Persistent Depressive Disorder-Related Effect of Sleep Disorder on the Highest Risk of Suicide in Taiwan, 2000–2015
Abstract
1. Introduction
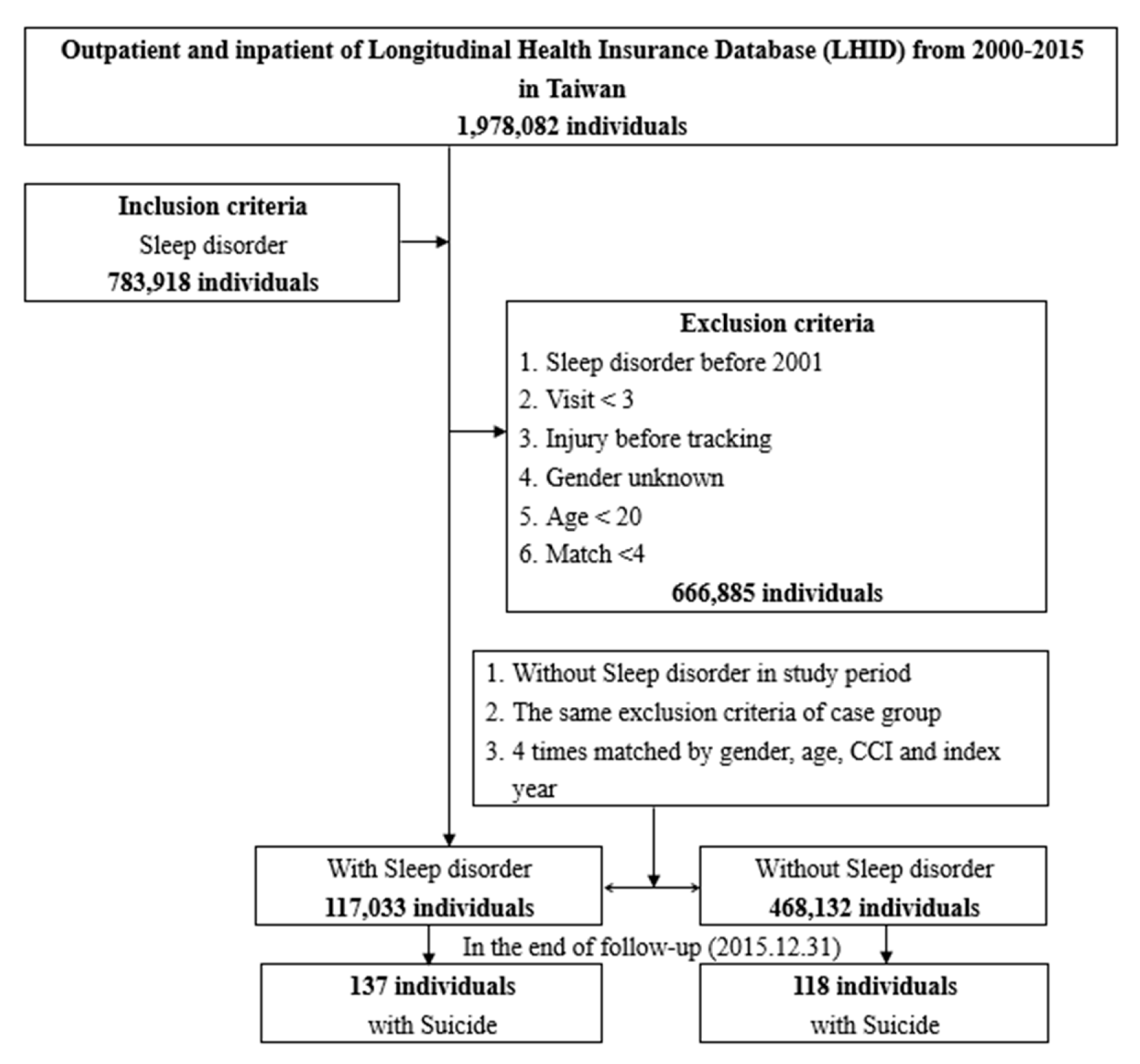
2. Method
2.1. Data Sources
2.2. Study Design
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Patients in the Study
3.2. Factors of Suicide at the End of Follow-Up Using Cox Regression
3.3. Comparison of the Risk of Suicide between the Sleep Disorder and Control Cohorts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chattu, V.K.; Manzar, M.D.; Kumary, S.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. The global problem of insufficient sleep and its serious public health implications. Healthcare 2018, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.C.; Fraser, K.; Rumana, N.; Abdullah, A.F.; Shahana, N.; Hanly, P.J.; Turin, T.C. Sleep disturbances among medical students: A global perspective. J. Clin. Sleep Med. 2015, 11, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.A.; Huecker, M.R. Sleep Deprivation. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547676/ (accessed on 19 July 2022).
- St-Onge, M.P.; Grandner, M.A.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L.; American Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; et al. Sleep duration and quality: Impact on lifestyle behaviors and cardiometabolic health: A scientific statement from the American Heart Association. Circulation 2016, 134, e367–e386. [Google Scholar] [CrossRef] [PubMed]
- Hershner, S.D.; Chervin, R.D. Causes and consequences of sleepiness among college students. Nat. Sci. Sleep 2014, 6, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Palatty, P.L.; Fernandes, E.; Suresh, S.; Baliga, M.S. Comparison of sleep pattern between medical and law students. Sleep Hypn. 2011, 13, 15–18. [Google Scholar]
- Praharaj, S.K.; Gupta, R.; Gaur, N. Clinical practice guideline on management of sleep disorders in the elderly. Indian J. Psychiatry 2018, 60 (Suppl. 3), S383–S396. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, E.C.; Cho, W.H.; Park, C.Y.; Choi, W.J.; Chang, H.S. Association between total sleep duration and suicidal ideation among the Korean general adult population. Sleep 2013, 36, 1563–1572, Erratum in Sleep 2013, 36, 2003. [Google Scholar] [CrossRef]
- World Health Organization. 2018. Available online: https://www.who.int/mental_health/prevention/suicide/suicideprevent/en/ (accessed on 13 July 2022).
- Karch, D.L.; Dahlberg, L.L.; Patel, N. Surveillance for violent deaths--National Violent Death Reporting System, 16 States, 2007. MMWR Surveill. Summ. 2010, 59, 1–50. [Google Scholar] [PubMed]
- Dodds, T.J. Prescribed benzodiazepines and suicide risk: A review of the literature. Prim. Care Companion CNS Disord. 2017, 19, 16r02037. [Google Scholar] [CrossRef] [PubMed]
- Bottino, S.M.; Bottino, C.M.; Regina, C.G.; Correia, A.V.; Ribeiro, W.S. Cyberbullying and adolescent mental health: Systematic review. Cad. Saude Publica 2015, 31, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Zalsman, G.; Hawton, K.; Wasserman, D.; van Heeringen, K.; Arensman, E.; Sarchiapone, M.; Carli, V.; Höschl, C.; Barzilay, R.; Balazs, J.; et al. Suicide prevention strategies revisited: 10-year systematic review. Lancet Psychiatry 2016, 3, 646–659. [Google Scholar] [CrossRef]
- Brådvik, L. Suicide risk and mental disorders. Int. J. Environ. Res. Public Health 2018, 15, 2028. [Google Scholar] [CrossRef] [PubMed]
- Young, K.S.; Sandman, C.F.; Craske, M.G. Positive and negative emotion regulation in adolescence: Links to anxiety and depression. Brain Sci. 2019, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- De Berardis, D.; Fornaro, M.; Orsolini, L.; Ventriglio, A.; Vellante, F.; Di Giannantonio, M. Emotional dysregulation in adolescents: Implications for the development of severe psychiatric disorders, substance abuse, and suicidal ideation and behaviors. Brain Sci. 2020, 10, 591. [Google Scholar] [CrossRef] [PubMed]
- Orsolini, L.; Latini, R.; Pompili, M.; Serafini, G.; Volpe, U.; Vellante, F.; Fornaro, M.; Valchera, A.; Tomasetti, C.; Fraticelli, S.; et al. Understanding the complex of suicide in depression: From research to clinics. Psychiatry Investig. 2020, 17, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Bilsen, J. Suicide and youth: Risk factors. Front. Psychiatry 2018, 9, 540. [Google Scholar] [CrossRef] [PubMed]
- Hawton, K.; Casañas, I.; Comabella, C.; Haw, C.; Saunders, K. Risk factors for suicide in individuals with depression: A systematic review. J. Affect. Disord. 2013, 147, 17–28. [Google Scholar] [CrossRef]
- Tsuno, N.; Besset, A.; Ritchie, K. Sleep and depression. J. Clin. Psychiatry 2005, 66, 1254–1269. [Google Scholar] [CrossRef]
- Perlis, M.L.; Giles, D.E.; Buysse, D.J.; Thase, M.E.; Tu, X.; Kupfer, D.J. Which depressive symptoms are related to which sleep electroencephalographic variables? Biol. Psychiatry 1997, 42, 904–913. [Google Scholar] [CrossRef]
- Hamilton, M. Frequency of symptoms in melancholia (depressive illness). Br. J. Psychiatry 1989, 154, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Franzen, P.L.; Buysse, D.J. Sleep disturbances and depression: Risk relationships for subsequent depression and therapeutic implications. Dialogues Clin. Neurosci. 2008, 10, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Kanwar, A.; Sim, L.A.; Prokop, L.J.; Wang, Z.; Benkhadra, K.; Murad, M.H. The association between sleep disturbances and suicidal behaviors in patients with psychiatric diagnoses: A systematic review and meta-analysis. Syst. Rev. 2014, 3, 18. [Google Scholar] [CrossRef]
- Porras-Segovia, A.; Perez-Rodriguez, M.M.; López-Esteban, P.; Courtet, P.; López-Castromán, J.; Cervilla, J.A.; Baca-García, E. Contribution of sleep deprivation to suicidal behaviour: A systematic review. Sleep Med. Rev. 2019, 44, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Jieyu, L.; Yongtai, H.; Yili, Z.; Yiru, C.; Wenshun, W.; Jixin, L.; Gengyi, L. A study on the application of development types of townships and urban areas in Taiwan to the sampling design of large-scale health surveys. J. Health Manag. 2006, 4, 1–22. [Google Scholar]
- Public Assistance Act, Article 4 (30 December 2015). Available online: http://law.moj.gov.tw/Eng/LawClass/LawAll.aspx?PCode=D0050078 (accessed on 22 December 2017).
- Nutt, D.; Wilson, S.; Paterson, L. Sleep disorders as core symptoms of depression. Dialogues Clin. Neurosci. 2022, 10, 329–336. [Google Scholar] [CrossRef]
- Fang, H.; Tu, S.; Sheng, J.; Shao, A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J. Cell. Mol. Med. 2019, 23, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Stickley, A.; Leinsalu, M.; DeVylder, J.E.; Inoue, Y.; Koyanagi, A. Sleep problems and depression among 237 023 community-dwelling adults in 46 low- and middle-income countries. Sci. Rep. 2019, 9, 12011. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Z.; Li, H.; Ding, K. Excessive daytime sleepiness in depression and obstructive sleep apnea: More than just an overlapping symptom. Front. Psychiatry 2021, 12, 710435. [Google Scholar] [CrossRef]
- Thase, M.E. Depression and sleep: Pathophysiology and treatment. Dialogues Clin. Neurosci. 2022, 8, 217–226. [Google Scholar] [CrossRef]
- World Health Organization. Depression. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 8 August 2022).
- Dumais, A.; Lesage, A.D.; Alda, M.; Rouleau, G.; Dumont, M.; Chawky, N.; Roy, M.; Mann, J.J.; Benkelfat, C.; Turecki, G. Risk factors for suicide completion in major depression: A case-control study of impulsive and aggressive behaviors in men. Am. J. Psychiatry 2005, 162, 2116–2124. [Google Scholar] [CrossRef]
- Kim, C.; Lesage, A.; Seguin, M.; Lipp, O.; Vanier, C.; Turecki, G. Patterns of comorbidity in male suicide completers. Psychol. Med. 2003, 33, 1299–1309. [Google Scholar] [CrossRef]
- King, T.L.; Shields, M.; Sojo, V.; Daraganova, G.; Currier, D.; O’Neil, A.; King, K.; Milner, A. Expressions of masculinity and associations with suicidal ideation among young males. BMC Psychiatry 2020, 20, 228. [Google Scholar] [CrossRef]
- Kielan, A.; Jaworski, M.; Mosiołek, A.; Chodkiewicz, J.; Święcicki, Ł.; Walewska-Zielecka, B. Factors related to the level of depression and suicidal behavior among men with diagnosed depression, physically ill men, and healthy men. Front. Psychiatry 2021, 12, 644097. [Google Scholar] [CrossRef]
- Orzechowska, A.; Zajaczkowska, M.; Talarowska, M.; Gałecki, P. Depression and ways of coping with stress: A preliminary study. Med. Sci. Monit. 2013, 19, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, S.; Xu, H. Systematic review and meta-analysis of the relationship between sleep disorders and suicidal behaviour in patients with depression. BMC Psychiatry 2019, 19, 303. [Google Scholar] [CrossRef]
- Agargun, M.Y.; Kara, H.; Solmaz, M. Subjective sleep quality and suicidality in patients with major depression. J. Psychiatr. Res. 1997, 31, 377–381. [Google Scholar] [CrossRef]
- Staner, L.; Kempenaers, C.; Simonnet, M.P.; Fransolet, L.; Mendlewicz, J. 5-HT2 receptor antagonism and slow-wave sleep in major depression. Acta Psychiatr. Scand. 1992, 86, 133–137. [Google Scholar] [CrossRef]
- Fau, J.M.; Touret, M. Indolamines and sleep: 5-HT or 5-HTP? Schweiz. Rundsch. Fur Med. Prax. 1988, 77, 6–9. [Google Scholar]
- Benson, K.L.; Faull, K.F.; Zarcone, V.P., Jr. The effects of age and serotonergic activity on slow-wave sleep in depressive illness. Biol. Psychiatry 1993, 33, 842–844. [Google Scholar] [CrossRef]
- Solomon, R.A.; Sharpley, A.L.; Cowen, P.J. Increased slow wave sleep with 5-HT2 receptor antagonists: Detection by ambulatory EEG recording and automatic sleep stage analysis. J. Psychopharmacol. 1989, 3, 125–129. [Google Scholar] [CrossRef]
- Linnoila, V.M.; Virkkunen, M. Aggression, suicidality, and serotonin. J. Clin. Psychiatry 1992, 53, 46–51. [Google Scholar] [PubMed]
- McCall, W.V.; Blocker, J.N.; D’Agostino, R., Jr.; Kimball, J.; Boggs, N.; Lasater, B.; Rosenquist, P.B. Insomnia severity is an indicator of suicidal ideation during a depression clinical trial. Sleep Med. 2010, 11, 822–827. [Google Scholar] [CrossRef]
- Riemann, D.; Krone, L.B.; Wulff, K.; Nissen, C. Sleep, insomnia, and depression. Neuropsychopharmacology 2020, 45, 74–89. [Google Scholar] [CrossRef]
- Bernert, R.A.; Luckenbaugh, D.A.; Duncan, W.C.; Iwata, N.G.; Ballard, E.D.; Zarate, C.A. Sleep architecture parameters as a putative biomarker of suicidal ideation in treatment-resistant depression. J. Affect. Disord. 2017, 208, 309–315. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Araujo, J.F. Sleep disorders and suicidal ideation in patients with depressive disorder. Psychiatry Res. 2007, 153, 131–136. [Google Scholar] [CrossRef]
- Agargun, M.Y.; Kara, H.; Solinaz, M. Sleep disturbances and suicidal behavior in patients with major depression. J. Clin. Psychiatry 1997, 58, 249–251. [Google Scholar]
- McIntyre, R.S.; O’Donovan, C. The human cost of not achieving full remission in depression. Can. J. Psychiatry 2004, 49, 10–16. [Google Scholar]
- Huang, Y.C.; Yu, C.P.; Wang, B.L.; Chung, R.J.; Lin, I.J.; Chung, C.H.; Sun, C.A.; Yu, P.C.; Huang, S.H.; Chien, W.C.; et al. Trend distribution of violent injuries in Taiwan from 2000 to 2015. Int. J. Environ. Res. Public Health 2022, 19, 7874. [Google Scholar] [CrossRef]
| Sleep Disorder | Total | With | Without | p-Value | |||
|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | |
| Total | 585,165 | 100 | 117,033 | 20.0 | 468,132 | 80.0 | |
| Gender | >0.999 | ||||||
| Female | 302,935 | 51.8 | 60,587 | 51.8 | 242,348 | 51.8 | |
| Male | 282,230 | 48.2 | 56,446 | 48.2 | 225,784 | 48.2 | |
| Age (mean ± SD, year) | 53.34 ± 15.74 | 53.43 ± 15.9 | 53.32 ± 15.69 | 0.089 | |||
| CCI | 1.64 ± 2.25 | 1.97 ± 2.41 | 1.56 ± 2.2 | <0.0001 | |||
| Age group (years) | 0.592 | ||||||
| 20–39 | 128,943 | 22.0 | 25888 | 22.1 | 103055 | 22.0 | |
| 40–64 | 323,232 | 55.2 | 64309 | 54.9 | 258923 | 55.3 | |
| ≥65 | 132,990 | 22.7 | 26836 | 22.9 | 106154 | 22.7 | |
| Low income | <0.0001 | ||||||
| Without | 580,429 | 99.2 | 115,740 | 98.9 | 464,689 | 99.3 | |
| With | 4736 | 0.8 | 1293 | 1.1 | 3443 | 0.7 | |
| Diabetes mellitus | <0.0001 | ||||||
| Without | 455,183 | 77.8 | 87,578 | 74.8 | 367,605 | 78.5 | |
| With | 129,982 | 22.2 | 29,455 | 25.2 | 100,527 | 21.5 | |
| Hypertension | <0.0001 | ||||||
| Without | 374,320 | 64.0 | 67,851 | 58.0 | 306,469 | 65.5 | |
| With | 210,845 | 36.0 | 49,182 | 42.0 | 161,663 | 34.5 | |
| Persistent depressive disorder | <0.0001 | ||||||
| Without | 536,620 | 91.7 | 89,743 | 76.7 | 446,877 | 95.5 | |
| With | 48,545 | 8.3 | 27,290 | 23.3 | 21,255 | 4.5 | |
| Chronic kidney disease | <0.0001 | ||||||
| Without | 555,977 | 95.0 | 110,516 | 94.4 | 445,461 | 95.2 | |
| With | 29,188 | 5.0 | 6517 | 5.6 | 22,671 | 4.8 | |
| Heart failure | <0.0001 | ||||||
| Without | 546,859 | 93.5 | 108,065 | 92.3 | 438,794 | 93.7 | |
| With | 38,306 | 6.5 | 8968 | 7.7 | 29,338 | 6.3 | |
| Chronic obstructive pulmonary disease and allied conditions | <0.0001 | ||||||
| Without | 377,009 | 64.4 | 67,670 | 57.8 | 309,339 | 66.1 | |
| With | 208,156 | 35.6 | 49,363 | 42.2 | 158,793 | 33.9 | |
| Disorders of lipoid metabolism | <0.0001 | ||||||
| Without | 392,131 | 67.0 | 70,870 | 60.6 | 321,261 | 68.6 | |
| With | 193,034 | 33.0 | 46,163 | 39.4 | 146,871 | 31.4 | |
| Diffuse diseases of connective tissue | <0.0001 | ||||||
| Without | 560,708 | 95.8 | 109,822 | 93.8 | 450,886 | 96.3 | |
| With | 24,457 | 4.2 | 7211 | 6.2 | 17,246 | 3.7 | |
| Season | 0.017 | ||||||
| Spring (3–5) | 14,820 | 2.5 | 2939 | 2.5 | 11,881 | 2.5 | |
| Summer (6–8) | 14,475 | 2.5 | 2732 | 2.3 | 11,743 | 2.5 | |
| Autumn (9–11) | 14,360 | 2.5 | 2832 | 2.4 | 11,528 | 2.5 | |
| Winter (12–2) | 541,510 | 92.5 | 108,530 | 92.7 | 432,980 | 92.5 | |
| Location | 0.004 | ||||||
| Northern Taiwan | 306,365 | 52.4 | 61,408 | 52.5 | 244,957 | 52.3 | |
| Middle Taiwan | 95,835 | 16.4 | 23,692 | 20.2 | 72,143 | 15.4 | |
| Southern Taiwan | 166,792 | 28.5 | 29,151 | 24.9 | 137,641 | 29.4 | |
| Eastern Taiwan | 12,756 | 2.2 | 2111 | 1.8 | 10,645 | 2.3 | |
| Missing data | 3417 | 0.6 | 671 | 0.6 | 2746 | 0.6 | |
| Urbanization level | 0.086 | ||||||
| 1 (Highest) | 175,076 | 29.9 | 35,910 | 30.7 | 139,166 | 29.7 | |
| 2 (Second) | 185,234 | 31.7 | 37,643 | 32.2 | 147,591 | 31.5 | |
| 3 (Third) | 172,603 | 29.5 | 34,492 | 29.5 | 138,111 | 29.5 | |
| 4 (Lowest) | 48,096 | 8.2 | 8161 | 7.0 | 39,935 | 8.5 | |
| Missing data | 4156 | 0.7 | 827 | 0.7 | 3329 | 0.7 | |
| Level of care | <0.0001 | ||||||
| Medical center | 53,116 | 9.1 | 13,597 | 11.6 | 39,519 | 8.4 | |
| Regional hospital | 70,499 | 12.0 | 17,235 | 14.7 | 53,264 | 11.4 | |
| Local hospital | 68,124 | 11.6 | 15,135 | 12.9 | 52,989 | 11.3 | |
| Clinic | 393,426 | 67.2 | 71,066 | 60.7 | 322,360 | 68.9 | |
| Variables | Adjusted HR | 95% CI | p-Value |
|---|---|---|---|
| Sleep disorder | |||
| Without | Reference | ||
| With | 1.429 | 1.073–1.905 | 0.015 |
| Gender | |||
| Female | Reference | ||
| Male | 1.297 | 1.001–1.681 | 0.049 |
| Age (years) | 0.946 | 0.935–0.957 | <0.0001 |
| CCI | 0.906 | 0.839–0.979 | 0.012 |
| Low income | |||
| Without | Reference | ||
| With | 0.809 | 0.257–2.543 | 0.717 |
| Diabetes mellitus | |||
| Without | Reference | ||
| With | 1.114 | 0.784–1.583 | 0.546 |
| Hypertension | |||
| Without | Reference | ||
| With | 1.123 | 0.809–1.558 | 0.489 |
| Persistent depressive disorder | |||
| Without | Reference | ||
| With | 7.195 | 5.378–9.626 | <0.0001 |
| Chronic kidney disease | |||
| Without | Reference | ||
| With | 1.016 | 0.559–1.845 | 0.960 |
| Heart failure | |||
| Without | Reference | ||
| With | 1.536 | 0.949–2.486 | 0.080 |
| Chronic obstructive pulmonary disease and allied conditions | |||
| Without | Reference | ||
| With | 1.064 | 0.811–1.396 | 0.653 |
| Disorders of lipoid metabolism | |||
| Without | Reference | ||
| With | 0.735 | 0.538–1.003 | 0.053 |
| Diffuse diseases of connective tissue | |||
| Without | Reference | ||
| With | 0.793 | 0.460–1.367 | 0.403 |
| Season | |||
| Spring (3–5) | Reference | ||
| Summer (6–8) | 0.855 | 0.426–1.715 | 0.659 |
| Autumn (9–11) | 0.902 | 0.455–1.787 | 0.7668 |
| Winter (12–2) | 0.063 | 0.036–0.108 | <0.0001 |
| Location | Had multicollinearity with urbanization level | ||
| Northern Taiwan | Had multicollinearity with urbanization level | ||
| Middle Taiwan | Had multicollinearity with urbanization level | ||
| Southern Taiwan | Had multicollinearity with urbanization level | ||
| Eastern Taiwan | Had multicollinearity with urbanization level | ||
| Urbanization level | |||
| 1 (Highest) | Reference | ||
| 2 (Second) | 0.872 | 0.634–1.201 | 0.402 |
| 3 (Third) | 0.944 | 0.685–1.303 | 0.728 |
| 4 (Lowest) | 1.351 | 0.875–2.087 | 0.175 |
| Hospital level | |||
| Medical center | 15.208 | 9.027–25.623 | <0.0001 |
| Regional hospital | 21.651 | 13.306–35.229 | <0.0001 |
| Local hospital | 23.282 | 14.230–38.091 | <0.0001 |
| Clinic | Reference | ||
| Sleep Disorder | Adjusted HR | 95%CI | p-Value |
|---|---|---|---|
| Variables | |||
| Total | 1.429 | 1.073–1.905 | 0.015 |
| Gender | |||
| Female | 1.172 | 0.814–1.686 | 0.394 |
| Male | 2.104 | 1.330–3.329 | 0.002 |
| Age group (years) | |||
| 20–39 | 1.053 | 0.620–1.789 | 0.849 |
| 40–64 | 1.924 | 1.293–2.862 | 0.001 |
| ≥65 | 1.211 | 0.626–2.343 | 0.569 |
| Low income | |||
| Without | 1.484 | 1.112–1.979 | 0.007 |
| With | 0.000 | 1.000 | |
| Diabetes mellitus | |||
| Without | 1.363 | 0.978–1.900 | 0.067 |
| With | 1.868 | 1.053–3.312 | 0.033 |
| Hypertension | |||
| Without | 1.655 | 1.140–2.401 | 0.008 |
| With | 1.250 | 0.797–1.961 | 0.330 |
| Persistent depressive disorder | |||
| Without | 1.174 | 0.820–1.680 | 0.382 |
| With | 2.050 | 1.350–3.112 | 0.001 |
| Chronic kidney disease | |||
| Without | 1.483 | 1.104–1.993 | 0.009 |
| With | 1.299 | 0.379–4.451 | 0.677 |
| Heart failure | |||
| Without | 1.447 | 1.068–1.959 | 0.017 |
| With | 1.686 | 0.717–3.967 | 0.231 |
| Chronic obstructive pulmonary disease and allied conditions | |||
| Without | 1.769 | 1.207–2.592 | 0.004 |
| With | 1.215 | 0.793–1.861 | 0.371 |
| Disorders of lipoid metabolism | |||
| Without | 1.715 | 1.207–2.437 | 0.003 |
| With | 1.145 | 0.702–1.867 | 0.587 |
| Diffuse diseases of connective tissue | |||
| Without | 1.526 | 1.137–2.046 | 0.005 |
| With | 1.022 | 0.284–3.677 | 0.974 |
| Season | |||
| Spring (3–5) | 1.061 | 0.374–3.007 | 0.912 |
| Summer (6–8) | 2.614 | 0.834–8.196 | 0.099 |
| Autumn (9–11) | 7.259 | 1.824–28.884 | 0.005 |
| Winter (12–2) | 1.244 | 0.903–1.713 | 0.183 |
| Urbanization level | |||
| 1 (Highest) | 1.502 | 0.900–2.505 | 0.119 |
| 2 (Second) | 1.085 | 0.632–1.862 | 0.767 |
| 3 (Third) | 2.071 | 1.219–3.518 | 0.007 |
| 4 (Lowest) | 1.255 | 0.531–2.968 | 0.604 |
| Hospital level | |||
| Medical center | 1.372 | 0.731–2.575 | 0.324 |
| Regional hospital | 1.463 | 0.927–2.308 | 0.102 |
| Local hospital | 1.371 | 0.820–2.290 | 0.229 |
| Clinic | 3.319 | 1.160–9.498 | 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, S.-H.; Cheng, C.-C.; Lin, I.-J.; Yu, C.-P.; Huang, Y.-C.; Huang, S.-H.; Sun, C.-A.; Fann, L.-Y.; Sheu, M.-Y.; Chien, W.-C. Persistent Depressive Disorder-Related Effect of Sleep Disorder on the Highest Risk of Suicide in Taiwan, 2000–2015. Int. J. Environ. Res. Public Health 2022, 19, 13169. https://doi.org/10.3390/ijerph192013169
Hsiao S-H, Cheng C-C, Lin I-J, Yu C-P, Huang Y-C, Huang S-H, Sun C-A, Fann L-Y, Sheu M-Y, Chien W-C. Persistent Depressive Disorder-Related Effect of Sleep Disorder on the Highest Risk of Suicide in Taiwan, 2000–2015. International Journal of Environmental Research and Public Health. 2022; 19(20):13169. https://doi.org/10.3390/ijerph192013169
Chicago/Turabian StyleHsiao, Sheng-Huang, Chih-Chien Cheng, Iau-Jin Lin, Chia-Peng Yu, Yao-Ching Huang, Shi-Hao Huang, Chien-An Sun, Li-Yun Fann, Miin-Yea Sheu, and Wu-Chien Chien. 2022. "Persistent Depressive Disorder-Related Effect of Sleep Disorder on the Highest Risk of Suicide in Taiwan, 2000–2015" International Journal of Environmental Research and Public Health 19, no. 20: 13169. https://doi.org/10.3390/ijerph192013169
APA StyleHsiao, S.-H., Cheng, C.-C., Lin, I.-J., Yu, C.-P., Huang, Y.-C., Huang, S.-H., Sun, C.-A., Fann, L.-Y., Sheu, M.-Y., & Chien, W.-C. (2022). Persistent Depressive Disorder-Related Effect of Sleep Disorder on the Highest Risk of Suicide in Taiwan, 2000–2015. International Journal of Environmental Research and Public Health, 19(20), 13169. https://doi.org/10.3390/ijerph192013169





