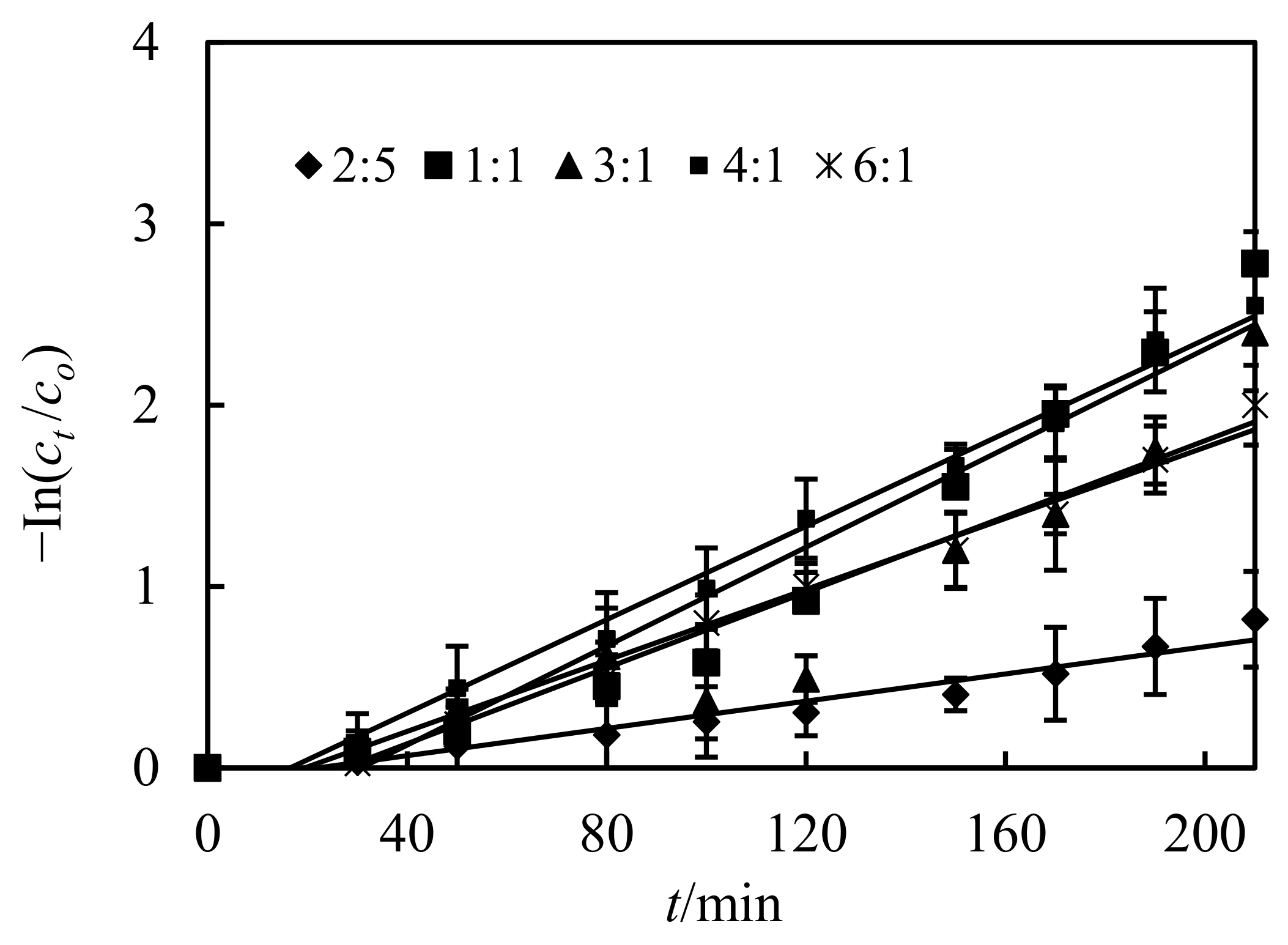
3.8. Impact of Ion Intensities in the Complemental Feeding Stage
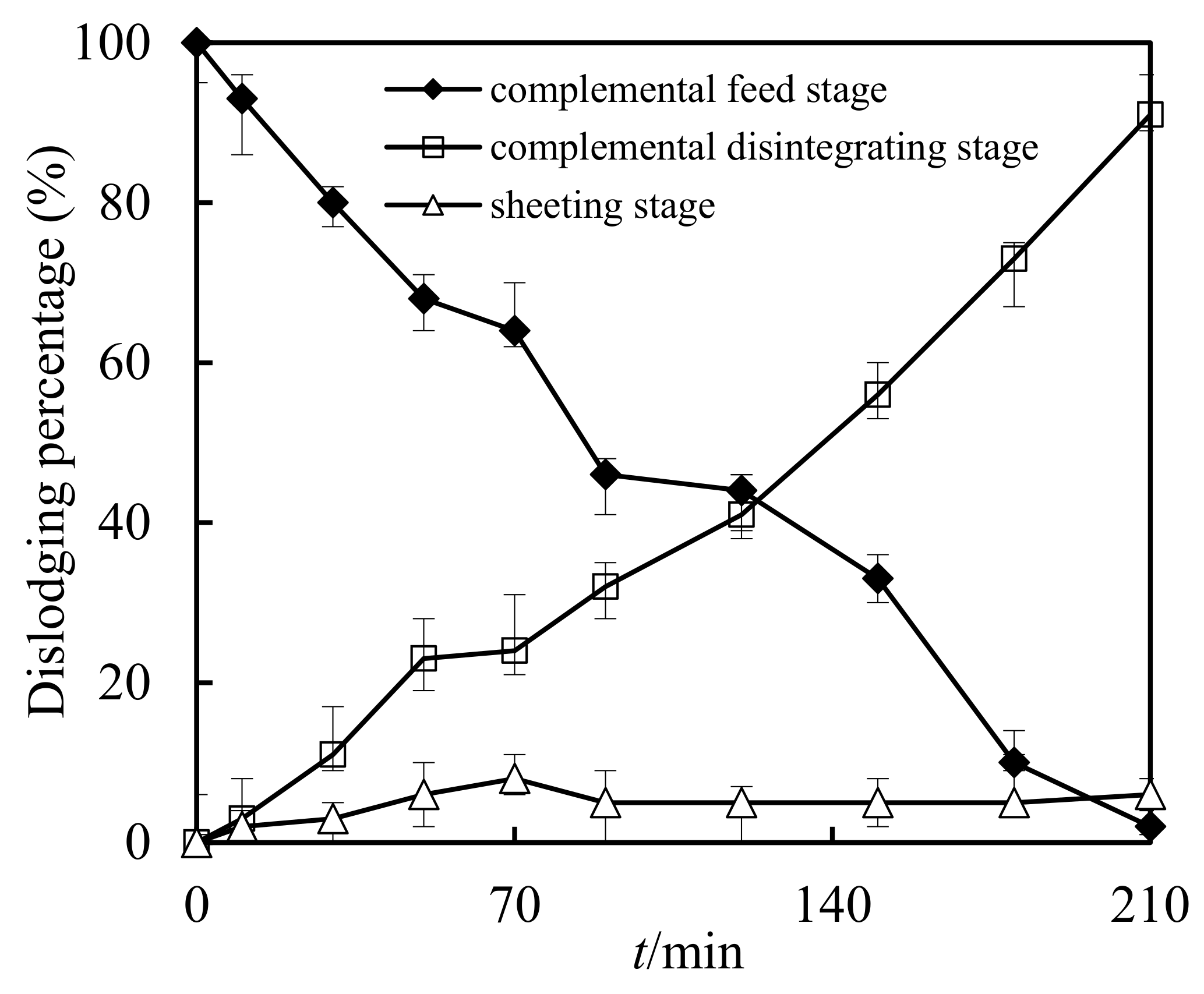
3.9. Retention in Sheeting Stage
4. Dynamic Analysis
5. Conclusions
- (1)
- The optimal conditions for dislodging Cd(II) were that the molarity of hydrochloric acid was 4.0 mol/L, S/F was 1:10, Cyanex-301 molarity was 0.150 mol/L, and S/D was 1:1 in the complemental disintegrating stage, initial molarity of Cd(II) was 3.2 × 10−4 mol/L, and optimal pH was 5.0 in the complemental feeding stage; when dislodging time was 210 min, the dislodging percentage was 92.9%.
- (2)
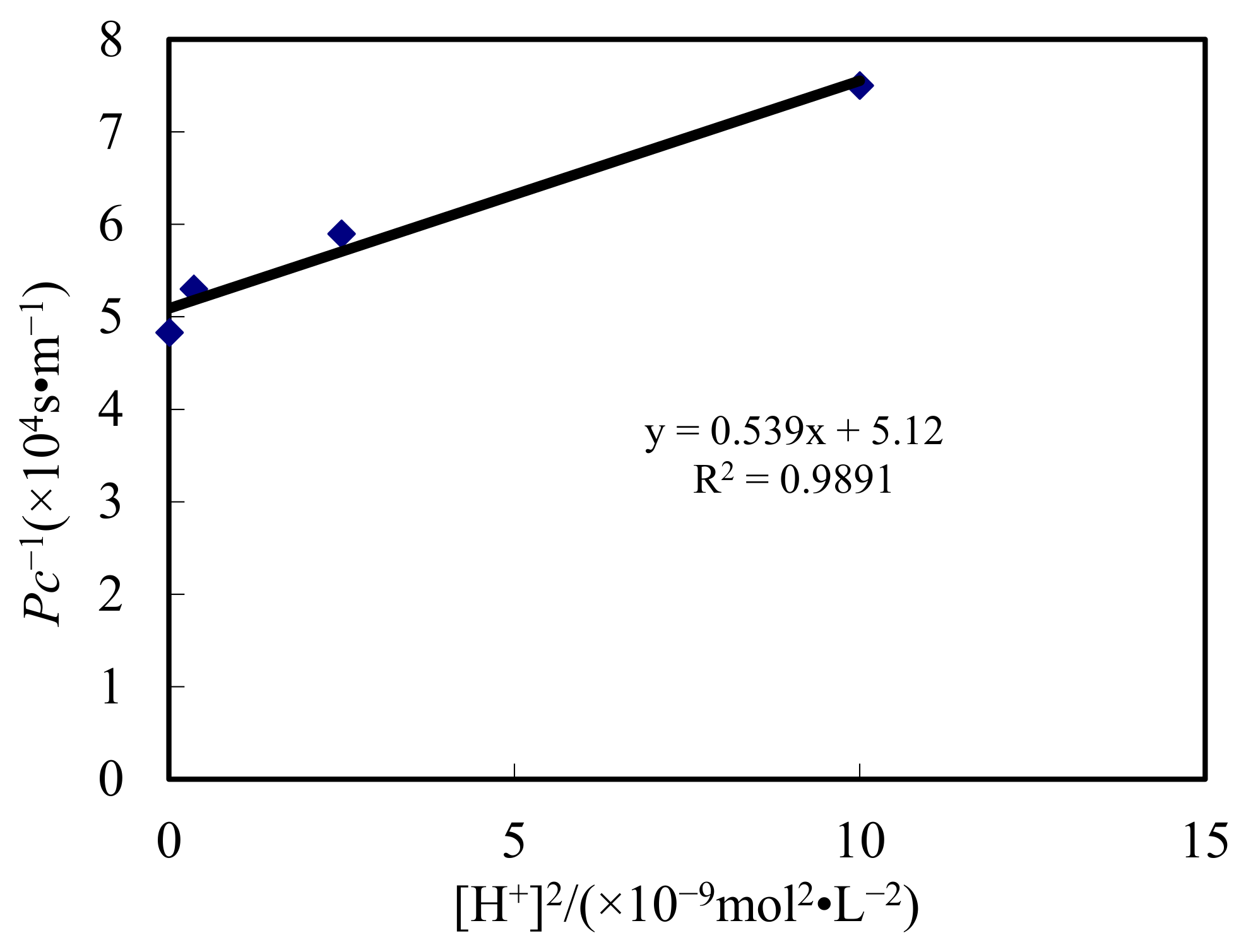
- Through this study, a relevance (modal) was established to elaborate the metallo-ion reaction and dislodging in the DSD. A new dynamic formula was inferred. The transferring constant of the sheeting stage and transferring layer thickness of the complemental feeding stage were gotten by using Straight line and Gradient method. They were 1.31 × 10−7 m2/s and 1.08 × 10−4 m.
- (3)
- In the DSD, large amounts of sheeting liquor were used; this could be complemented by the loss of Cyanex-301 in the sheeting device. As a result, the Cd(II) dislodging percentage is increased, the constancy of sheeting is increased, and the life of the sheeting is extended.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, B.; Zhou, F.J.; Yang, F.; Lian, J.J.; Ye, T.R.; Wu, H.Y.; Hui, A.Y. Enhanced sequestration of molybdenum(VI) using composite constructed wetlands and responses of microbial communities. Water Sci. Technol. 2022, 85, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.S.; Zhang, G.Q.; Zeng, L.; Zhou, Q.; Li, Z.; Zhang, D.; Cao, Z.; Guan, W.; Li, Q.; Xiao, L. Study on dislodging of molybdenum from ammonium tungstate liquors using dissolvant dislodging with quaternary ammonium salt extractant. Hydrometallugy 2019, 186, 218–225. [Google Scholar] [CrossRef]
- Yang, X.J.; Fane, A.G.; MacNaughton, S. Removal and recovery of heavy metals from slops by supported liquid membranes. Water Sci. Technol. 2001, 43, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; World Cancer Reports; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Pei, L.; Wang, L.M.; Ma, Z.Y. Modelling of Ce(IV) Transport through a Dispersion Combined Liquid Membrane with Carrier p507. Front. Env. Sci. Eng. 2014, 8, 503–509. [Google Scholar] [CrossRef]
- Kandah, M.I.; Meunier, J.L. Removal of cadmium ions from water by multi-walled carbon nanotubes. J. Hazard. Mater. 2007, 146, 283–288. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Pei, L.; Yao, B.H.; Zhang, C.J. Transport of Tm(III) through dispersion implanted fluid sheeting including Cyanex-301 in kerosene as the carrier. Sep. Purif. Technol. 2009, 65, 220–227. [Google Scholar] [CrossRef]
- Wu, X.S.; Zhang, G.Q.; Zeng, L.; Guan, W.; Xiao, L. Continuous dissolvant dislodging operations for the dislodging of molybdenum from ammonium tungstate liquor with quaternary ammonium salt extractant. Hydrometallugy 2020, 195, 105401. [Google Scholar] [CrossRef]
- Kasra-Kermanshahi, R.; Tajer-Mohammad-Ghazvini, P.; Bahrami-Bavani, M. A biotechnological strategy for molybdenum dislodging using acidithiobacillusferrooxidans. Appl. Biochem. Biotechnol. 2020, 193, 884–895. [Google Scholar] [CrossRef]
- Valenzuela, F.; Fonseca, C.; Basualto, C.; Correa, O.; Tapia, C.; Sapag, J. Removal of copper ions from a wastewater by a fluid emulsion sheeting method. Miner. Eng. 2015, 18, 33–40. [Google Scholar] [CrossRef]
- Ho, W. Engineering Membranes for Environmental and Energy Applications. In Asian Pacific Confederation of Chemical Engineering Congress Program and Abstracts Asian Pacific Confederation of Chemical Engineers Congress Program and Abstracts; The Society of Chemical Engineers: Tokyo, Japan, 2004; p. 225. [Google Scholar]
- Pei, L.; Wang, L.M. Transport behavior of divalent lead ions through disphase supplying supported liquid membrane with PC-88A as mobile carrier. Int. J. Chem. React. Eng. 2012, 10, 1–27. [Google Scholar] [CrossRef]
- Pei, L.; Wang, L.M.; Guo, W.; Zhao, N. Study on a Novel Disphase Supplying Supported Liquid Membrane for Transport Behavior of Divalent Nickel Ions. Chin. J. Chem. Eng. 2012, 20, 633–640. [Google Scholar] [CrossRef]
- Albarak, Z. Carrier-mediated fluid sheeting systems for lead (II) ion separations. Chem. Pap. 2020, 74, 77–88. [Google Scholar] [CrossRef]
- Ahmad, A.; Tariq, S.; Zaman, J.U.; Perales, A.I.M.; Mubashir, M.; Luque, R. Recent trends and challenges with the synthesis of sheetings: Industrial opportunities towards environmental remediation. Chemosphere 2022, 135634. [Google Scholar] [CrossRef]
- Pei, L.; Sun, L. Impact Factors on Migration of Molybdenum(VI) from the Simulated Trade Effluent Using Membrane Chemical Reactor Combined with Carrier in the Mixed Renewal Solutions. Toxics 2022, 10, 438. [Google Scholar] [CrossRef]
- Tang, Y.; Wei, L.; Wan, J.; Wang, Y.; Yang, X. Two-stage recovery of s-adenosylmethionine using supported liquid membranes with strip dispersion. Process Biochem. 2013, 48, 1980–1991. [Google Scholar] [CrossRef]
- Bhatluri, K.K.; Manna, M.S.; Ghoshal, A.K.; Saha, P. Separation of cadmium and lead from wastewater using implanted fluid sheeting integrated with in-situ electrodeposition. Electrochim. Acta 2017, 229, 1–7. [Google Scholar] [CrossRef]
- Li, F.P.; Wang, Y.; Mao, L.C.; Tao, H.; Chen, M. Molybdenum background and pollution levels in the TaipuRiver, China. Environ. Chem. Lett. 2022, 20, 1009–1015. [Google Scholar] [CrossRef]
- Foong, C.Y.; Wirzal, M.D.H.; Bustam, M.A. A review on nanofibers sheeting with amino-based ionic fluid for heavy metal dislodging. J. Mol. Liq. 2020, 297, 111793. [Google Scholar] [CrossRef]
- He, D.S.; Ma, M.; Wang, H.; Zhou, P. Impact of dynamics synergiston transport of Copper(II) through a fluid sheeting including P507 in Kerosene. Can. J. Chem. 2001, 79, 1213–1219. [Google Scholar] [CrossRef]
- Pichler, T.; Koopmann, S. Should monitoring of molybdenum (Mo) in groundwater, drinking water and well permitting made mandatory? Environ. Sci. Technol. 2020, 54, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Qiu, Z.F.; Yang, J.; Cao, L.; Zhang, W. Recovery of Mo and Ni from spent acrylonitrile catalysts using an oxidation leaching-chemical precipitation technique. Hydrometallurgy 2016, 164, 64–70. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Zhang, M.; Yu, W.X.; Li, J.; Kong, D. Ecotoxicological risk ranking of 19 metals in the lower Yangtze River of China based on their threats to aquatic wildlife. Sci. Total Environ. 2022, 812, 152370. [Google Scholar] [CrossRef]
- Zeng, L.Q.; Zhao, Z.W.; Huo, G.S.; Wang, X.; Pu, H. Mechanism of selective precipitation of molybdenum from tungstate liquor. JOM 2020, 72, 800–805. [Google Scholar] [CrossRef]
- Hamza, M.F.; Roux, J.C.; Guibai, E. Metal valorization from the waste produced in the manufacturing of Co/Mo catalysts: Leaching and selective precipitation. J. Mater. Cycles Waste Manag. 2019, 21, 525–538. [Google Scholar] [CrossRef]
- Davies, T.D.; Pickard, J.; Hall, K.J. Acute molybdenum toxicity to rainbow trout and other fish. J. Environ. Eng. Sci. 2005, 4, 481–485. [Google Scholar] [CrossRef]
- Bina, G.; Akash, D.; Poonma, M. Extraction and recovery of cadmium using Cyanex 923. Hydrometallurgy 2001, 61, 65–71. [Google Scholar]
- Yesil, H.; Tugtas, A.E. Removal of heavy metals from leaching effluents of sewage sludge via supported liquid membranes. Sci. Total Environ. 2019, 693, 133608. [Google Scholar] [CrossRef]
- Solongo, T.; Fukushi, K.; Altansukh, O.; Takahashi, Y.; Akehi, A.; Baasansuren, G.; Hasebe, N. Distribution and chemical speciation of molybdenum in river and pond sediments affected by mining activity in Erdenet City, Mongolia. Minerals 2018, 8, 288. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.X.; Wan, D.J.; Tian, H.X.; He, W.; Wang, Z.; Liu, Q. Pollution assessment of heavy metals in soils and plants around a Molybdenum mine in Central China. Pol. J. Environ. Stud. 2019, 28, 123–133. [Google Scholar] [CrossRef]
- Song, Z.X.; Song, G.F.; Tnag, W.Z.; Yan, D.; Zhao, Y.; Zhu, Y.; Ma, Y. Molybdenum contamination dispersion from mining site to a reservoir. Ecotoxicol. Environ. Saf. 2021, 208, 111631. [Google Scholar] [CrossRef] [PubMed]
- Monesi, M.; Khatibi, M.; Rahbar-Kelishami, A. A simulation study of an electro-membrane extraction for enhancement of the ion transport via tailoring the electrostatic properties. Sci. Rep. 2022, 12, 16482–16501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Z.Y.; Zhan, K.; Sun, R.; Sheng, Z.; Wang, M.; Hou, X. Two dimensional nanomaterial-based separation membranes. Electrophoresis 2019, 40, 2029–2040. [Google Scholar] [CrossRef] [PubMed]
- Mal’tseva, E.E.; Blokhin, A.A.; Murashkin, Y.V. Sorptiveparation of molybdenum(VI)from rhenium-containing solutions. Russ. J. Appl. Chem. 2017, 90, 528–532. [Google Scholar] [CrossRef]
- Hao, Z.; Wang, Z.; Zhang, W.; Ho, W.W. Supported liquid membranes for recovery of cephalexin. J. Membr. Sci. 2014, 468, 90–97. [Google Scholar] [CrossRef]
- Li, Y.; Cui, C.; Ren, X.; Li, Y. Solvent extraction of chromium(vi) from hydrochloric acid solution with trialkylamine/kerosene. Desalin. Water Treat. 2015, 54, 191–199. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, L.; Wang, C. Diphasic Sheeting Device with Cyanex-301 for Dislodging Feature of Divalent Cadmium from Industrial Effluent. Int. J. Environ. Res. Public Health 2022, 19, 13281. https://doi.org/10.3390/ijerph192013281
Pei L, Wang C. Diphasic Sheeting Device with Cyanex-301 for Dislodging Feature of Divalent Cadmium from Industrial Effluent. International Journal of Environmental Research and Public Health. 2022; 19(20):13281. https://doi.org/10.3390/ijerph192013281
Chicago/Turabian StylePei, Liang, and Chunhui Wang. 2022. "Diphasic Sheeting Device with Cyanex-301 for Dislodging Feature of Divalent Cadmium from Industrial Effluent" International Journal of Environmental Research and Public Health 19, no. 20: 13281. https://doi.org/10.3390/ijerph192013281
APA StylePei, L., & Wang, C. (2022). Diphasic Sheeting Device with Cyanex-301 for Dislodging Feature of Divalent Cadmium from Industrial Effluent. International Journal of Environmental Research and Public Health, 19(20), 13281. https://doi.org/10.3390/ijerph192013281









