Enhanced Production of Bacterial Cellulose from Miscanthus as Sustainable Feedstock through Statistical Optimization of Culture Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. BC Production
2.3. Experimental Design
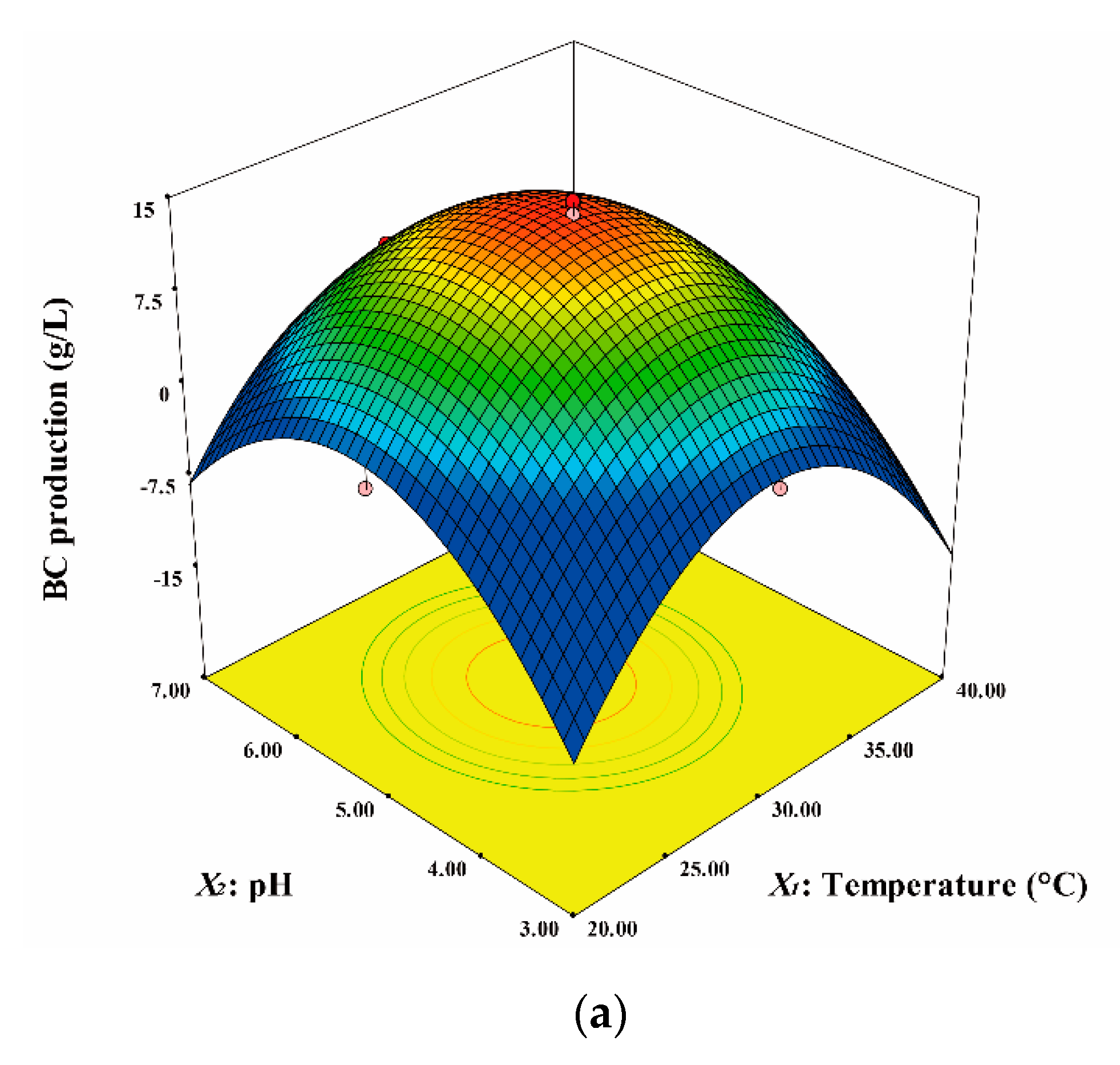
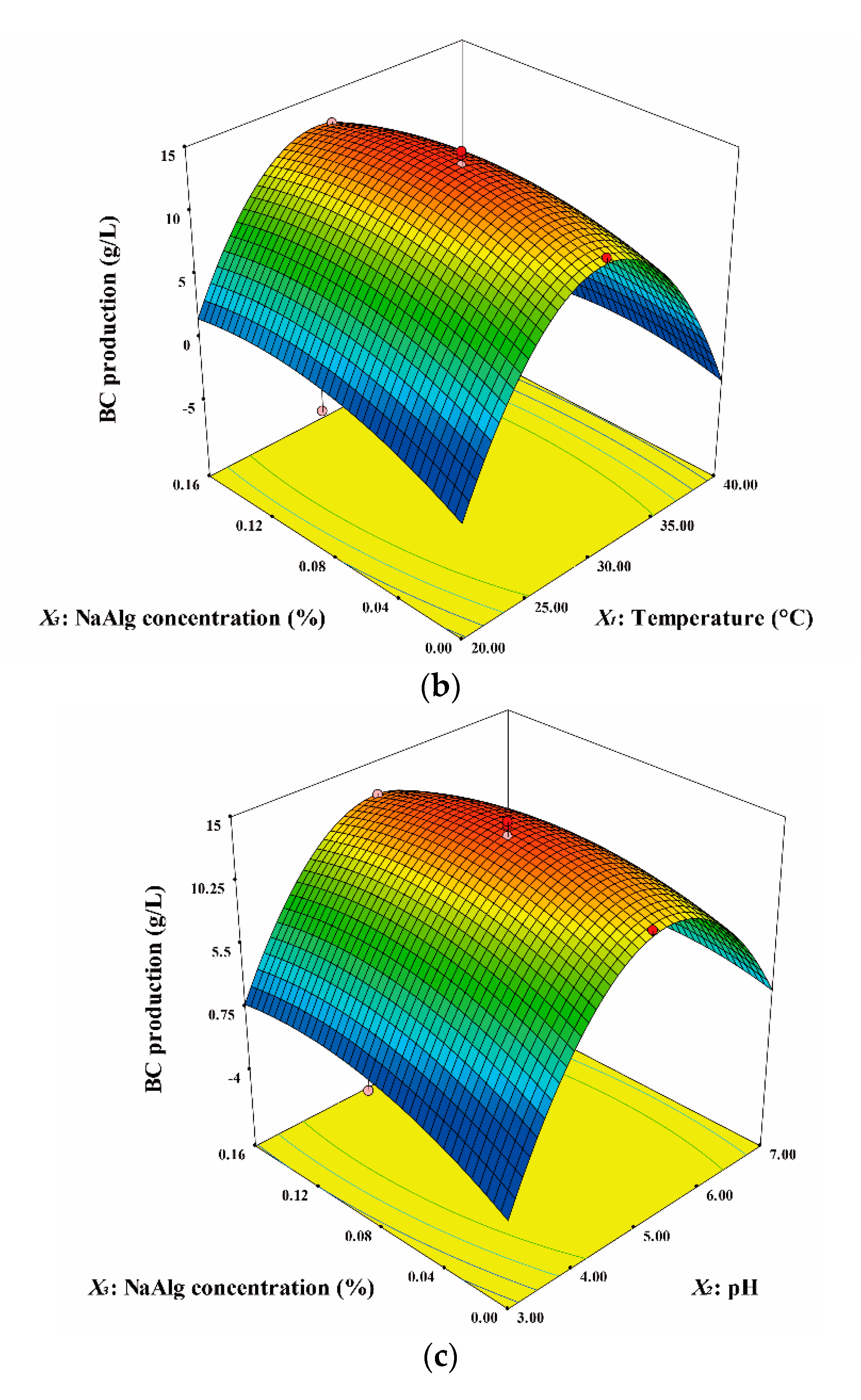
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shin, M.; Seo, J.; Baek, Y.; Lee, T.; Jang, M.; Park, C. Novel and Efficient Synthesis of Phenethyl Formate via Enzymatic Esterification of Formic Acid. Biomolecules 2020, 10, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.H.; Chun, Y.; Jang, Y.W.; Lee, S.K.; Kim, H.R.; Lee, J.H.; Kim, S.W.; Park, C.; Yoo, H.Y. Fabrication of Functional Bioelastomer for Food Packaging from Aronia (Aronia melanocarpa) Juice Processing By-Products. Foods 2020, 9, 1565. [Google Scholar] [CrossRef] [PubMed]
- Li, G. Performance Evaluation of Low Global Warming Potential Working Fluids as R134a Alternatives for Two-Stage Centrifugal Chiller Applications. Korean J. Chem. Eng. 2021, 38, 1438–1451. [Google Scholar] [CrossRef]
- Herrmann, A.; Sauerborn, R.; Nilsson, M. The Role of Health in Households’ Balancing Act for Lifestyles Compatible with the Paris Agreement—Qualitative Results from Mannheim, Germany. Int. J. Environ. Res. Public Health 2020, 17, 1297. [Google Scholar] [CrossRef] [Green Version]
- Kostas, E.T.; Adams, J.M.M.; Ruiz, H.A.; Durán-Jiménez, G.; Lye, G.J. Macroalgal Biorefinery Concepts for the Circular Bioeconomy: A Review on Biotechnological Developments and Future Perspectives. Renew. Sustain. Energ. Rev. 2021, 151, 111553. [Google Scholar] [CrossRef]
- An, H.-E.; Lee, K.H.; Jang, Y.W.; Kim, C.-B.; Yoo, H.Y. Improved Glucose Recovery from Sicyos angulatus by NaOH Pretreatment and Application to Bioethanol Production. Processes 2021, 9, 245. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Kim, S.W. The Next-Generation Biomass for Biorefining. BioResources 2021, 16, 2188–2191. [Google Scholar] [CrossRef]
- Kim, H.; Son, J.; Lee, J.; Yoo, H.Y.; Lee, T.; Jang, M.; Oh, J.-M.; Park, C. Improved Production of Bacterial Cellulose through Investigation of Effects of Inhibitory Compounds from Lignocellulosic Hydrolysates. GCB Bioenergy 2021, 13, 436–444. [Google Scholar] [CrossRef]
- Naomi, R.; Bt Hj Idrus, R.; Fauzi, M.B. Plant- vs. Bacterial-Derived Cellulose for Wound Healing: A Review. Int. J. Environ. Res. Public Health 2020, 17, 6803. [Google Scholar] [CrossRef]
- Ho Jin, Y.; Lee, T.; Kim, J.R.; Choi, Y.-E.; Park, C. Improved Production of Bacterial Cellulose from Waste Glycerol through Investigation of Inhibitory Effects of Crude Glycerol-Derived Compounds by Gluconacetobacter xylinus. J. Ind. Eng. Chem. 2019, 75, 158–163. [Google Scholar] [CrossRef]
- Skiba, E.A.; Budaeva, V.V.; Ovchinnikova, E.V.; Gladysheva, E.K.; Kashcheyeva, E.I.; Pavlov, I.N.; Sakovich, G.V. A Technology for Pilot Production of Bacterial Cellulose from Oat Hulls. Chem. Eng. J. 2020, 383, 123128. [Google Scholar] [CrossRef]
- Lin, S.; Loira Calvar, I.; Catchmark, J.M.; Liu, J.; Demirci, A.; Cheng, K. Biosynthesis, Production and Applications of Bacterial Cellulose. Cellulose 2013, 20, 2191–2219. [Google Scholar] [CrossRef]
- Raval, A.A.; Raval, U.G.; Sayyed, R.Z. Utilization of Industrial Waste for the Sustainable Production of Bacterial Cellulose. Environ. Sustain. 2020, 3, 427–435. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, X.; Ma, M.; Hu, C.; Seidi, F.; Yin, S.; Xiao, H. Recent Advances on the Bacterial Cellulose-Derived Carbon Aerogels. J. Mater. Chem. C 2021, 9, 818–828. [Google Scholar] [CrossRef]
- Fernandes, I.A.A.; Pedro, A.C.; Ribeiro, V.R.; Bortolini, D.G.; Ozaki, M.S.C.; Maciel, G.M.; Haminiuk, C.W.I. Bacterial Cellulose: From Production Optimization to New Applications. Int. J. Biol. Macromol. 2020, 164, 2598–2611. [Google Scholar] [CrossRef]
- Sperotto, G.; Stasiak, L.G.; Godoi, J.P.M.G.; Gabiatti, N.C.; De Souza, S.S. A Review of Culture Media for Bacterial Cellulose Production: Complex, Chemically Defined and Minimal Media Modulations. Cellulose 2021, 28, 2649–2673. [Google Scholar] [CrossRef]
- Chukwuma, O.B.; Rafatullah, M.; Tajarudin, H.A.; Ismail, N. A Review on Bacterial Contribution to Lignocellulose Breakdown into Useful Bio-Products. Int. J. Environ. Res. Public Health 2021, 18, 6001. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, S.K.; Lee, J.; Kim, S.; Park, C.; Kim, S.W.; Yoo, H.Y. Improvement of Enzymatic Glucose Conversion from Chestnut Shells through Optimization of KOH Pretreatment. Int. J. Environ. Res. Public Health 2021, 18, 3772. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Yang, X.; Kim, D.S.; Lee, S.K.; Lotrakul, P.; Prasongsuk, S.; Punnapayak, H.; Kim, S.W. Evaluation of the Overall Process on Bioethanol Production from Miscanthus Hydrolysates Obtained by Dilute Acid Pretreatment. Biotechnol. Bioprocess Eng. 2016, 21, 733–742. [Google Scholar] [CrossRef]
- Shepherd, A.; Littleton, E.; Clifton-Brown, J.; Martin, M.; Hastings, A. Projections of Global and UK Bioenergy Potential from Miscanthus × Giganteus—Feedstock Yield, Carbon Cycling and Electricity Generation in the 21st Century. GCB Bioenergy 2020, 12, 287–305. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Erickson, L.; Stefanovska, T.; Popelka, J.; Hettiarachchi, G.; Davis, L.; Trögl, J. Potential Phytomanagement of Military Polluted Sites and Biomass Production Using Biofuel Crop Miscanthus × Giganteus. Environ. Pollut. 2019, 249, 330–337. [Google Scholar] [CrossRef]
- Jang, Y.W.; Lee, K.H.; Yoo, H.Y. Improved Sugar Recovery from Orange Peel by Statistical Optimization of Thermo-Alkaline Pretreatment. Processes 2021, 9, 409. [Google Scholar] [CrossRef]
- Lee, K.H.; Jang, Y.W.; Kim, H.; Ki, J.S.; Yoo, H.Y. Optimization of Lutein Recovery from Tetraselmis suecica by Response Surface Methodology. Biomolecules 2021, 11, 182. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoo, H.Y.; Lee, S.K.; Chun, Y.; Kim, H.R.; Bankeeree, W.; Lotrakul, P.; Punnapayak, H.; Prasongsuk, S.; Kim, S.W. Significant Impact of Casein Hydrolysate to Overcome the Low Consumption of Glycerol by Klebsiella aerogenes ATCC 29007 and Its Application to Bioethanol Production. Energy Convers. Manag. 2020, 221, 113181. [Google Scholar] [CrossRef]
- Islam, M.U.; Ullah, M.W.; Khan, S.; Shah, N.; Park, J.K. Strategies for Cost-Effective and Enhanced Production of Bacterial Cellulose. Int. J. Biol. Macromol. 2017, 102, 1166–1173. [Google Scholar] [CrossRef]
- Zhou, L.L.; Sun, D.P.; Hu, L.Y.; Li, Y.W.; Yang, J.Z. Effect of Addition of Sodium Alginate on Bacterial Cellulose Production by Acetobacter Xylinum. J. Ind. Microbiol. Biotechnol. 2007, 34, 483–489. [Google Scholar] [CrossRef]
- Cheng, K.C.; Catchmark, J.M.; Demirci, A. Effect of Different Additives on Bacterial Cellulose Production by Acetobacter Xylinum and Analysis of Material Property. Cellulose 2009, 16, 1033–1045. [Google Scholar] [CrossRef]
- Kim, H.-D.; Choi, J.-I. Medium Optimization and Proteome Analysis of Protease Production by Janthinobacterium sp. Biotechnol. Bioprocess Eng. 2020, 25, 787–794. [Google Scholar] [CrossRef]
- Kim, H.; Yoo, H.Y.; Park, N.; Kim, H.; Lee, J.; Baek, Y.; Lee, T.; Oh, J.M.; Cho, J.; Park, C. Enhanced l-Lysine into 1,5-Diaminopentane Conversion via Statistical Optimization of Whole-Cell Decarboxylation System. Polymers 2019, 11, 1372. [Google Scholar] [CrossRef] [Green Version]
- Saadat, F.; Zerafat, M.M.; Foorginezhad, S. Adsorption of Copper Ions from Aqueous Media Using Montmorillonite-Al2O3 Nano-Adsorbent Incorporated with Fe3O4 for Facile Separation. Korean J. Chem. Eng. 2020, 37, 2273–2286. [Google Scholar] [CrossRef]
- Lee, K.H.; Jang, Y.W.; Lee, J.; Kim, S.; Park, C.; Yoo, H.Y. Statistical Optimization of Alkali Pretreatment to Improve Sugars Recovery from Spent Coffee Grounds and Utilization in Lactic Acid Fermentation. Processes 2021, 9, 494. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.P.; Carrère, H. Do Furanic and Phenolic Compounds of Lignocellulosic and Algae Biomass Hydrolyzate Inhibit Anaerobic Mixed Cultures? A Comprehensive Review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef] [PubMed]
- Soeiro, V.S.; Tundisi, L.L.; Novaes, L.C.L.; Mazzola, P.G.; Aranha, N.; Grotto, D.; Júnior, J.M.O.; Komatsu, D.; Gama, F.M.P.; Chaud, M.V.; et al. Production of bacterial cellulose nanocrystals via enzymatic hydrolysis and evaluation of their coating on alginate particles formed by ionotropic gelation. Carbohydr. Polym. Technol. Appl. 2021, 2, 100155. [Google Scholar] [CrossRef]
- Abdelraof, M.; Hasanin, M.S.; El-Saied, H. Ecofriendly green conversion of potato peel wastes to high productivity bacterial cellulose. Carbohydr. Polym. 2019, 211, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-H.H.; Huang, C.-Y.Y.; Shieh, C.-J.J.; Wang, H.-M.M.D.; Tseng, C.-Y.Y. Hydrolysis of Orange Peel with Cellulase and Pectinase to Produce Bacterial Cellulose using Gluconacetobacter xylinus. Waste Biomass Valorization 2019, 10, 85–93. [Google Scholar] [CrossRef]
- Wang, Q.; Nnanna, P.C.; Shen, F.; Huang, M.; Tian, D.; Hu, J.; Zeng, Y.; Yang, G.; Deng, S. Full utilization of sweet sorghum for bacterial cellulose production: A concept of material crop. Ind. Crops Prod. 2021, 162, 113256. [Google Scholar] [CrossRef]
- Mikkelsen, D.; Flanagan, B.M.; Dykes, G.A.; Gidley, M.J. Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus strain ATCC 53524. J. Appl. Microbiol. 2009, 107, 576–583. [Google Scholar] [CrossRef]
| Variables | Unit | Symbol | Coded Variable Levels | ||||
|---|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | |||
| Temperature | °C | X1 | 20 | 25 | 30 | 35 | 40 |
| Initial pH | X2 | 3 | 4 | 5 | 6 | 7 | |
| NaAlg concentration | %, w/v | X3 | 0.00 | 0.04 | 0.08 | 0.12 | 0.16 |
| Std. | Coded Variable Levels | BC Production (g/L) | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Actual | Predicted | |
| 1 | −1 | −1 | −1 | 6.98 | 5.91 |
| 2 | 1 | −1 | −1 | 4.64 | 5.23 |
| 3 | −1 | 1 | −1 | 8.75 | 8.48 |
| 4 | 1 | 1 | −1 | 4.97 | 6.78 |
| 5 | −1 | −1 | 1 | 9.62 | 8.09 |
| 6 | 1 | −1 | 1 | 5.49 | 6.05 |
| 7 | −1 | 1 | 1 | 9.30 | 8.99 |
| 8 | 1 | 1 | 1 | 4.57 | 5.92 |
| 9 | −2 | 0 | 0 | 0.00 | 1.73 |
| 10 | 2 | 0 | 0 | 0.00 | −2.02 |
| 11 | 0 | −2 | 0 | 0.00 | 0.86 |
| 12 | 0 | 2 | 0 | 4.46 | 3.31 |
| 13 | 0 | 0 | −2 | 11.59 | 11.20 |
| 14 | 0 | 0 | 2 | 12.43 | 12.53 |
| 15 | 0 | 0 | 0 | 14.36 | 13.92 |
| 16 | 0 | 0 | 0 | 13.44 | 13.92 |
| 17 | 0 | 0 | 0 | 14.63 | 13.92 |
| 18 | 0 | 0 | 0 | 13.16 | 13.92 |
| 19 | 0 | 0 | 0 | 13.59 | 13.92 |
| 20 | 0 | 0 | 0 | 14.63 | 13.92 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 474.26 | 9 | 52.70 | 25.28 | <0.0001 |
| 14.03 | 1 | 14.03 | 6.73 | 0.0267 | |
| 5.97 | 1 | 5.97 | 2.86 | 0.1214 | |
| 1.77 | 1 | 1.77 | 0.85 | 0.3791 | |
| 0.53 | 1 | 0.53 | 0.25 | 0.6257 | |
| 0.94 | 1 | 0.94 | 0.45 | 0.5182 | |
| 1.39 | 1 | 1.39 | 0.67 | 0.4331 | |
| 310.76 | 1 | 310.76 | 149.08 | <0.0001 | |
| 220.01 | 1 | 220.01 | 105.55 | <0.0001 | |
| 6.62 | 1 | 6.62 | 3.18 | 0.1051 | |
| Residual | 20.85 | 10 | 2.08 | ||
| Lack of Fit | 18.73 | 5 | 3.75 | 8.85 | 0.0159 |
| Pure Error | 2.12 | 5 | 0.42 | ||
| Cor Total | 495.10 | 19 |
| Parameters | Goal | Importance | Predicted | Actual |
|---|---|---|---|---|
| Temperature (°C) | In range | – | 29.24 | 29 |
| Initial pH | In range | – | 5.09 | 5.1 |
| NaAlg concentration (%, w/v) | In range | – | 0.09 | 0.09 |
| Bacterial cellulose production (g/L) | Maximize | 3 | 14.07 | 14.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, J.; Lee, K.H.; Lee, T.; Kim, H.S.; Shin, W.H.; Oh, J.-M.; Koo, S.-M.; Yu, B.J.; Yoo, H.Y.; Park, C. Enhanced Production of Bacterial Cellulose from Miscanthus as Sustainable Feedstock through Statistical Optimization of Culture Conditions. Int. J. Environ. Res. Public Health 2022, 19, 866. https://doi.org/10.3390/ijerph19020866
Son J, Lee KH, Lee T, Kim HS, Shin WH, Oh J-M, Koo S-M, Yu BJ, Yoo HY, Park C. Enhanced Production of Bacterial Cellulose from Miscanthus as Sustainable Feedstock through Statistical Optimization of Culture Conditions. International Journal of Environmental Research and Public Health. 2022; 19(2):866. https://doi.org/10.3390/ijerph19020866
Chicago/Turabian StyleSon, Jemin, Kang Hyun Lee, Taek Lee, Hyun Soo Kim, Weon Ho Shin, Jong-Min Oh, Sang-Mo Koo, Byung Jo Yu, Hah Young Yoo, and Chulhwan Park. 2022. "Enhanced Production of Bacterial Cellulose from Miscanthus as Sustainable Feedstock through Statistical Optimization of Culture Conditions" International Journal of Environmental Research and Public Health 19, no. 2: 866. https://doi.org/10.3390/ijerph19020866
APA StyleSon, J., Lee, K. H., Lee, T., Kim, H. S., Shin, W. H., Oh, J.-M., Koo, S.-M., Yu, B. J., Yoo, H. Y., & Park, C. (2022). Enhanced Production of Bacterial Cellulose from Miscanthus as Sustainable Feedstock through Statistical Optimization of Culture Conditions. International Journal of Environmental Research and Public Health, 19(2), 866. https://doi.org/10.3390/ijerph19020866











