A New Model Algorithm for Estimating the Inhalation Exposure Resulting from the Spraying of (Semi)-Volatile Binary Liquid Mixtures (SprayEva)
Abstract
1. Introduction
2. Methods
2.1. General Mass Balance Equations
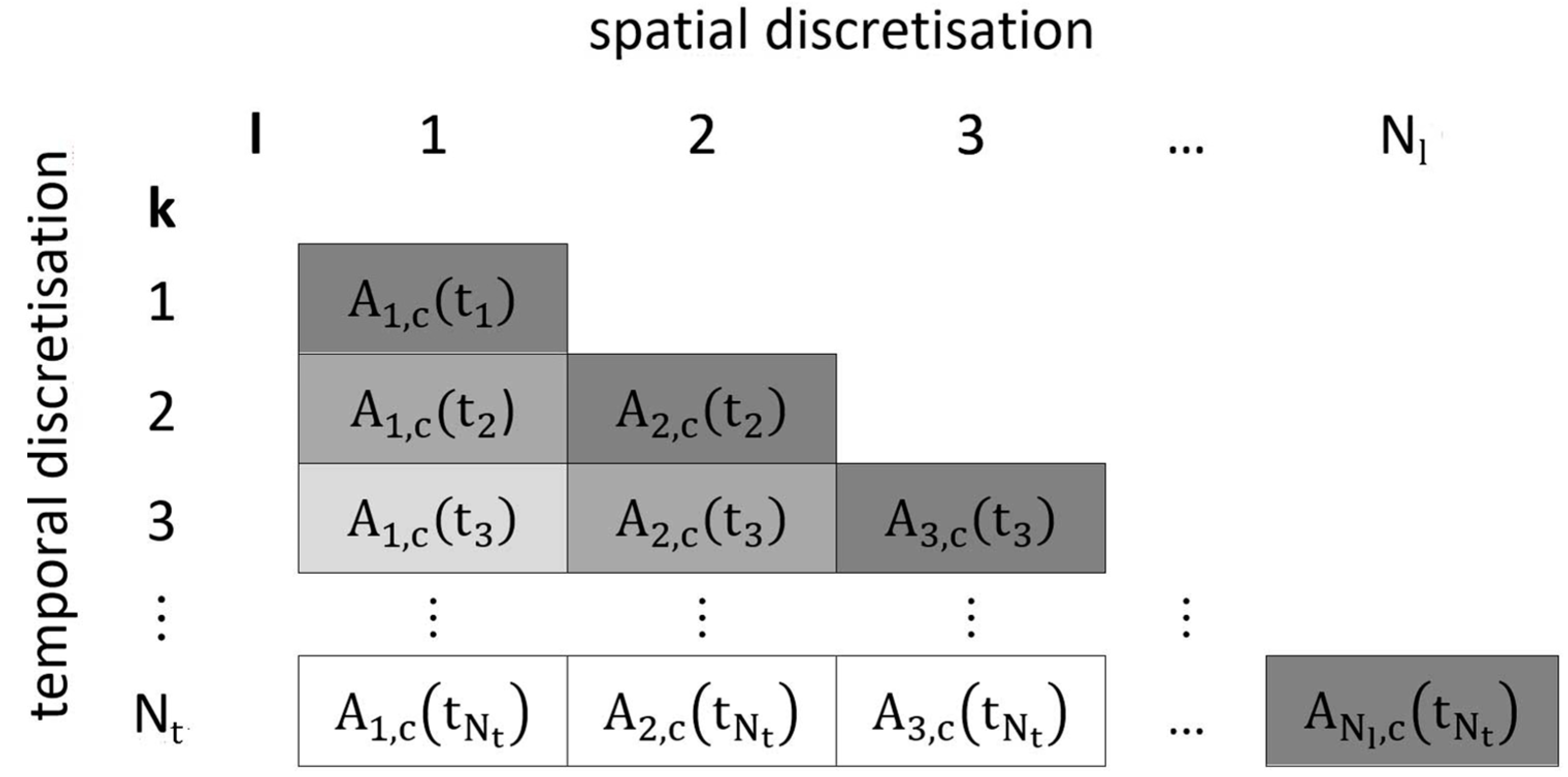
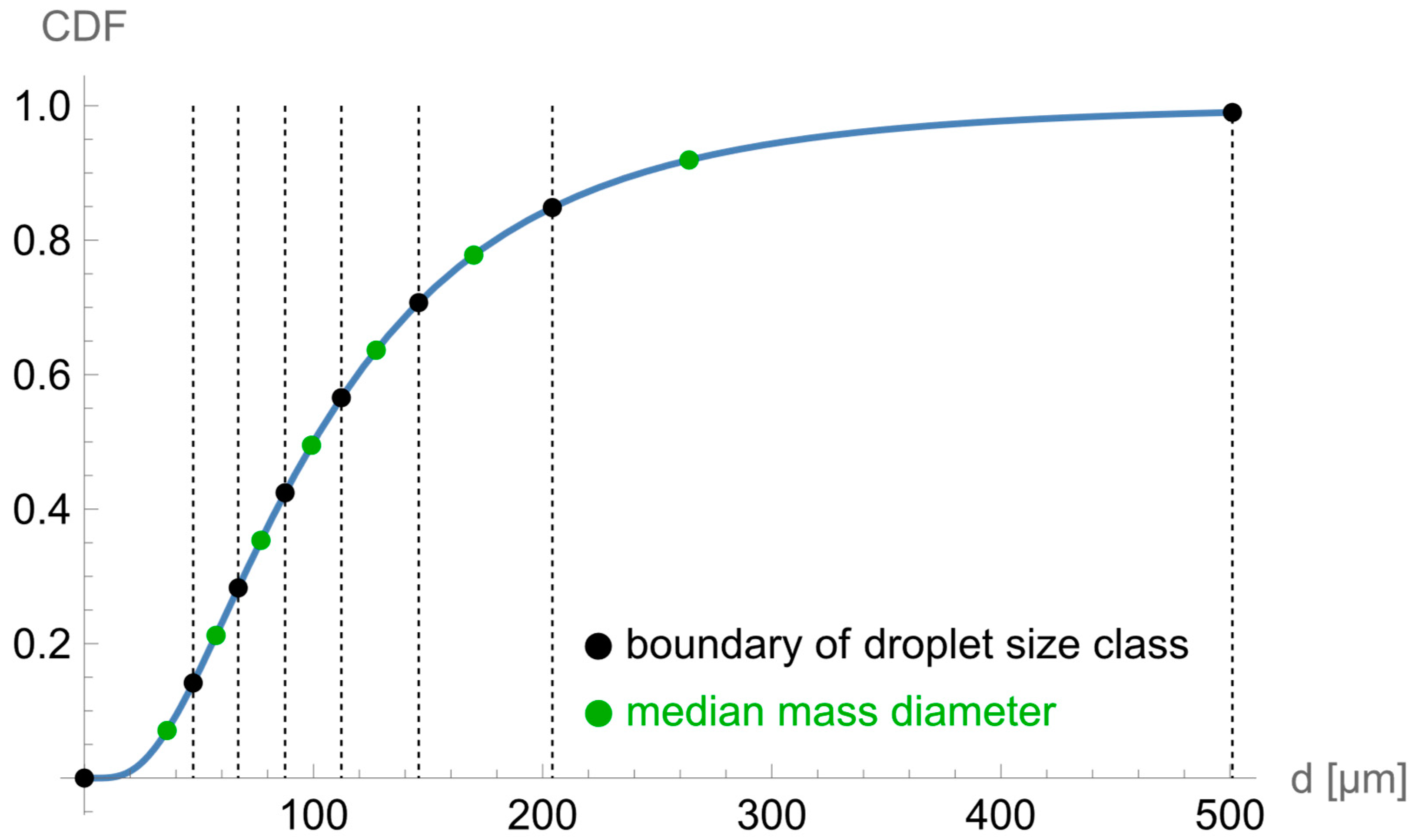
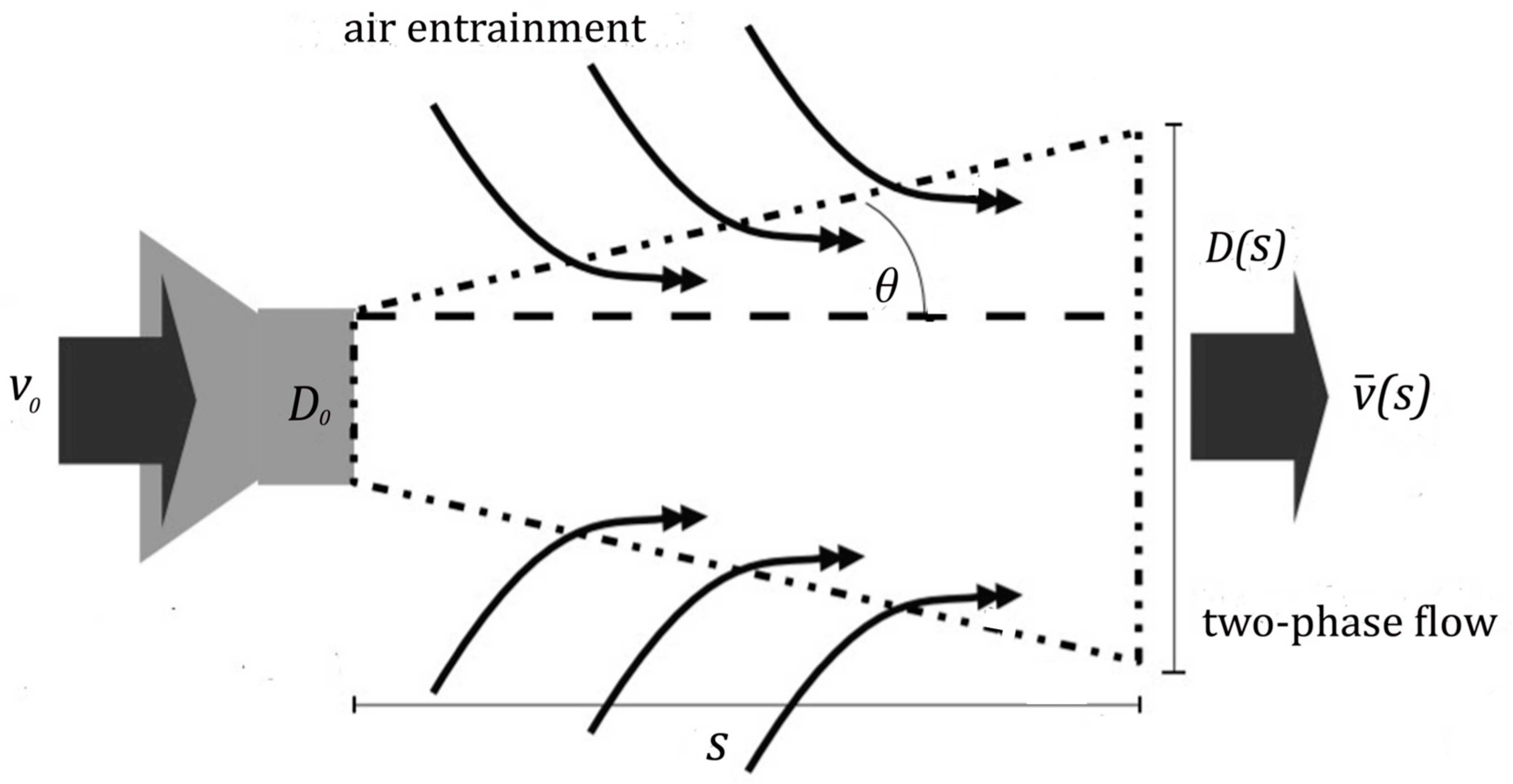
2.2. Discretisation Approach
2.3. Numerical Approximation Using Euler’s Method
2.4. Mass Loss Due to Ventilation
2.5. Mass Loss Due to Droplet Settling
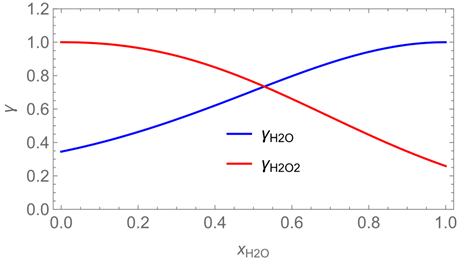
2.6. Evaporation of Two-Component Droplets
2.7. Evaporation from the Floor
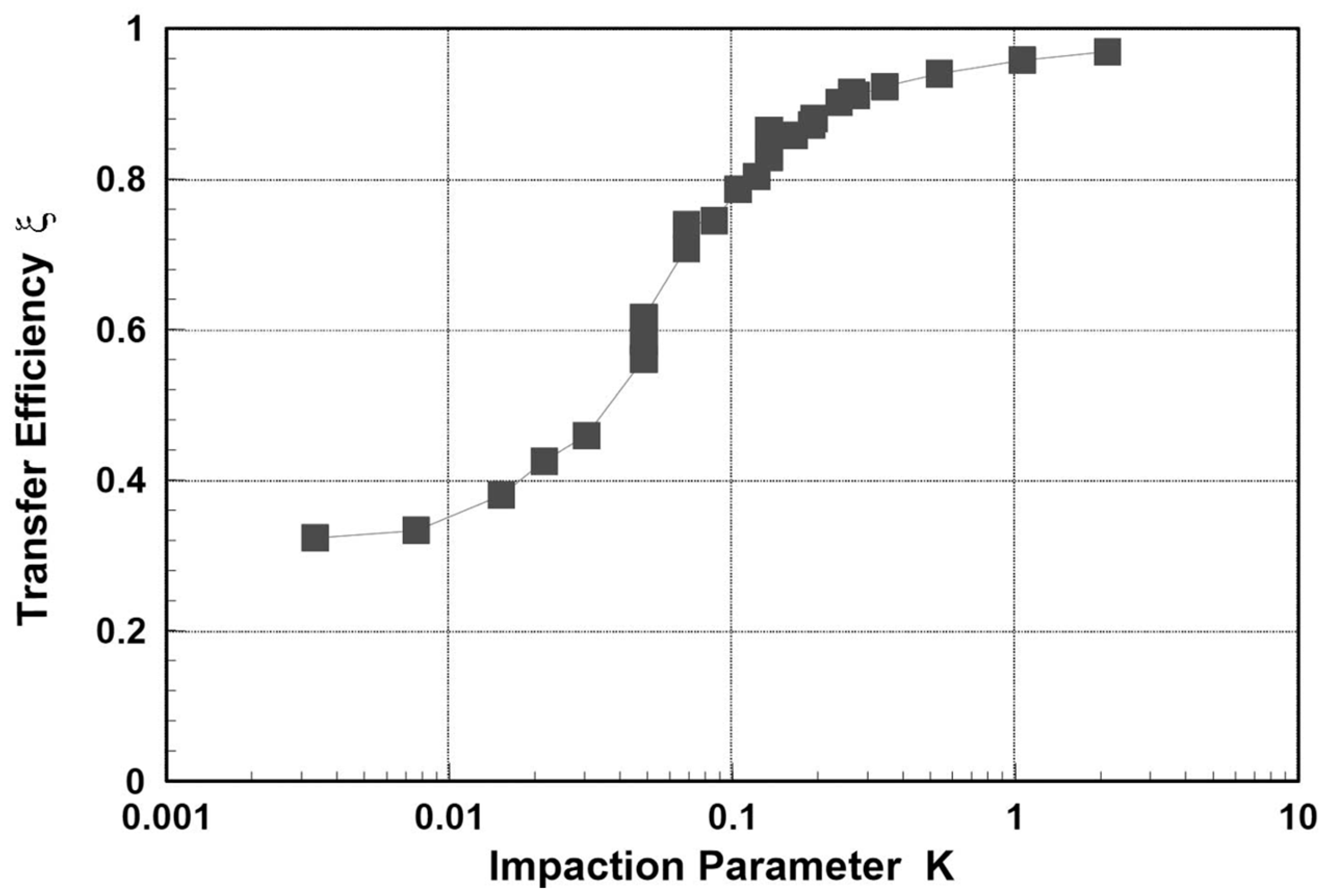
2.8. Impaction Module
- -
- The initial droplet velocity v0 is equal to the velocity of the liquid at the injector tip.
- -
- A full-cone turbulent round jet ensuing from a circular nozzle is assumed.
- -
- Air entrainment beyond the vicinity of the nozzle is considered.
- -
- The droplet size distribution is constant during the transport process.
- -
- The approach is restricted to medium volatile mixtures that should not exceed the vapour pressure of water.
2.9. Evaporation from Sprayed Walls
3. Results
3.1. Example Calculation
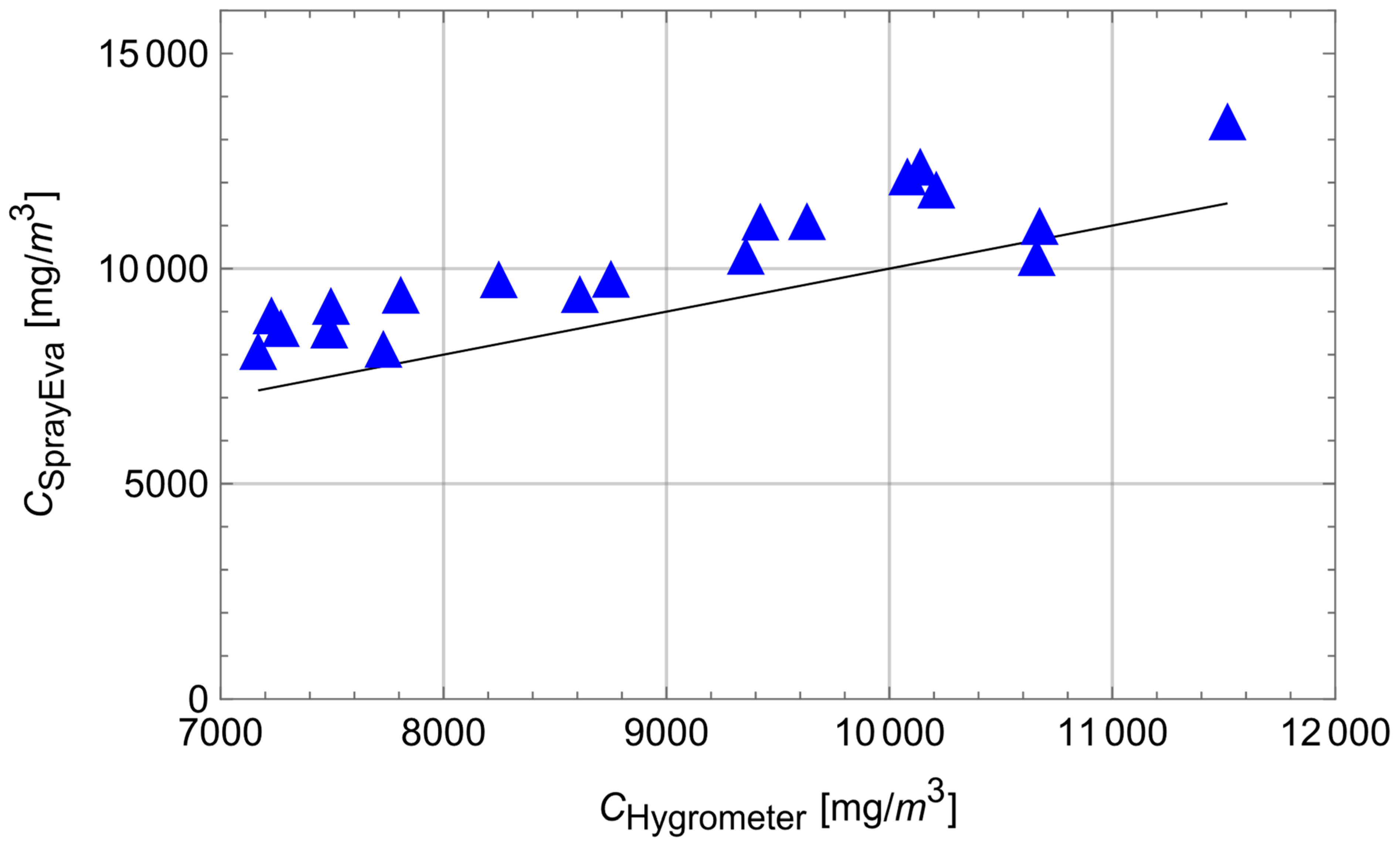
3.2. Comparison with Measured Data
- 5 min measurements prior to spraying;
- 7 min spraying (4 × 1 min spraying with 1 min interruption in between);
- 30 min follow-up measurements.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Indices: | |
| i | counting index for particular substances |
| l | counting index for particular release pulse |
| k | counting index for particular time steps |
| m | counting index for the application cycle |
| c | counting index for the droplet size class |
| room | parameters referring to the air space in the room in case of well-mixed-room models |
| inh | refers to the inhalable fraction |
| dr | parameters referring to droplet properties |
| pr | parameters referring to product properties |
| vent | parameters referring the air exchanged by ventilation |
| ev | parameters indicating an evaporating fraction |
| app | refers to the application duration |
| expo | refers to the total simulated exposure duration |
| init | initial value (for product amounts applied or air concentrations) |
| os | parameters referring to the overspray |
| sed | parameters referring to sedimentation |
| Parameters and Units: | |
| p | vapour pressure [Pa] |
| p* | vapour pressure of a pure substance [Pa] |
| ρ | density [kg/m3] |
| x | molar fraction |
| V | liquid molar volume [m3/mol] |
| γ | activity coefficient |
| W | wall surface area of the applied product [m2] |
| F | floor area [m2] |
| βi | mass transfer coefficient of component i [m/s] |
| R | ideal gas constant (8.3145 Pa m3 K−1 mol−1) |
| T | temperature [K] |
| V | volume of an air space [m3] |
| v | velocity [m/s] |
| g | gravitational acceleration [m/s2] |
| molecular diffusion coefficient in air of component i [m2/s] | |
| v | kinematic viscosity of air [m2/s] |
| η | dynamic viscosity of the air [kg/(m s)] |
| λ | stopping distance [m] |
| D | spray diameter [m] |
| ξ | transfer efficiency |
| Mi | molecular weight of component i [kg/mol] |
| Vi | molar volume of component i [m3/mol] |
| n | molar amount [mol] |
| m | mass [kg] |
| Wi | weight fraction of component i |
| SC | the initial surface coverage of the product [kg/m2] |
| rr | release rate of the sprayed product [kg/s] |
| A | aerosol concentration [kg/m3] |
| d | droplet diameter [m] |
| dm | mass median diameter [m] |
| GSD | geometric standard deviation |
| θ | opening angle of spray cone [grad] |
| s | distance nozzle wall [m] |
| C | vapour concentration [kg/m3] |
| RH | relative humidity [%] |
| t | time [s] |
| Q | air exchange rate [m3/s] |
| wr | work rate (rate at which the surface is covered with product) [m2/s] |
| Nt | number of time steps |
| NΔA | number of area elements |
| IM | integer multiples of Δt |
| Nc | number of droplet size classes |
| r | Pearson correlation coefficient |
| bias | absolute bias |
| rel.bias | relative bias [%] |
References
- Koch, W.; Berger-Preiß, E.; Boehncke, A.; Könnecker, G.; Mangelsdorf, I. Arbeitsplatzbelastungen bei der Verwendung von Biozidprodukten—Teil 1. Inhalative und Dermale Expositionsdaten für das Versprühen von Flüssigen Biozidprodukten; Bundesanstalt für Arbeitsschutz und Arbeitsmedizin: Dortmund, Germany, 2004. [Google Scholar]
- McNally, K.; Gorce, J.-P.; Goede, H.A.; Schinkel, J.; Warren, N. Calibration of the dermal advanced reach tool (dART) mechanistic model. Ann. Work Expo. Health 2019, 63, 637–650. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and the Council [EP]. Regulation (EU) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH); European Parliament and the Council: Strasbourg, France, 2006.
- European Parliament and the Council. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC); European Parliament and the Council: Strasbourg, France, 2009.
- European Parliament and the Council [EP]. Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 Concerning the Making Available on the Market and Use of Biocidal Products; European Parliament and the Council: Strasbourg, France, 2012.
- DIN EN 13936:2014-04; Exposition am Arbeitsplatz—Messung eines als Mischung aus luftgetragenen Partikeln und Dampf vorliegenden chemischen Arbeitsstoffes. Anforderungen und Prüfverfahren: Berlin, Germany, 2014.
- Hahn, S.; Meyer, J.; Roitzsch, M.; Delmaar, C.; Koch, W.; Schwarz, J.; Heiland, A.; Schendel, T.; Jung, C.; Schlüter, U. Modelling Exposure by Spraying Activities—Status and Future Needs. Int. J. Environ. Res. Public Health 2021, 18, 7737. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. TNsG Technical Notes for Guidance: Human Exposure to Biocidal Products—Guidance on Exposure Estimation; ECHA: Helsinki, Finland, 2002. [Google Scholar]
- European Chemicals Agency. Guidance on Information Requirements and Chemical Safety Assessment—Chapter R.14: Occupational Exposure Assessment; ECHA: Helsinki, Finland, 2016. [Google Scholar]
- Cresti, R.; Cabella, R.; Attias, L.; Rubbiani, M. Professional exposure to biocides: A comparison of human exposure models for surface disinfectants. Int. J. Environ. Health 2011, 5, 221–245. [Google Scholar] [CrossRef]
- Delmaar, J.E.; Park, M.V.D.Z.; van Engelen, J.G.M. ConsExpo 4.0 Consumer Exposure and Uptake Models Program Manual; RIVM Report 320104004/2005; RIVM: Utrecht, The Netherlands, 2005. [Google Scholar]
- Koch, W.; Behnke, W.; Berger-Preiß, E.; Kock, H.; Gerling, S.; Hahn, S.; Schröder, K. Validation of an EDP Assisted Model for Assessing Inhalation Exposure and Dermal Exposure during Spraying Processes; Bundesanstalt für Arbeitsschutz und Arbeitsmedizin: Dortmund, Germany, 2012. [Google Scholar]
- Tischer, M.; Roitzsch, M. Estimating Inhalation Exposure Resulting from Evaporation of Volatile Multicomponent Mixtures Using Different Modelling Approaches. Int. J. Environ. Res. Public Health 2022, 19, 1957. [Google Scholar] [CrossRef] [PubMed]
- Hinds, W.C. Aerosol Technology, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Dahmen, W.; Reusken, A. Numerik für Ingenieure und Naturwissenschaftler; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- ISO 7708:1995; Air Quality—Particle Size Fraction Definitions for Health-Related Sampling. International Standards Organisation: Geneva, Switzerland, 1995.
- Heinsohn, R.J. General Ventilation Well-Mixed Mode (Chapter 5). In Industrial Ventilation Engineering Principles; John Wiley & Sons, Inc.: New York, NY, USA, 1991. [Google Scholar]
- Keil, C.B.; Simmons, C.E.; Anthony, T. Mathematical Models for Estimating Occupational Exposure to Chemicals, 2nd ed.; American Industrial Hygiene Association: Fairfax, VA, USA, 2009. [Google Scholar]
- Arnold, S.F.; Shao, Y.; Ramachandran, G. Evaluation of the well mixed room and near-field far-field models in occupational settings. J. Occup. Environ. Hyg. 2017, 9, 694–702. [Google Scholar] [CrossRef]
- Baughmann, A.V.; Gadgil, A.; Nazaroff, W.W. Mixing of a Point Source Pollutant by Natural Convection Flow within a Room. Indoor Air 1994, 4, 114–122. [Google Scholar] [CrossRef]
- Abattan, S.F.; Lavoue, J.; Halle, S.; Bahloul Ali Drolet, D. Modeling occupational exposure to solvent vapors using the Two-Zone (near-field/far-field) model: A literature review. J. Occup. Environ. Hyg. 2021, 18, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Wilms, J. Evaporation of Multicomponent Droplets; Institut für Thermodynamik der Luft- und Raumfahrt Universität: München, Germany, 2005. [Google Scholar]
- Hubbard, G.L.; Denny, V.E.; Mills, A.F. Droplet evaporation: Effects of transient and variable properties. Int. J. Heat Mass Transfer. 1975, 18, 1003–1008. [Google Scholar] [CrossRef]
- Gmehling, J.; Weidlich, U.; Lehmann, E.; Fröhlich, N. Verfahren zur Berechnung von Luftkonzentrationen bei Freisetzung von Stoffen aus flüssigen Produktgemischen (Teil 1). Staub-Reinhaltung der Luft 1989, 49, 227–230. [Google Scholar]
- Weidlich, U.; Gmehling, J. Expositionsabschätzung–Ein Methodenvergleich mit Hinweisen für Die Praktische Anwendung; Schriftenreihe der Bundesanstalt für Arbeitsschutz, Fb 488: Dortmund, Germany, 1986. [Google Scholar]
- Pozorski, J.; Sashin, S.S.; Wachawczyk, M.; Crua, C.; Kennaird, D.; Heikal, M. Spray penetration in a turbulent flow. Flow Turbul. Combust. 2002, 68, 153–165. [Google Scholar] [CrossRef]
- Sazhin, S.S.; Feng, G.; Heikal, M.R. A model for fuel spray penetration. Fuel 2001, 80, 2171–2180. [Google Scholar] [CrossRef]
- Medrano, F.F.; Fukumoto, Y.; Velte, C.M.; Hodzic, A. Mass entrainment rate of an ideal momentum turbulent round jet. J. Phys. Soc. Jpn. 2017, 86, 3. [Google Scholar] [CrossRef]
- Su, K.; Yao, S.C. Numerical studies of sprays impacting normally on an infinite plate. In Proceedings of the 15th Brazillian Congress of Mechanical Engineering, Aguas de Lindoia, Sao Paulo, Brazil, 22–26 November 1999. [Google Scholar]
- ECHA, Regulation (EU) No 528/2012 concerning the making available on the market and use of biocidal products Product Assessment Report of A Biocidal Product (Family) for National Authorisation Applications (Submitted by the Evaluating Competent Authority) BELOX Product Types 2, 3, 4, 5 Hydrogen Peroxide as Included in the Union List of Approved Active Substances Case Number in R4BP: [BC-KC029711-56] Evaluating Competent Authority: SI Date: February 2020. Available online: https://echa.europa.eu/documents/10162/1e1085f8-5cd7-e878-d79b-3ccd18ed0996 (accessed on 16 July 2022).
- Koch, W.; Dunkhorst, W.; Lödding, H. Design and Performance of a New Personal Aerosol Monitor. Aerosol Sci. Technol. 1999, 31, 231. [Google Scholar] [CrossRef]
- Hornung, R.W. Statistical evaluation of exposure assessment strategies. Appl. Occup. Environ. Hyg. 1991, 6, 516–520. [Google Scholar] [CrossRef]
- Hofstetter, E.; Spencer, J.W.; Hiteshew, K.; Coutu, M.; Nealley, M. Evaluation of recommended REACH exposure modelling tools and near-field, far-field model in assessing occupational exposure to toluene from spray paint. Ann. Occup. Hyg. 2013, 57, 210–220. [Google Scholar] [PubMed]
- Spencer, J.W.; Plisko, M.J.; Occup, J. A Comparison Study Using a Mathematical Model and Actual Exposure Monitoring for Estimating Solvent Exposures During the Disassembly of Metal Parts. Environ. Hyg. 2007, 4, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Nicas, M.; Neuhaus, J. Predicting Benzene Vapor Concentrations with a Near Field/Far Field Model. J. Occup. Environ. Hyg. 2008, 5, 599–608. [Google Scholar] [CrossRef]
- Zhang, Y.; Banerjee, S.; Yang, R.; Lungu, C.; Ramachandran, G. Bayesian Modeling of Exposure and Airflow Using Two-Zone Models. Ann. Occup. Hyg. 2009, 53, 409–424. [Google Scholar]
- Ganser, G.H.; Hewett, P. Models for nearly every occasion: Part II—Two box models. J. Occup. Environ. Hyg. 2017, 14, 58–71. [Google Scholar] [CrossRef]
- Hewett, P.; Ganser, G.H. Models for nearly every occasion: Part I—One box models. J. Occup. Environ. Hyg. 2017, 14, 49–57. [Google Scholar] [CrossRef]
- Wolfram Research, Inc. Mathematica, Version 12.3; Wolfram Research: Champaign, IL, USA, 2020. [Google Scholar]

| Troom = 293 K | Tdr = 283.8 K |
| Vroom = 150 m3 | hroom = 2.5 m |
| Qvent = 0.125 m3/s | s = 0.4 m |
| νair = 0.5 m/s | = 0 mg/m3 |
| tapp = 300 s | texpo = 18,000 s |
| W = 20 m2 | SC = 0.06 kg/m2 |
| rr = 8 g/s | θ = 25 deg |
| dm = 250 µm (hollow cone) | GSD = 1.8 |
| D0 = 10−3 m | v0 = 12.7 m/s |
| p*H2O = 2308 Pa at 293 K | p*H2O2 = 179 Pa at 293 K |
| p*H2O =1276 Pa at 283.8 K | p*H2O2 = 88 Pa at 283.8 K |
| wpr,H2O = 0.926 | wpr,H2O2 = 0.074 |
| DH2O = 2.4 × 10−5 m2/s | MH2O=18 × 10−3 kg/mol |
| DH2O2 = 1.8 × 10−5 m2/s | MH2O2=34 × 10−3 kg/mol |
| βH2O = 2.4 × 10−3 m/s | βH2O2 = 2.2 × 10−3 m/s |
| η = 1.82 × 10−5 kg/(m∙s) | ν = 1.53 × 10−5 m2/s |
 | |
| № | H2O | H2O2 |
|---|---|---|
| 1 | 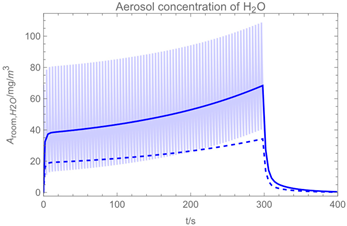 | 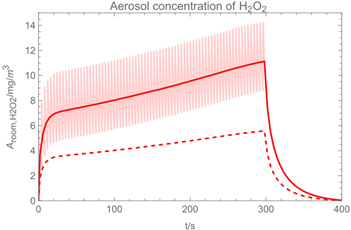 |
| 2 | 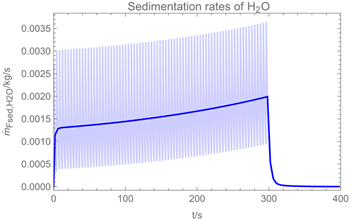 |  |
| 3 | 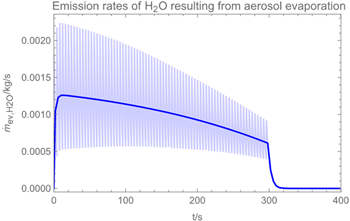 | 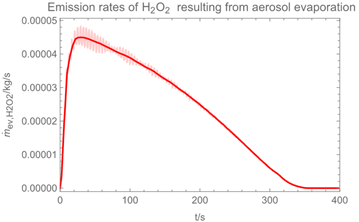 |
| 4 | 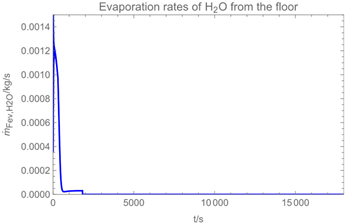 |  |
| 5 | 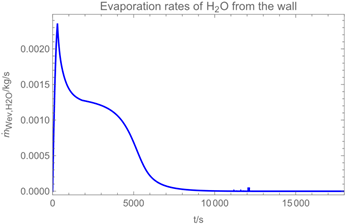 | 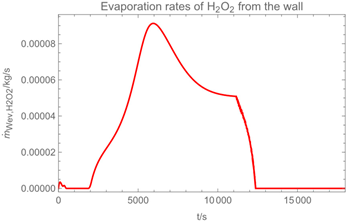 |
| 6 | 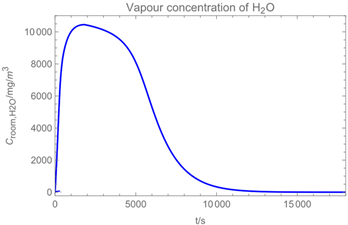 | 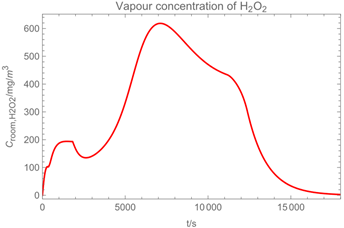 |
| Legend |  |  |
| Type of Nozzle | Release Rate | MMD | Temp. | Init. RH | Respicon | SprayEva | Hygrometer | SprayEva |
|---|---|---|---|---|---|---|---|---|
| [g/s] | [µm] | °C | % | Inhalable Fraction [mg/m3] | H2O Vapour [mg/m3] | |||
| Flat fan | 19.6 | 400 | 16.1 | 38 | 0.32 | 0.43 | 7227 | 9031 |
| Flat fan | 15.4 | 470 | 16.3 | 38 | 0.3 | 0.29 | 7729 | 8252 |
| Flat fan | 10.0 | 600 | 16.6 | 42 | 0.03 | 0.11 | 7168 | 8200 |
| Flat fan | 5.0 | 250 | 16.7 | 44 | 0.3 | 0.34 | 7494 | 9250 |
| Flat fan | 4.2 | 400 | 17.0 | 44 | 0.13 | 0.12 | 7487 | 8700 |
| Flat fan | 2.7 | 500 | 17.5 | 45 | 0.04 | 0.056 | 7269 | 8735 |
| Flat fan | 10.4 | 340 | 18.3 | 39 | 0.34 | 0,31 | 8247 | 9870 |
| Flat fan | 8.3 | 380 | 17.7 | 43 | 0.16 | 0.22 | 7807 | 9510 |
| Flat fan | 5.4 | 420 | 19.3 | 43 | 0.06 | 0.14 | 8750 | 9880 |
| Hollow cone | 14.2 | 280 | 18.0 | 43 | 0.71 | 0.58 | 9630 | 11,220 |
| Hollow cone | 11.3 | 300 | 17.9 | 40 | 0.45 | 0.42 | 9421 | 11,210 |
| Hollow cone | 6.0 | 290 | 18.1 | 40 | 0.26 | 0.28 | 8611 | 9525 |
| Hollow cone | 9.8 | 230 | 18.2 | 42 | 0.72 | 0.79 | 10,673 | 11,110 |
| Hollow cone | 8.1 | 270 | 17.7 | 45 | 0.4 | 0.41 | 10,660 | 10,385 |
| Hollow cone | 5.8 | 340 | 19.2 | 45 | 0.16 | 0.19 | 9356 | 10,415 |
| Cold fogger | 5.5 | 130 | 20.1 | 45 | 1.87 | 2.32 | 10,210 | 11,954 |
| Cold fogger | 13.3 | 140 | 19.0 | 40 | 3.39 | 4.69 | 11,516 | 13,540 |
| Cold fogger | 7.9 | 110 | 18.6 | 42 | 4.41 | 5.12 | 10,138 | 12,488 |
| Therm. fogger | 7.3 | 50 | 21.4 | 33 | 15.7 | 17.1 | 10,080 | 12,261 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tischer, M.; Meyer, J. A New Model Algorithm for Estimating the Inhalation Exposure Resulting from the Spraying of (Semi)-Volatile Binary Liquid Mixtures (SprayEva). Int. J. Environ. Res. Public Health 2022, 19, 13182. https://doi.org/10.3390/ijerph192013182
Tischer M, Meyer J. A New Model Algorithm for Estimating the Inhalation Exposure Resulting from the Spraying of (Semi)-Volatile Binary Liquid Mixtures (SprayEva). International Journal of Environmental Research and Public Health. 2022; 19(20):13182. https://doi.org/10.3390/ijerph192013182
Chicago/Turabian StyleTischer, Martin, and Jessica Meyer. 2022. "A New Model Algorithm for Estimating the Inhalation Exposure Resulting from the Spraying of (Semi)-Volatile Binary Liquid Mixtures (SprayEva)" International Journal of Environmental Research and Public Health 19, no. 20: 13182. https://doi.org/10.3390/ijerph192013182
APA StyleTischer, M., & Meyer, J. (2022). A New Model Algorithm for Estimating the Inhalation Exposure Resulting from the Spraying of (Semi)-Volatile Binary Liquid Mixtures (SprayEva). International Journal of Environmental Research and Public Health, 19(20), 13182. https://doi.org/10.3390/ijerph192013182







