Left Ventricular, Left Atrial and Right Ventricular Strain Modifications after Maximal Exercise in Elite Ski-Mountaineering Athletes: A Feasibility Speckle Tracking Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Echocardiographic Examination
2.3. Statistical Analyses
3. Results
3.1. Baseline Athletes’ Characteristics and Echocardiographic Assessment at Rest
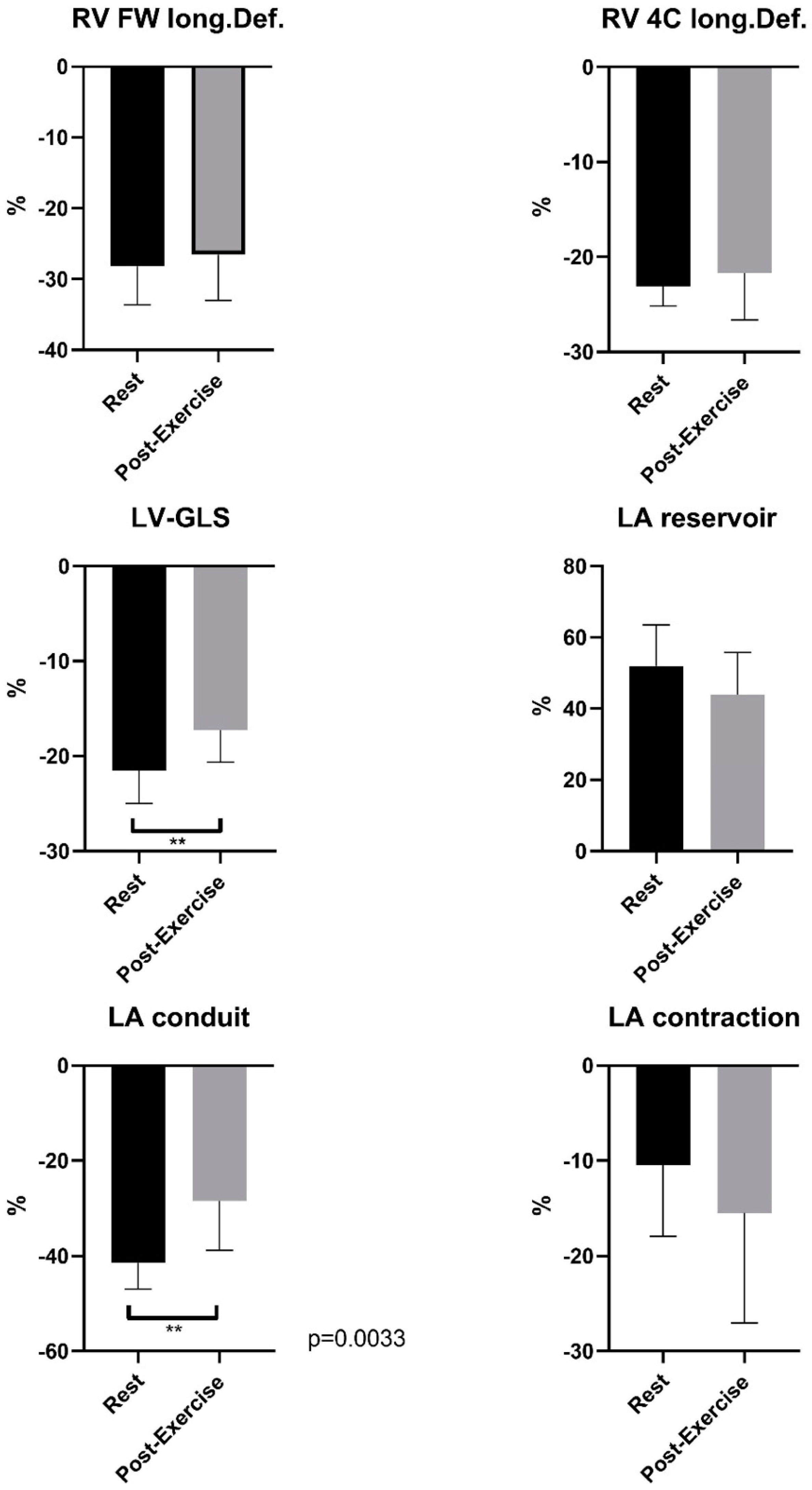
3.2. Speckle Tracking Analysis of the Right and Left Heart at Rest and Post-Exercise–Sport-Specific Functional Cardiac Remodeling
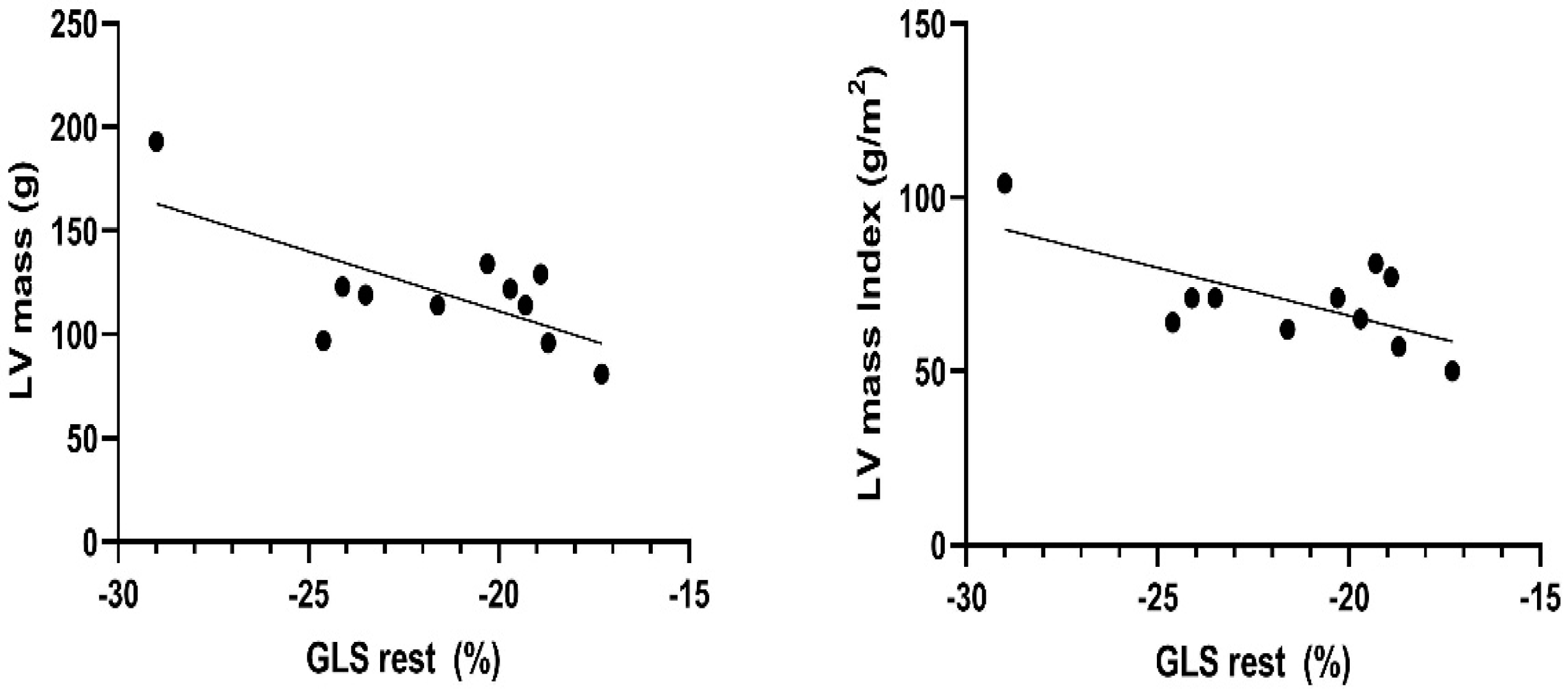
3.3. Sport-Specific Functional Cardiac Remodeling–Univariante Relationships between Morphological Echocardiographic Characteristics and Functional Remodeling as Speckle Tracking Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ski Mountaineering Added to the Milano Cortina 2026 Sports Programme. Available online: https://olympics.com/Ioc/News/Ski-Mountaineering-Added-to-the-Milano-Cortina-2026-Sports-Programme (accessed on 20 July 2021).
- Faiss, R.; von Orelli, C.; Dériaz, O.; Millet, G.P. Responses to Exercise in Normobaric Hypoxia: Comparison of Elite and Recreational Ski Mountaineers. Int. J. Sports Physiol. Perform. 2014, 9, 978–984. [Google Scholar] [CrossRef]
- Praz, C.; Léger, B.; Kayser, B. Energy Expenditure of Extreme Competitive Mountaineering Skiing. Eur. J. Appl. Physiol. 2014, 114, 2201–2211. [Google Scholar] [CrossRef]
- Duc, S.; Cassirame, J.; Durand, F. Physiology of Ski Mountaineering Racing. Int. J. Sports Med. 2011, 32, 856–863. [Google Scholar] [CrossRef]
- Schöffl, V.R.; Bösl, T.; Lutter, C. Ski Mountaineering: Sports Medical Considerations for This New Olympic Sport. Br. J. Sports Med. 2022, 56, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Wüstenfeld, J.; Zimmermann, L.; Schöffl, V.; Schöffl, I. Physiological Aspects of World Elite Competitive German Winter Sport Athletes. Int. J. Environ. Res. Public Health 2022, 19, 5620. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Schöffl, I.; Schöffl, V.; Zimmermann, L.; Eckstein, M.L.; Moser, O.; Wüstenfeld, J. Physiological Effects of Training in Elite German Winter Sport Athletes: Sport Specific Remodeling Determined Using Echocardiographic Data and CPET Performance Parameters. J. Cardiovasc. Dev. Dis. 2022, 9, 235. [Google Scholar] [CrossRef]
- Zimmermann, P.; Moser, O.; Eckstein, M.L.; Wüstenfeld, J.; Schöffl, V.; Zimmermann, L.; Braun, M.; Schöffl, I. Athlete’s Heart in Elite Biathlon, Nordic Cross—Country and Ski-Mountaineering Athletes: Cardiac Adaptions Determined Using Echocardiographic Data. J. Cardiovasc. Dev. Dis. 2021, 9, 8. [Google Scholar] [CrossRef]
- Schöffl, V.; Pöppelmeier, O.; Emmler, J.; Schöffl, I.; Küpper, T.; Lutter, C. Ski Mountaineering–Evaluation of a Sports Specific Performance Diagnosis. Sportverletz. Sportschaden 2018, 32, 233–242. [Google Scholar] [CrossRef]
- Unnithan, V.B.; Beaumont, A.; Rowland, T.W.; Sculthorpe, N.; George, K.; Lord, R.; Oxborough, D. The Influence of Training Status on Right Ventricular Morphology and Segmental Strain in Elite Pre-Adolescent Soccer Players. Eur. J. Appl. Physiol. 2021, 121, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Caselli, S.; Montesanti, D.; Autore, C.; di Paolo, F.M.; Pisicchio, C.; Squeo, M.R.; Musumeci, B.; Spataro, A.; Pandian, N.G.; Pelliccia, A. Patterns of Left Ventricular Longitudinal Strain and Strain Rate in Olympic Athletes. J. Am. Soc. Echocardiogr. 2015, 28, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Moneghetti, K.J.; Singh, T.; Hedman, K.; Christle, J.W.; Kooreman, Z.; Kobayashi, Y.; Bouajila, S.; Amsallem, M.; Wheeler, M.; Gerche, A.L.; et al. Echocardiographic Assessment of Left Ventricular Remodeling in American Style Footballers. Int. J. Sports Med. 2020, 41, 27–35. [Google Scholar] [CrossRef]
- D’Andrea, A.; Galderisi, M.; Sciomer, S.; Nistri, S.; Agricola, E.; Ballo, P.; Buralli, S.; D’Errico, A.; Losi, M.A.; Mele, D.; et al. Echocardiographic Evaluation of the Athlete’s Heart: From Morphological Adaptations to Myocardial Function. G Ital. Cardiol. 2009, 10, 533–544. [Google Scholar]
- Artis, N.J.; Oxborough, D.L.; Williams, G.; Pepper, C.B.; Tan, L.B. Two-Dimensional Strain Imaging: A New Echocardiographic Advance with Research and Clinical Applications. Int. J. Cardiol. 2008, 123, 240–248. [Google Scholar] [CrossRef]
- Utomi, V.; Oxborough, D.; Whyte, G.P.; Somauroo, J.; Sharma, S.; Shave, R.; Atkinson, G.; George, K. Systematic Review and Meta-Analysis of Training Mode, Imaging Modality and Body Size Influences on the Morphology and Function of the Male Athlete’s Heart. Heart 2013, 99, 1727–1733. [Google Scholar] [CrossRef]
- Gruca, M.M.; Cheema, B.; Garg, G.; Ryan, J.; Thomas, J.D.; Rigolin, V.H.; Zielinski, A.R.; Puthumana, J.J. Strain Echocardiography to Describe Left Ventricular Function Pre- and Postexercise in Elite Basketball Athletes: A Feasibility Study. Echocardiography 2021, 38, 1165–1172. [Google Scholar] [CrossRef]
- Forsythe, L.; George, K.; Oxborough, D. Speckle Tracking Echocardiography for the Assessment of the Athlete’s Heart: Is It Ready for Daily Practice? Curr. Treat. Options Cardiovasc. Med. 2018, 20, 83. [Google Scholar] [CrossRef]
- la Gerche, A.; Jurcut, R.; Voigt, J.-U. Right Ventricular Function by Strain Echocardiography. Curr. Opin. Cardiol. 2010, 25, 430–436. [Google Scholar] [CrossRef]
- Yingchoncharoen, T.; Agarwal, S.; Popović, Z.B.; Marwick, T.H. Normal Ranges of Left Ventricular Strain: A Meta-Analysis. J. Am. Soc. Echocardiogr. 2013, 26, 185–191. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Caselli, S.; Solari, M.; Pelliccia, A.; Cameli, M.; Focardi, M.; Padeletti, M.; Corrado, D.; Bonifazi, M.; Mondillo, S. Novel Echocardiographic Techniques for the Evaluation of Athletes’ Heart: A Focus on Speckle-Tracking Echocardiography. Eur. J. Prev. Cardiol. 2016, 23, 437–446. [Google Scholar] [CrossRef]
- Rupwate, R.U.; Chitaley, M.; Kamat, S.R. Cardiopulmonary Functional Changes in Acute Acclimatisation to High Altitude in Mountaineers. Eur. J. Epidemiol. 1990, 6, 266–272. [Google Scholar] [CrossRef]
- Gaston, A.-F.; Marti Peiro, A.; Hapkova, I.; Durand, F. Exploring Physiological Parameters in Ski Mountaineering during World Cup Races. Int. J. Perform. Anal. Sport 2019, 19, 275–288. [Google Scholar] [CrossRef]
- Lasshofer, M.; Seifert, J.; Wörndle, A.-M.; Stöggl, T. Physiological Responses and Predictors of Performance in a Simulated Competitive Ski Mountaineering Race. J. Sports Sci. Med. 2021, 20, 250–257. [Google Scholar] [CrossRef]
- Unnithan, V.B.; Rowland, T.; Lindley, M.R.; Roche, D.M.; Garrard, M.; Barker, P. Cardiac Strain during Upright Cycle Ergometry in Adolescent Males. Echocardiography 2015, 32, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, L.; Hellard, P.; Millet, G.P.; Roels, B.; Richalet, J.P.; Fouillot, J.P. Heart Rate Variability and Performance at Two Different Altitudes in Well-Trained Swimmers. Int. J. Sports Med. 2006, 27, 226–231. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schmitt, L.; Fouillot, J.-P.; Millet, G.P.; Robach, P.; Nicolet, G.; Brugniaux, J.; Richalet, J.-P. Altitude, Heart Rate Variability and Aerobic Capacities. Int. J. Sports Med. 2008, 29, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Sevre, K.; Bendz, B.; Hankø, E.; Nakstad, A.R.; Hauge, A.; Kåsin, J.I.; Lefrandt, J.D.; Smit, A.J.; Eide, I.; Rostrup, M. Reduced Autonomic Activity during Stepwise Exposure to High Altitude. Acta Physiol. Scand. 2001, 173, 409–417. [Google Scholar] [CrossRef]
- Schmitt, L.; Willis, S.J.; Coulmy, N.; Millet, G.P. Effects of Different Training Intensity Distributions Between Elite Cross-Country Skiers and Nordic-Combined Athletes During Live High-Train Low. Front. Physiol. 2018, 9, 932. [Google Scholar] [CrossRef] [PubMed]
- Harriss, D.J.; MacSween, A.; Atkinson, G. Ethical Standards in Sport and Exercise Science Research: 2020 Update. Int. J. Sports Med. 2019, 40, 813–817. [Google Scholar] [CrossRef]
- Evangelista, A.; Flachskampf, F.; Lancellotti, P.; Badano, L.; Aguilar, R.; Monaghan, M.; Zamorano, J.; Nihoyannopoulos, P. European Association of Echocardiography Recommendations for Standardization of Performance, Digital Storage and Reporting of Echocardiographic Studies. Eur. J. Echocardiogr. 2008, 9, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Bierig, M.; Devereux, R.; Flachskampf, F.; Foster, E.; Pellikka, P.; Picard, M.; Roman, M.; Seward, J.; Shanewise, J. Recommendations for Chamber Quantification. Eur. J. Echocardiogr. 2006, 7, 79–108. [Google Scholar] [CrossRef]
- Hagendorff, A.; Fehske, W.; Flachskampf, F.A.; Helfen, A.; Kreidel, F.; Kruck, S.; la Rosée, K.; Tiemann, K.; Voigt, J.-U.; von Bardeleben, R.S.; et al. Manual Zur Indikation U.Und Durchführung Der Echokardiographie–Update 2020 Der Deutschen Gesellschaft Für Kardiologie. Kardiologe 2020, 14, 396–431. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of Adult Transthoracic Echocardiography Reporting in Agreement with Recent Chamber Quantification, Diastolic Function, and Heart Valve Disease Recommendations: An Expert Consensus Document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of Left Atrial, Right Ventricular, and Right Atrial Deformation Imaging Using Two-Dimensional Speckle Tracking Echocardiography: A Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Zimmermann, P.; Moser, O.; Edelmann, F.; Schöffl, V.; Eckstein, M.L.; Braun, M. Electrical and Structural Adaption of Athlete’s Heart and the Impact on Training and Recovery Management in Professional Basketball Players: A Retrospective Observational Study. Front. Physiol. 2022, 13, 739753. [Google Scholar] [CrossRef]
- Klaeboe, L.G.; Edvardsen, T. Echocardiographic Assessment of Left Ventricular Systolic Function. J. Echocardiogr. 2019, 17, 10–16. [Google Scholar] [CrossRef]
- D’Andrea, A.; Radmilovic, J.; Carbone, A.; Mandoli, G.E.; Santoro, C.; Evola, V.; Bandera, F.; D’Ascenzi, F.; Bossone, E.; Galderisi, M.; et al. Speckle Tracking Evaluation in Endurance Athletes: The “Optimal” Myocardial Work. Int. J. Cardiovasc. Imaging 2020, 36, 1679–1688. [Google Scholar] [CrossRef]
- Schattke, S.; Xing, Y.; Lock, J.; Brechtel, L.; Schroeckh, S.; Spethmann, S.; Baumann, G.; Borges, A.C.; Knebel, F. Increased Longitudinal Contractility and Diastolic Function at Rest in Well-Trained Amateur Marathon Runners: A Speckle Tracking Echocardiography Study. Cardiovasc. Ultrasound 2014, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Binnetoğlu, F.K.; Babaoğlu, K.; Altun, G.; Kayabey, Ö. Effects That Different Types of Sports Have on the Hearts of Children and Adolescents and the Value of Two-Dimensional Strain-Strain-Rate Echocardiography. Pediatr. Cardiol. 2014, 35, 126–139. [Google Scholar] [CrossRef]
- Shave, R.; George, K.; Whyte, G.; Middleton, N.; Hart, E.; Artis, N.; Oxborough, D. A Comparison of Doppler, Tissue Doppler Imaging, and Strain Rate Imaging in the Assessment of Postexercise Left Ventricular Function. Appl. Physiol. Nutr. Metab. 2009, 34, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.M.; Yamada, A.; Haseler, L.J.; Kavanagh, J.J.; Koerbin, G.; Chan, J.; Sabapathy, S. Altered Ventricular Mechanics after 60 Min of High-Intensity Endurance Exercise: Insights from Exercise Speckle-Tracking Echocardiography. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H875–H883. [Google Scholar] [CrossRef]
- Santoro, A.; Alvino, F.; Antonelli, G.; Cameli, M.; Bertini, M.; Molle, R.; Mondillo, S. Left Ventricular Strain Modifications after Maximal Exercise in Athletes: A Speckle Tracking Study. Echocardiography 2015, 32, 920–927. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Tsai, H.-H.; Fu, T.-C.; Hsu, C.-C.; Wang, J.-S. High-Intensity Interval Training Improves Left Ventricular Contractile Function. Med. Sci. Sports Exerc. 2019, 51, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Forsythe, L.; Somauroo, J.; George, K.; Oxborough, D. The Impact of Preload Reduction with Head-up Tilt Testing on Longitudinal and Transverse Left Ventricular Mechanics: A Study Utilizing Deformation Volume Analysis. Echo Res. Pract. 2018, 5, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Żebrowska, A.; Mikołajczyk, R.; Waśkiewicz, Z.; Gąsior, Z.; Mizia-Stec, K.; Kawecki, D.; Rosemann, T.; Nikolaidis, P.T.; Knechtle, B. Left Ventricular Systolic Function Assessed by Speckle Tracking Echocardiography in Athletes with and without Left Ventricle Hypertrophy. J. Clin. Med. 2019, 8, 687. [Google Scholar] [CrossRef] [PubMed]
- Oxborough, D.; Birch, K.; Shave, R.; George, K. “Exercise-Induced Cardiac Fatigue”—A Review of the Echocardiographic Literature. Echocardiography 2010, 27, 1130–1140. [Google Scholar] [CrossRef]
- Banks, L.; Sasson, Z.; Busato, M.; Goodman, J.M. Impaired Left and Right Ventricular Function Following Prolonged Exercise in Young Athletes: Influence of Exercise Intensity and Responses to Dobutamine Stress. J. Appl. Physiol. 2010, 108, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Knackstedt, C.; Hildebrandt, U.; Schmidt, K.; Syrocki, L.; Lang, A.; Bjarnason-Wehrens, B.; Schummers, G.; Stapf, D.; Becker, M.; Predel, H.G. Analysis of Right and Left Ventricular Deformation in Former World Class Swimmers: Evaluation Using Speckle Tracking. J. Sports Med. Phys. Fitness 2015, 55, 978–987. [Google Scholar]
- Smolarek, D.; Gruchała, M.; Sobiczewski, W. Echocardiographic Evaluation of Right Ventricular Systolic Function: The Traditional and Innovative Approach. Cardiol. J. 2017, 24, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Bjerring, A.W.; Landgraff, H.E.; Leirstein, S.; Aaeng, A.; Ansari, H.Z.; Saberniak, J.; Murbræch, K.; Bruun, H.; Stokke, T.M.; Haugaa, K.H.; et al. Morphological Changes and Myocardial Function Assessed by Traditional and Novel Echocardiographic Methods in Preadolescent Athlete’s Heart. Eur. J. Prev. Cardiol. 2018, 25, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, T.G.; Curry, B.A.; Wright, S.P.; Meah, V.L.; Yousef, Z.; Eves, N.D.; Shave, R.E.; Stembridge, M. Right Ventricular Function and Region-Specific Adaptation in Athletes Engaged in High-Dynamic Sports: A Meta-Analysis. Circ. Cardiovasc. Imaging 2021, 14, e012315. [Google Scholar] [CrossRef]
- Christou, G.A.; Pagourelias, E.D.; Anifanti, M.A.; Sotiriou, P.G.; Koutlianos, N.A.; Tsironi, M.P.; Andriopoulos, P.I.; Christou, K.A.; Kouidi, E.J.; Deligiannis, A.P. Exploring the Determinants of the Cardiac Changes after Ultra-Long Duration Exercise: The Echocardiographic Spartathlon Study. Eur. J. Prev. Cardiol. 2020, 27, 1467–1477. [Google Scholar] [CrossRef]
- Oxborough, D.; Sharma, S.; Shave, R.; Whyte, G.; Birch, K.; Artis, N.; Batterham, A.M.; George, K. The Right Ventricle of the Endurance Athlete: The Relationship between Morphology and Deformation. J. Am. Soc. Echocardiogr. 2012, 25, 263–271. [Google Scholar] [CrossRef]
- Zaidi, A.; Knight, D.S.; Augustine, D.X.; Harkness, A.; Oxborough, D.; Pearce, K.; Ring, L.; Robinson, S.; Stout, M.; Willis, J.; et al. Echocardiographic Assessment of the Right Heart in Adults: A Practical Guideline from the British Society of Echocardiography. Echo Res. Pract. 2020, 7, G19–G41. [Google Scholar] [CrossRef]
- King, G.; Almuntaser, I.; Murphy, R.T.; la Gerche, A.; Mahoney, N.; Bennet, K.; Clarke, J.; Brown, A. Reduced Right Ventricular Myocardial Strain in the Elite Athlete May Not Be a Consequence of Myocardial Damage. “Cream Masquerades as Skimmed Milk”. Echocardiography 2013, 30, 929–935. [Google Scholar] [CrossRef]
- Rimensberger, C.; Carlen, F.; Brugger, N.; Seiler, C.; Wilhelm, M. Right Ventricular Adaptations and Arrhythmias in Amateur Ultra-Endurance Athletes. Br. J. Sports Med. 2014, 48, 1179–1184. [Google Scholar] [CrossRef]
- Ujka, K.; Bastiani, L.; D’Angelo, G.; Catuzzo, B.; Tonacci, A.; Mrakic-Sposta, S.; Vezzoli, A.; Giardini, G.; Pratali, L. Enhanced Right-Chamber Remodeling in Endurance Ultra-Trail Athletes Compared to Marathon Runners Detected by Standard and Speckle-Tracking Echocardiography. Front. Physiol. 2017, 8, 527. [Google Scholar] [CrossRef]
- Qasem, M.; George, K.; Somauroo, J.; Forsythe, L.; Brown, B.; Oxborough, D. Influence of Different Dynamic Sporting Disciplines on Right Ventricular Structure and Function in Elite Male Athletes. Int. J. Cardiovasc. Imaging 2018, 34, 1067–1074. [Google Scholar] [CrossRef]
- Zaidi, A.; Ghani, S.; Sharma, R.; Oxborough, D.; Panoulas, V.F.; Sheikh, N.; Gati, S.; Papadakis, M.; Sharma, S. Physiological Right Ventricular Adaptation in Elite Athletes of African and Afro-Caribbean Origin. Circulation 2013, 127, 1783–1792. [Google Scholar] [CrossRef]
- Lakatos, B.K.; Kiss, O.; Tokodi, M.; Tősér, Z.; Sydó, N.; Merkely, G.; Babity, M.; Szilágyi, M.; Komócsin, Z.; Bognár, C.; et al. Exercise-Induced Shift in Right Ventricular Contraction Pattern: Novel Marker of Athlete’s Heart? Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1640–H1648. [Google Scholar] [CrossRef]
- Sanz-de la Garza, M.; Rubies, C.; Batlle, M.; Bijnens, B.H.; Mont, L.; Sitges, M.; Guasch, E. Severity of Structural and Functional Right Ventricular Remodeling Depends on Training Load in an Experimental Model of Endurance Exercise. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H459–H468. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; la Gerche, A.; Golia, E.; Teske, A.J.; Bossone, E.; Russo, M.G.; Calabrò, R.; Baggish, A.L. Right Heart Structural and Functional Remodeling in Athletes. Echocardiography 2015, 32 (Suppl. 1), S11–S22. [Google Scholar] [CrossRef] [PubMed]
- la Gerche, A.; Burns, A.T.; Mooney, D.J.; Inder, W.J.; Taylor, A.J.; Bogaert, J.; Macisaac, A.I.; Heidbüchel, H.; Prior, D.L. Exercise-Induced Right Ventricular Dysfunction and Structural Remodelling in Endurance Athletes. Eur. Heart J. 2012, 33, 998–1006. [Google Scholar] [CrossRef]
- Lord, R.; Somauroo, J.; Stembridge, M.; Jain, N.; Hoffman, M.D.; George, K.; Jones, H.; Shave, R.; Haddad, F.; Ashley, E.; et al. The Right Ventricle Following Ultra-Endurance Exercise: Insights from Novel Echocardiography and 12-Lead Electrocardiography. Eur. J. Appl. Physiol. 2015, 115, 71–80. [Google Scholar] [CrossRef]
- Nemes, A.; Domsik, P.; Kalapos, A.; Orosz, A.; Oszlánczi, M.; Török, L.; Balogh, L.; Márton, J.; Forster, T.; Lengyel, C. Volumetric and Functional Assessment of the Left Atrium in Young Competitive Athletes without Left Ventricular Hypertrophy: The MAGYAR-Sport Study. J. Sports Med. Phys. Fitness 2017, 57, 900–906. [Google Scholar] [CrossRef]
- Caminiti, G.; Perrone, M.A.; Iellamo, F.; D’Antoni, V.; Catena, M.; Franchini, A.; Volterrani, M. Acute Left Atrial Response to Different Eccentric Resistance Exercise Loads in Patients with Heart Failure with Middle Range Ejection Fraction: A Pilot Study. J. Pers. Med. 2022, 12, 689. [Google Scholar] [CrossRef]
- Cuspidi, C.; Tadic, M.; Sala, C.; Gherbesi, E.; Grassi, G.; Mancia, G. Left Atrial Function in Elite Athletes: A Meta-analysis of Two-dimensional Speckle Tracking Echocardiographic Studies. Clin. Cardiol. 2019, 42, 579–587. [Google Scholar] [CrossRef]
- Lentini, A.C.; McKelvie, R.S.; McCartney, N.; Tomlinson, C.W.; MacDougall, J.D. Left Ventricular Response in Healthy Young Men during Heavy-Intensity Weight-Lifting Exercise. J. Appl. Physiol. 1993, 75, 2703–2710. [Google Scholar] [CrossRef]
- Anwar, A.M.; Geleijnse, M.L.; Soliman, O.I.I.; Nemes, A.; Cate, F.J.t. Left Atrial Frank Starling Law Assessed by Real-Time, Three-Dimensional Echocardiographic Left Atrial Volume Changes. Heart 2007, 93, 1393–1397. [Google Scholar] [CrossRef]
- Hart, E.; Shave, R.; Middleton, N.; George, K.; Whyte, G.; Oxborough, D. Effect of Preload Augmentation on Pulsed Wave and Tissue Doppler Echocardiographic Indices of Diastolic Function after a Marathon. J. Am. Soc. Echocardiogr. 2007, 20, 1393–1399. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Perez-Quilis, C.; Lippi, G.; Cervellin, G.; Leischik, R.; Löllgen, H.; Serrano-Ostáriz, E.; Lucia, A. Atrial Fibrillation in Highly Trained Endurance Athletes-Description of a Syndrome. Int. J. Cardiol. 2017, 226, 11–20. [Google Scholar] [CrossRef]
- D’Andrea, A.; Bossone, E.; Radmilovic, J.; Caso, P.; Calabrò, R.; Russo, M.G.; Galderisi, M. The Role of New Echocardiographic Techniques in Athlete’s Heart. F1000Research 2015, 4, 289. [Google Scholar] [CrossRef]
- Perry, R.; Swan, A.L.; Hecker, T.; de Pasquale, C.G.; Selvanayagam, J.B.; Joseph, M.X. The Spectrum of Change in the Elite Athlete’s Heart. J. Am. Soc. Echocardiogr. 2019, 32, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Gjerdalen, G.F.; Hisdal, J.; Solberg, E.E.; Andersen, T.E.; Radunovic, Z.; Steine, K. Atrial Size and Function in Athletes. Int. J. Sports Med. 2015, 36, 1170–1176. [Google Scholar] [CrossRef]
- Kooreman, Z.; Giraldeau, G.; Finocchiaro, G.; Kobayashi, Y.; Wheeler, M.; Perez, M.; Moneghetti, K.; Oxborough, D.; George, K.P.; Myers, J.; et al. Athletic Remodeling in Female College Athletes: The “Morganroth Hypothesis” Revisited. Clin. J. Sport Med. 2019, 29, 224–231. [Google Scholar] [CrossRef]
| Ski-Mo Male n = 8 | Ski-Mo Female n = 3 | |
|---|---|---|
| Age (years) | 20.5 ± 2.4 | 19 ± 0 |
| Height (cm) | 177.8 ± 4.6 | 164.0 ± 20.0 |
| Weight (kg) | 63.6 ± 6.1 | 52.1 ± 5.9 |
| BMI (kg/m2) | 20.1 ± 1.4 | 19.3 ± 0.4 |
| resting blood pressure systolic/diastolic (mmHg) | 119 ± 6.1 85 ± 3.2 | 110 ± 5.2 71 ± 2.3 |
| resting heart rate (bpm) | 41 ± 3.6 | 44 ± 2.5 |
| BSA (body surface area m2) | 1.79 ± 0.1 | 1.79 ± 0.1 |
| Ski-Mo Male n = 8 | Ski-Mo Female n = 3 | Reference Value Male | Reference Value Female | |
|---|---|---|---|---|
| LV edd (mm) | 47.13 ± 4.64 | 43.67 ± 2.31 | 42–58 | 38–52 |
| LV Mass Index (g/m2) | 71.38 ± 15.50 | 67.33 ± 12.35 | 49–115 | 43–95 |
| Relative wall Thickness RWT | 0.34 ± 0.05 | 0.37 ± 0.05 | ||
| IVSd (mm) | 8.25 ± 1.28 | 7.33 ± 0.58 | 6–10 | 6–9 |
| LVPWs (mm) | 8.00 ± 0.93 | 8.00 ± 1.00 | 6–10 | 6–9 |
| E/A | 1.98 ± 0.32 | 1.90 ± 0.20 | ||
| E/E′ | 5.08 ± 2.18 | 5.53 ± 0.62 | ||
| LAVI (mL/m2) | 28.13 ± 8.17 | 36.00 ± 3.00 | ||
| RA (cm2) | 15.00 ± 2.39 | 14.00 ± 2.65 | ||
| LV − EFrest (%) | 61.38 ± 4.17 | 59.00 ± 1.00 | 52–72 | 54–72 |
| LV − EFpost-stress (%) | 70.00 ± 2.88 | 68.33 ± 0.58 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmermann, P.; Eckstein, M.L.; Moser, O.; Schöffl, I.; Zimmermann, L.; Schöffl, V. Left Ventricular, Left Atrial and Right Ventricular Strain Modifications after Maximal Exercise in Elite Ski-Mountaineering Athletes: A Feasibility Speckle Tracking Study. Int. J. Environ. Res. Public Health 2022, 19, 13153. https://doi.org/10.3390/ijerph192013153
Zimmermann P, Eckstein ML, Moser O, Schöffl I, Zimmermann L, Schöffl V. Left Ventricular, Left Atrial and Right Ventricular Strain Modifications after Maximal Exercise in Elite Ski-Mountaineering Athletes: A Feasibility Speckle Tracking Study. International Journal of Environmental Research and Public Health. 2022; 19(20):13153. https://doi.org/10.3390/ijerph192013153
Chicago/Turabian StyleZimmermann, Paul, Max L. Eckstein, Othmar Moser, Isabelle Schöffl, Lukas Zimmermann, and Volker Schöffl. 2022. "Left Ventricular, Left Atrial and Right Ventricular Strain Modifications after Maximal Exercise in Elite Ski-Mountaineering Athletes: A Feasibility Speckle Tracking Study" International Journal of Environmental Research and Public Health 19, no. 20: 13153. https://doi.org/10.3390/ijerph192013153
APA StyleZimmermann, P., Eckstein, M. L., Moser, O., Schöffl, I., Zimmermann, L., & Schöffl, V. (2022). Left Ventricular, Left Atrial and Right Ventricular Strain Modifications after Maximal Exercise in Elite Ski-Mountaineering Athletes: A Feasibility Speckle Tracking Study. International Journal of Environmental Research and Public Health, 19(20), 13153. https://doi.org/10.3390/ijerph192013153









