The Effects of Dietary Nitrate Supplementation on Explosive Exercise Performance: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Quality Assessment
2.3. Data Extraction
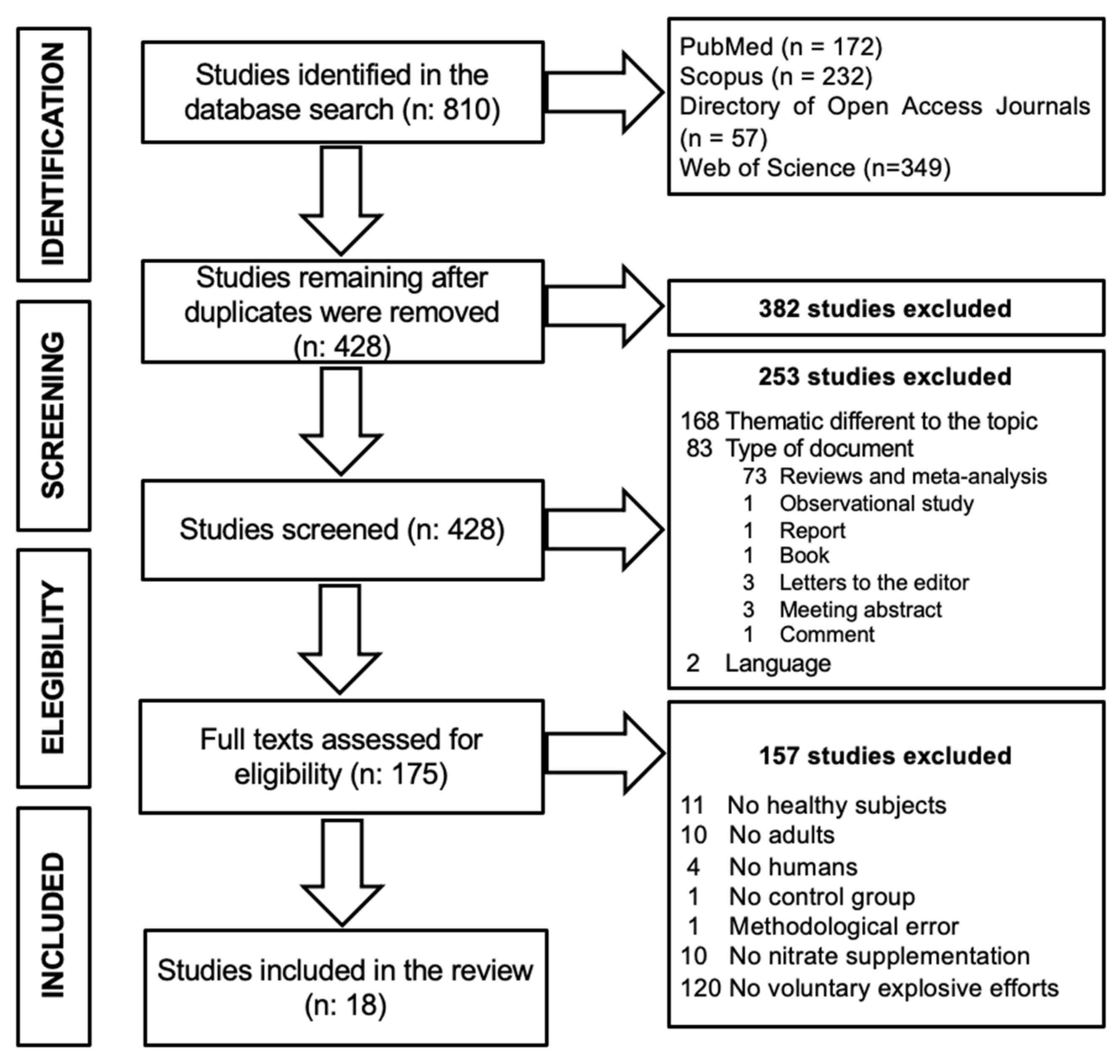
3. Results
3.1. Study Selection
3.2. Study Characteristics
4. Discussion
4.1. The Effects of Dietary Nitrate on Explosive Sprinting Exercise Performance
4.2. The Effects of Dietary Nitrate on Explosive Resistance Exercise Performance
4.3. Potential Factors Influencing Nitrate-Induced Improvements in Explosive Exercise
4.3.1. Nitric Oxide Bioavailability: Supplementation Strategies
4.3.2. Nitric Oxide Bioavailability: Skeletal Muscle Modulations and Storage
4.3.3. Nitric Oxide Bioavailability: Oral Microbiome
4.3.4. Nitric Oxide Bioavailability: Sex Differences
4.3.5. Methodology
4.4. Candidate Physiological Mechanisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stone, M.H.; Moir, G.; Glaister, M.; Sanders, R. How much strength is necessary? Phys. Ther. Sport 2002, 3, 88–96. [Google Scholar] [CrossRef]
- Baker, J.S.; McCormick, M.C.; Robergs, R.A. Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. J. Nutr. Metab. 2010, 2010, 905612. [Google Scholar] [CrossRef] [Green Version]
- Cormie, P.; McGuigan, M.R.; Newton, R.U. Developing maximal neuromuscular power: Part 1—Biological basis of maximal power production. Sports Med. 2011, 41, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Nance, S. The Relation Between Strength and Power in Professional Rugby League Players. J. Strength Cond. Res. 1999, 13, 224–229. [Google Scholar] [CrossRef]
- Bigland-Ritchie, B.; Woods, J.J. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve 1984, 7, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; Little, J.P.; MacDonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Nance, S.; Moore, M. The load that maximizes the average mechanical power output during explosive bench press throws in highly trained athletes. J. Strength Cond. Res. 2001, 15, 20–24. [Google Scholar]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Knapik, J.J.; Steelman, R.A.; Hoedebecke, S.S.; Austin, K.G.; Farina, E.K.; Lieberman, H.R. Prevalence of Dietary Supplement Use by Athletes: Systematic Review and Meta-Analysis. Sports Med. 2016, 46, 103–123. [Google Scholar] [CrossRef] [Green Version]
- Wylie, L.J.; Kelly, J.; Bailey, S.J.; Blackwell, J.R.; Skiba, P.F.; Winyard, P.G.; Jeukendrup, A.E.; Vanhatalo, A.; Jones, A.M. Beetroot juice and exercise: Pharmacodynamic and dose-response relationships. J. Appl. Physiol. 2013, 115, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Gilchrist, M.; Winyard, P.G.; Benjamin, N. Dietary nitrate—Good or bad? Nitric Oxide 2010, 22, 104–109. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on Blood Pressure of Reduced Dietary Sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Boorsma, R.K.; Whitfield, J.; Spriet, L.L. Beetroot Juice Supplementation Does Not Improve Performance of Elite 1500-m Runners. Med. Sci. Sports Exerc. 2014, 46, 2326–2334. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Meissner, G. Physiology of nitric oxide in skeletal muscle. Physiol. Rev. 2001, 81, 209–237. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Spiegelhalder, B.; Eisenbrand, G.; Preussmann, R. Influence of dietary nitrate on nitrite content of human saliva: Possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet. Toxicol. 1976, 14, 545–548. [Google Scholar] [CrossRef]
- Benjamin, N.; O’Driscoll, F.; Dougall, H.; Duncan, C.; Smith, L.; Golden, M.; McKenzie, H. Stomach NO synthesis. Nature 1994, 368, 502. [Google Scholar] [CrossRef]
- Hogg, N. The Biochemistry and Physiology of S-Nitrosothiols. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 585–600. [Google Scholar] [CrossRef]
- Modin, A.; Bjorne, H.; Herulf, M.; Alving, K.; Weitzberg, E.; Lundberg, J. Nitrite-derived nitric oxide: A possible mediator of ‘acidic-metabolic’ vasodilation. Acta Physiol. Scand. 2001, 171, 9–16. [Google Scholar] [CrossRef]
- Castello, P.R.; David, P.S.; McClure, T.; Crook, Z.; Poyton, R.O. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: Implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006, 3, 277–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hlinský, T.; Kumstát, M.; Vajda, P. Effects of Dietary Nitrates on Time Trial Performance in Athletes with Different Training Status: Systematic Review. Nutrients 2020, 12, 2734. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Valverde, D.; Montoya-Rodríguez, J.; Azofeifa-Mora, C.; Sanchez-Urena, B. Effectiveness of beetroot juice derived nitrates supplementation on fatigue resistance during repeated-sprints: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 61, 3395–3406. [Google Scholar] [CrossRef]
- Juan, A.F.S.; Dominguez, R.; Lago-Rodríguez, Á.; Montoya, J.J.; Tan, R.; Bailey, S.J. Effects of Dietary Nitrate Supplementation on Weightlifting Exercise Performance in Healthy Adults: A Systematic Review. Nutrients 2020, 12, 2227. [Google Scholar] [CrossRef] [PubMed]
- Senefeld, J.W.; Wiggins, C.C.; Regimbal, R.J.; Dominelli, P.B.; Baker, S.E.; Joyner, M.J. Ergogenic Effect of Nitrate Supplementation: A Systematic Review and Meta-analysis. Med. Sci. Sports Exerc. 2020, 52, 2250–2261. [Google Scholar] [CrossRef]
- Domínguez, R.; Maté-Muñoz, J.L.; Cuenca, E.; García-Fernández, P.; Mata-Ordoñez, F.; Lozano-Estevan, M.C.; Veiga-Herreros, P.; da Silva, S.F.; Garnacho-Castaño, M.V. Effects of beetroot juice supplementation on intermittent high-intensity exercise efforts. J. Int. Soc. Sports Nutr. 2018, 15, 2. [Google Scholar] [CrossRef] [Green Version]
- Calvo, J.L.; Alorda-Capo, F.; Pareja-Galeano, H.; Jiménez, S.L. Influence of Nitrate Supplementation on Endurance Cyclic Sports Performance: A Systematic Review. Nutrients 2020, 12, 1796. [Google Scholar] [CrossRef]
- Hernández, A.; Schiffer, T.A.; Ivarsson, N.; Cheng, A.J.; Bruton, J.D.; Lundberg, J.O.; Weitzberg, E.; Westerblad, H. Dietary nitrate increases tetatnic [Ca2+]i and contractile forcé in mouse fast-twitch muscle. J. Physiol. 2012, 590, 3575–3583. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Gandra, P.G.; Jones, A.M.; Hogan, M.C.; Nogueira, L. Incubation with sodium nitrite attenuates fatigue development in intact single mouse fibres at physiological PO2. J. Physiol. 2019, 597, 5429–5443. [Google Scholar] [CrossRef]
- Whitfield, J.; Gamu, D.; Heigenhauser, G.J.F.; van Loon, L.J.C.; Spriet, L.L.; Tupling, A.R.; Holloway, G.P. Beetroot Juice Increases Human Muscle Force without Changing Ca2+-Handling Proteins. Med. Sci. Sports Exerc. 2017, 49, 2016–2024. [Google Scholar] [CrossRef]
- Bailey, S.J.; Fulford, J.; Vanhatalo, A.; Winyard, P.G.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J. Appl. Physiol. 2010, 109, 135–148. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, S.K.; Hirai, D.M.; Copp, S.W.; Holdsworth, C.T.; Allen, J.D.; Jones, A.M.; Musch, T.I.; Poole, D.C. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J. Physiol. 2013, 591, 547–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, S.J.; Varnham, R.L.; DiMenna, F.J.; Breese, B.C.; Wylie, L.J.; Jones, A.M. Inorganic nitrate supplementation improves muscle oxygenation, O2 uptake kinetics, and exercise tolerance at high but not low pedal rates. J. Appl. Physiol. 2015, 118, 1396–1405. [Google Scholar] [CrossRef]
- Breese, B.C.; McNarry, M.; Marwood, S.; Blackwell, J.R.; Bailey, S.; Jones, A.M. Beetroot juice supplementation speeds O2 uptake kinetics and improves exercise tolerance during severe-intensity exercise initiated from an elevated metabolic rate. Am. J. Physiol. Integr. Comp. Physiol. 2013, 305, R1441–R1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coggan, A.R.; Peterson, L. Dietary Nitrate Enhances the Contractile Properties of Human Skeletal Muscle. Exerc. Sport Sci. Rev. 2018, 46, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.H.; Sim, A.; Burns, S.F. The Effect of Beetroot Ingestion on High-Intensity Interval Training: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3674. [Google Scholar] [CrossRef]
- Coggan, A.R.; Leibowitz, J.L.; Kadkhodayan, A.; Thomas, D.P.; Ramamurthy, S.; Spearie, C.A.; Waller, S.; Farmer, M.; Peterson, L. Effect of acute dietary nitrate intake on maximal knee extensor speed and power in healthy men and women. Nitric Oxide 2015, 48, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Coggan, A.R.; Leibowitz, J.L.; Spearie, C.A.; Kadkhodayan, A.; Thomas, D.P.; Ramamurthy, S.; Mahmood, K.; Park, S.; Waller, S.; Farmer, M.; et al. Acute Dietary Nitrate Intake Improves Muscle Contractile Function in Patients with Heart Failure: A Double-Blind, Placebo-Controlled, Randomized Trial. Circ. Hearth Fail 2015, 8, 914–920. [Google Scholar] [CrossRef] [Green Version]
- Coggan, A.R.; Broadstreet, S.R.; Mikhalkova, D.; Bole, I.; Leibowitz, J.L.; Kadkhodayan, A.; Park, S.; Thomas, D.P.; Thies, D.; Peterson, L. Dietary nitrate-induced increases in human muscle power: High versus low responders. Physiol. Rep. 2018, 6, e13575. [Google Scholar] [CrossRef]
- Jonvik, K.L.; Hoogervorst, D.; Peelen, H.B.; De Niet, M.; Verdijk, L.B.; Van Loon, L.J.C.; Van Dijk, J.-W. The impact of beetroot juice supplementation on muscular endurance, maximal strength and countermovement jump performance. Eur. J. Sport Sci. 2021, 21, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, E.; Jodra, P.; Pérez-López, A.; González-Rodríguez, L.G.; Fernandes Da Silva, S.; Veiga-Herreros, P.; Domínguez, R. Effects of beetroot juice supplementation on performance and fatigue in a 30-s all-out sprint exercise: A randomized, double-blind cross-over study. Nutrients 2018, 10, 1222. [Google Scholar] [CrossRef] [Green Version]
- Domínguez, R.; Cuenca, E.; Maté-Muñoz, J.L.; García-Fernández, P.; Serra-Paya, N.; Estevan, M.C.L.; Herreros, P.V.; Garnacho-Castaño, M.V. Effects of Beetroot Juice Supplementation on Cardiorespiratory Endurance in Athletes. A Systematic Review. Nutrients 2017, 9, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jodra, P.; Domínguez, R.; Sanchez-Oliver, A.J.; Veiga-Herreros, P.; Bailey, S.J. Effect of Beetroot Juice Supplementation on Mood, Perceived Exertion, and Performance During a 30-Second Wingate Test. Int. J. Sports Physiol. Perform. 2020, 15, 243–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimer, E.G.; Peterson, L.; Coggan, A.R.; Martin, J. Increase in Maximal Cycling Power With Acute Dietary Nitrate Supplementation. Int. J. Sports Physiol. Perform. 2016, 11, 715–720. [Google Scholar] [CrossRef] [Green Version]
- Jonvik, K.; Nyakayiru, J.; Van Dijk, J.; Maase, K.; Ballak, S.; Senden, J.; van Loon, L.J.; Verdijk, L. Repeated-sprint performance and plasma responses following beetroot juice supplementation do not differ between recreational, competitive and elite sprint athletes. Eur. J. Sport Sci. 2018, 18, 524–533. [Google Scholar] [CrossRef]
- Williams, T.D.; Martin, M.P.; Mintz, J.A.; Rogers, R.R.; Ballmann, C.G. Effect of Acute Beetroot Juice Supplementation on Bench Press Power, Velocity, and Repetition Volume. J. Strength Cond. Res. 2020, 34, 924–928. [Google Scholar] [CrossRef]
- Ranchal-Sanchez, A.; Diaz-Bernier, V.M.; Alonso De La Florida-Villagran, C.; Llorente-Cantarero, F.J.; Campos-Perez, J.; Jurado-Castro, J.M. Acute effects of beetroot juice supplements on resistance training: A randomized double-blind crossover. Nutrients 2020, 12, 1912. [Google Scholar] [CrossRef]
- Henneman, E.; Somjen, G.; Carpenter, D.O. Excitability and Inhibitibility of Motoneurons of Different Sizes. J. Neurophysiol. 1965, 28, 599–620. [Google Scholar] [CrossRef]
- Wylie, L.; Bailey, S.; Kelly, J.; Blackwell, J.R.; Vanhatalo, A.; Jones, A.M. Influence of beetroot juice supplementation on intermittent exercise performance. Eur. J. Appl. Physiol. 2016, 116, 415–425. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Grgic, J. Caffeine ingestion enhances Wingate performance: A meta-analysis. Eur. J. Sport Sci. 2018, 18, 219–225. [Google Scholar] [CrossRef]
- Van Tulder, M.; Furlan, A.; Bombardier, C.; Bouter, L. Updated Method Guidelines for Systematic Reviews in the Cochrane Collaboration Back Review Group. Spine 2003, 28, 1290–1299. [Google Scholar] [CrossRef] [Green Version]
- McCrary, J.M.; Ackermann, B.; Halaki, M. A systematic review of the effects of upper body warm-up on performance and injury. Br. J. Sports Med. 2015, 49, 935–942. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge Academic: New York, NY, USA, 1988. [Google Scholar]
- Kokkinoplitis, K.; Chester, N. The effect of beetroot juice on repeated sprint performance and muscle force production. J. Phys. Educ. Sport 2014, 14, 242–247. [Google Scholar]
- López-Samanes, Á.; Pérez-López, A.; Moreno-Pérez, V.; Nakamura, F.Y.; Acebes-Sánchez, J.; Quintana-Milla, I.; Sánchez-Oliver, A.J.; Moreno-Pérez, D.; Fernández-Elías, V.E.; Domínguez, R. Effects of Beetroot Juice Ingestion on Physical Performance in Highly Competitive Tennis Players. Nutrients 2020, 12, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; The PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, C.; Wylie, L.J.; Fulford, J.; Kelly, J.; Black, M.I.; McDonagh, S.T.; Jeukendrup, A.E.; Vanhatalo, A.; Jones, A.M. Dietary nitrate improves sprint performance and cognitive function during prolonged intermittent exercise. Eur. J. Appl. Physiol. 2015, 115, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Vanhatalo, A.; Jell, H.; Fulford, J.; Carter, J.; Nyman, L.; Bailey, S.J.; Jones, A.M. Dietary nitrate supplementation improves sprint and high-intensity intermittent running performance. Nitric Oxide 2016, 61, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Buck, C.L.; Henry, T.; Guelfi, K.; Dawson, B.; Mc Naughton, L.R.; Wallman, K. Effects of sodium phosphate and beetroot juice supplementation on repeated-sprint ability in females. Eur. J. Appl. Physiol. 2015, 115, 2205–2213. [Google Scholar] [CrossRef]
- Smith, K.; Muggeridge, D.J.; Easton, C.; Ross, M.D. An acute dose of inorganic dietary nitrate does not improve high-intensity, intermittent exercise performance in temperate or hot and humid conditions. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 119, 723–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kent, G.L.; Dawson, B.; McNaughton, L.R.; Cox, G.; Burke, L.M.; Peeling, P. The effect of beetroot juice supplementation on repeat-sprint performance in hypoxia. J. Sports Sci. 2019, 37, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Berntzen, B.; Davison, G.W.; West, D.J.; Howatson, G.; Stevenson, E.J. Effects of Beetroot Juice on Recovery of Muscle Function and Performance between Bouts of Repeated Sprint Exercise. Nutrients 2016, 8, 506. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, A.; Castillo, D.; Raya-González, J.; Domínguez, R.; Bailey, S.J. Beetroot juice supplementation increases concentric and eccentric muscle power output. Original investigation. J. Sci. Med. Sport 2020, 24, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Tillin, N.A.; Moudy, S.; Nourse, K.M.; Tyler, C.J. Nitrate Supplement Benefits Contractile Forces in Fatigued but Not Unfatigued Muscle. Med. Sci. Sports Exerc. 2018, 50, 2122–2131. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.J.; Baur, D.A.; Spicer, M.T.; Vukovich, M.; Ormsbee, M.J. The effect of six days of dietary nitrate supplementation on performance in trained CrossFit athletes. J. Int. Soc. Sports Nutr. 2016, 13, 39. [Google Scholar] [CrossRef] [Green Version]
- Haider, G.; Folland, J.P. Nitrate Supplementation Enhances the Contractile Properties of Human Skeletal Muscle. Med. Sci. Sports Exerc. 2014, 46, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, S.; Ramaglia, M.; Bellistri, G.; Pavei, G.; Pugliese, L.; Montorsi, M.; Rasica, L.; Marzorati, M. Aerobic Fitness Affects the Exercise Performance Responses to Nitrate Supplementation. Med. Sci. Sports Exerc. 2015, 47, 1643–1651. [Google Scholar] [CrossRef] [Green Version]
- Wilkerson, D.P.; Hayward, G.M.; Bailey, S.J.; Vanhatalo, A.; Blackwell, J.R.; Jones, A.M. Influence of acute dietary nitrate supplementation on 50 mile time trial performance in well-trained cyclists. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 112, 4127–4134. [Google Scholar] [CrossRef]
- Bond, H.; Morton, L.; Braakhuis, A. Dietary Nitrate Supplementation Improves Rowing Performance in Well-Trained Rowers. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 251–256. [Google Scholar] [CrossRef]
- Trappe, S.; Luden, N.; Minchev, K.; Raue, U.; Jemiolo, B.; Trappe, T.A. Skeletal muscle signature of a champion sprint runner. J. Appl. Physiol. 2015, 118, 1460–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.M.; Loenneke, J.P.; Jo, E.; Wilson, G.J.; Zourdos, M.C.; Kim, J.-S. The Effects of Endurance, Strength, and Power Training on Muscle Fiber Type Shifting. J. Strength Cond. Res. 2012, 26, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rosell, D.; Blanco, F.P.; Aagaard, P.; González-Badillo, J.J. Physiological and methodological aspects of rate of force development assessment in human skeletal muscle. Clin. Physiol. Funct. Imaging 2018, 38, 743–762. [Google Scholar] [CrossRef] [PubMed]
- Wootton-Beard, P.C.; Ryan, L. Combined use of Multiple Methodologies for the Measurement of Total Antioxidant Capacity in UK Commercially Available Vegetable Juices. Plant Foods Hum. Nutr. 2012, 67, 142–147. [Google Scholar] [CrossRef]
- Gago, B.; Lundberg, J.O.; Barbosa, R.M.; Laranjinha, J. Red wine-dependent reduction of nitrite to nitric oxide in the stomach. Free Radic. Biol. Med. 2007, 43, 1233–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flueck, J.L.; Bogdanova, A.; Mettler, S.; Perret, C. Is beetroot juice more effective than sodium nitrate? The effects of equimolar nitrate dosages of nitrate-rich beetroot juice and sodium nitrate on oxygen consumption during exercise. Appl. Physiol. Nutr. Metab. 2016, 41, 421–429. [Google Scholar] [CrossRef]
- Pawlak-Chaouch, M.; Boissière, J.; Gamelin, F.X.; Cuvelier, G.; Berthoin, S.; Aucouturier, J. Effect of dietary nitrate supplementation on metabolic rate during rest and exercise in human: A systematic review and a meta-analysis. Nitric Oxide 2016, 53, 65–76. [Google Scholar] [CrossRef]
- Reid, M.B. Redox interventions to increase exercise performance. J. Physiol. 2015, 594, 5125–5133. [Google Scholar] [CrossRef] [Green Version]
- Jonvik, K.L.; Nyakayiru, J.; Van Dijk, J.-W.; Wardenaar, F.C.; Van Loon, L.J.; Verdijk, L.B. Habitual Dietary Nitrate Intake in Highly Trained Athletes. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Glaister, M.; Pattison, J.R.; Muniz-Pumares, D.; Patterson, S.; Foley, P. Effects of Dietary Nitrate, Caffeine, and Their Combination on 20-km Cycling Time Trial Performance. J. Strength Cond. Res. 2015, 29, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Karampelas, D.; Antonopoulos, K.; Michailidis, Y.; Mitrotasios, M.; Mandroukas, A.; Metaxas, T. Comparison of Ergogenic Effects of Caffeine and Nitrate Supplementation on Speed, Power and Repeated Sprint Performance of Soccer Players. Physiologia 2021, 1, 2. [Google Scholar] [CrossRef]
- Lane, S.C.; Hawley, J.A.; Desbrow, B.; Jones, A.M.; Blackwell, J.R.; Ross, M.L.; Zemski, A.J.; Burke, L.M. Single and combined effects of beetroot juice and caffeine supplementation on cycling time trial performance. Appl. Physiol. Nutr. Metab. 2014, 39, 1050–1057. [Google Scholar] [CrossRef] [Green Version]
- Callahan, M.J.; Parr, E.; Hawley, J.; Burke, L.M. Single and Combined Effects of Beetroot Crystals and Sodium Bicarbonate on 4-km Cycling Time Trial Performance. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 271–278. [Google Scholar] [CrossRef]
- Nyakayiru, J.; van Loon, L.J.; Verdijk, L.B. Could intramuscular storage of dietary nitrate contribute to its ergogenic effect? A mini-review. Free Radic. Biol. Med. 2020, 152, 295–300. [Google Scholar] [CrossRef]
- Gilliard, C.N.; Lam, J.K.; Cassel, K.S.; Park, J.W.; Schechter, A.N.; Piknova, B. Effect of dietary nitrate levels on nitrate fluxes in rat skeletal muscle and liver. Nitric Oxide 2018, 75, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Piknova, B.; Park, J.W.; Swanson, K.M.; Dey, S.; Noguchi, C.T.; Schechter, A.N. Skeletal muscle as an endogenous nitrate reservoir. Nitric Oxide 2015, 47, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Piknova, B.; Park, J.W.; Lam, K.K.J.; Schechter, A.N. Nitrate as a source of nitrite and nitric oxide during exercise hyperemia in rat skeletal muscle. Nitric Oxide 2016, 55-56, 54–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wylie, L.J.; Park, J.W.; Vanhatalo, A.; Kadach, S.; Black, M.I.; Stoyanov, Z.; Schechter, A.N.; Jones, A.M.; Piknova, B. Human skeletal muscle nitrate store: Influence of dietary nitrate supplementation and exercise. J. Physiol. 2019, 597, 5565–5576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyde, E.R.; Andrade, F.; Vaksman, Z.; Parthasarathy, K.; Jiang, H.; Parthasarathy, D.K.; Torregrossa, A.C.; Tribble, G.; Kaplan, H.B.; Petrosino, J.F.; et al. Metagenomic Analysis of Nitrate-Reducing Bacteria in the Oral Cavity: Implications for Nitric Oxide Homeostasis. PLoS ONE 2014, 9, e88645. [Google Scholar] [CrossRef] [Green Version]
- Govoni, M.; Jansson, E.Å.; Weitzberg, E.; Lundberg, J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 2008, 19, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Petersson, J.; Carlström, M.; Schreiber, O.; Phillipson, M.; Christoffersson, G.; Jägare, A.; Roos, S.; Jansson, E.Å.; Persson, A.E.G.; Lundberg, J.O.; et al. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic. Biol. Med. 2009, 46, 1068–1075. [Google Scholar] [CrossRef]
- Bailey, S.J.; Blackwell, J.R.; Wylie, L.J.; Holland, T.; Winyard, P.G.; Jones, A.M. Improvement in blood pressure after short-term inorganic nitrate supplementation is attenuated in cigarette smokers compared to non-smoking controls. Nitric Oxide 2016, 61, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhatalo, A.; L’Heureux, J.E.; Kelly, J.; Blackwell, J.R.; Wylie, L.J.; Fulford, J.; Winyard, P.G.; Williams, D.W.; van der Giezen, M.; Jones, A.M. Network analysis of nitrate-sensitive oral microbiome reveals interactions with cognitive function and cardiovascular health across dietary interventions. Redox Biol. 2021, 41, 101933. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.; Bieuzen, F.; Bleakley, C. Where are all the female participants in Sports and Exercise Medicine research? Eur. J. Sport Sci. 2014, 14, 847–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickham, K.A.; Spriet, L.L. No longer beeting around the bush: A review of potential sex differences with dietary nitrate supplementation. Appl. Physiol. Nutr. Metab. 2019, 44, 915–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranauskas, M.N.; Freemas, J.A.; Tan, R.; Carter, S.J. Moving beyond inclusion: Methodological considerations for the menstrual cycle and menopause in research evaluating effects of dietary nitrate on vascular function. Nitric Oxide 2021, 118, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Rathod, K.S.; Khambata, R.S.; Bahra, M.; Velmurugan, S.; Purba, A.; Watson, D.S.; Barnes, M.R.; Wade, W.G.; Ahluwalia, A. Sex differences in the nitrate-nitrite-NO• pathway: Role of oral nitrate-reducing bacteria. Free Radic. Biol. Med. 2018, 126, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Villanueva, A.; Bishop, D.; Hamer, P. Reproducibility of a 6-s maximal cycling sprint test. J. Sci. Med. Sport 2007, 10, 323–326. [Google Scholar] [CrossRef]
- Bishop, D.; Spencer, M.; Duffield, R.; Lawrence, S. The validity of a repeated spring ability test. J. Sci. Med. Sport 2001, 4, 19–29. [Google Scholar] [CrossRef]
- Glaister, M.; Stone, M.H.; Stewart, A.M.; Hughes, M.; Moir, G.L. The reliability and validity of fatigue measures during short-duration maximal-intensity intermittent cycling. J. Strength Cond. Res. 2004, 18, 459–462. [Google Scholar]
- Sim, A.Y.; Dawson, B.T.; Guelfi, K.J.; E Wallman, K.; Young, W.B. Effects of Static Stretching in Warm-Up on Repeated Sprint Performance. J. Strength Cond. Res. 2009, 23, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Darrall-Jones, J.D.; Jones, B.; Roe, G.; Till, K. Reliability and Usefulness of Linear Sprint Testing in Adolescent Rugby Union and League Players. J. Strength Cond. Res. 2016, 30, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.; Lawrence, S.; Rechichi, C.; Bishop, D.; Dawson, B.; Goodman, C. Time-motion analysis of elite field hockey, with special reference to repeated-sprint activity. J. Sports Sci. 2004, 22, 843–850. [Google Scholar] [CrossRef]
- Spencer, M.; Bishop, D.; Dawson, B.; Goodman, C. Physiological and Metabolic Responses of Repeated-Sprint Activities. Sports Med. 2005, 35, 1025–1044. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S. Redox signaling: Nitrosylation and related target interactions of nitric oxide. Cell 1994, 78, 931–936. [Google Scholar] [CrossRef]
- Whitfield, J.; Ludzki, A.; Heigenhauser, G.J.F.; Senden, J.M.G.; Verdijk, L.; van Loon, L.J.; Spriet, L.L.; Holloway, G.P. Beetroot juice supplementation reduces whole body oxygen consumption but does not improve indices of mitochondrial efficiency in human skeletal muscle. J. Physiol. 2016, 594, 421–435. [Google Scholar] [CrossRef] [Green Version]
- Vanhatalo, A.; Jones, A.M.; Blackwell, J.R.; Winyard, P.G.; Fulford, J. Dietary nitrate accelerates postexercise muscle metabolic recovery and O2 delivery in hpoxia. J. Appl. Physiol. 2014, 117, 1460–1470. [Google Scholar] [CrossRef] [Green Version]

| Parameter | Inclusion Criteria |
|---|---|
| Population | Adult healthy population |
| Intervention | Acute and/or chronic Supplementation with NO3− |
| Comparison | A placebo condition (supplementation depleted on NO3−) |
| Outcome | Variables related to performance of explosive efforts (≤6 s [2]) |
| Setting | Randomized double-blind placebo-controlled studies |
| Reference | Subjects | Supplementation | Exercise Protocol | Results |
|---|---|---|---|---|
| Buck et al. [61] | 13 female amateur basketball and soccer players | 6 d of BR supplementation (NO3− 6 mmol per day) | Before, during, and after 60 min in a simulated team-game: 6 × 20 m running sprints, 25 s rest | ↔ Best sprint time (set 1): −0.3% (3.68 ± 0.26 vs. 3.69 ± 0.25 s; d = 0.04 [−0.85 to 0.77]) ↔ Best sprint time (set 2): −0.8% (3.77 ± 0.32 vs. 3.80 ± 0.25 s; d = 0.11 [−0.91 to 0.71]) ↔ Best sprint time (set 3): +1.1% (3.81 ± 0.32 vs. 3.77 ± 0.25 s; d = 0.14 [−0.68 to 0.95]) |
| Rimer et al. [44] | 13 competitive athletes (female, n = 2, male, n = 11) | 2.5 h prior to exercise acute BR ingestion (NO3− 11.2 mmol) | 4 × 3 to 4 s cycling sprints, 2 min rest | ↑ Pmax: +6 ± 2.6 vs. 2 ± 3.8% (d = 1.21 [0.31 to 2.07]) ↑ RPMopt: +6.5 ± 11.4 vs. 0.3 ± 4.1% (d = 0.79 [−0.14 to 1.54]) |
| Smith et al. [62] | 12 male recreationally active athletes | 3 h prior to exercise acute BR ingestion (NO3− 6.2 mmol) | 20 × 6 s cycling sprints in temperate (22.5 °C) and hot environmental conditions (30 °C), 114 s rest | ↔ Ppeak (hot): −6.0% (659 ± 100 vs. 683 ± 139 W; d = 0.21 [−1.04 to 0.66]) ↔ Pmean (temperate): −1.6% (562 ± 120 vs. 571 ± 124 W; d = 0.08 [−0.92 to 0.78]) ↔ Pmean (hot): −5.9% (543 ± 29 vs. 575 ±39 W; d = 0.97 [−11.79 to −0.01]) ↔ Total work (temperate): −1.5% (67.44 ± 14.39 vs. 68.46 ± 15.07 kJ; d = 0.07 [−0.91 to 0.78]) ↔ Total work (hot): −5.6% (66.07 ± 10.84 vs. 69.74 ± 15.13 kJ; d = 0.03 [−1.12 to 0.58]) |
| Thompson et al. [59] | 16 male recreational team-sport players | 2.5 h prior to exercise and 7 d of BR supplementation (NO3− 12.8 mmol per day) | 2 × 40 min cycling sprints, 15 min rest 10 × 6 s sprints, 100 s rest at 35% VO2max + 14 s passive rest 5 × 4 s sprints, 16 s rest at 35% VO2max 10 × 6 s sprints, 100 s rest at 35% VO2max + 14 s passive rest | ↑ Total work: +3.5% (123 ± 19 vs. 119 ± 17 kJ; d = 0.23 [−0.51 to 0.94]) |
| Wylie et al. [49] | 10 male recreational team-sport players | 2.5 h prior to exercise and 3 to 5 d of BR supplementation (NO3− 8.2 mmol per day) | 24 × 6 s cycling sprints, 24 s rest | ↔ Ppeak (mean): +1.3% (792 ± 159 vs. 782 ± 154 W; d = 0.07 [−0.88 to 1.00]) ↑ Pmean (sprints 1–6): +7.3% (694 ± 125 vs. 647 ± 122 W; d = 0.39 [−0.58 to 1.31]) ↔ Pmean (sprints 7–12): +3.9% (560 ± 100 vs. 539 ± 112 W; d = 0.20 [−0.75 to 1.13]) ↔ Pmean (sprints 13–18): +5.3% (518 ± 111 vs. 492 ± 121 W; d = 0.23 [−0.73 to 1.16]) ↔ Pmean(sprints 19–24): +4.8% (500 ± 114 vs. 477 ± 119 W; d = 0.20 [−0.75 to 1.13]) ↑ Pmean (mean): +5.4% (568 ± 136 vs. 539 ± 136 W; d = 0.22 [−0.74 to 1.15]) |
| Kent et al. [63] | 12 male team sport players | 2 h prior to exercise acute BR ingestion (NO3− 13 mmol) | 4 sets of 9 × 4 s cycling sprints with 16 s active + 6 s passive rest, interspersed with 3 min rest (3000 m simulated altitude) | ↔ Ppeak (set 1): −2.4% (1185 ± 172 vs. 1214 ± 179 W; d = 0.17 [−1.01 to 0.69]) ↔ Ppeak (set 2): −1.0% (1157 ± 178 vs. 1181 ± 163 W; d = 0.15 [−0.98 to 0.71]) ↔ Ppeak (set 3): −0.5% (1159 ± 186 vs. 1165 ± 160 W; d = 0.04 [−0.88 to 0.81]) ↔ Ppeak (set 4): −1.0% (1152 ± 194 vs. 1164 ± 139 W; d = 0.07 [−0.92 to 0.78]) ↔ Pmean (set 1): −2.7% (807 ± 144 vs. 829 ± 144 W; d = 0.16 [−1.00 to 0.70]) ↔ Pmean (set 2): +3.1% (794 ± 156 vs. 770 ± 142 W; d = 0.17 [−0.69 to 1.00]) ↔ Pmean (set 3): +2.1% (809 ± 150 vs. 792 ± 131 W; d = 0.15 [−0.73 to 0.96]) ↔ Pmean (set 4): −1.0% (779 ± 156 vs. 804 ± 122 W; d = 0.19 [−1.02 to 0.68]) ↔ Total work (set 1): −2.7% (29.0 ± 5.18 vs. 29.8 ± 5.19 J; d = 0.16 [−1.00 to 0.70]) ↔ Total work (set 2): −0.5% (28.5 ± 5.61 vs. 28.7 ± 5.10 J; d = 0.04 [−0.88 to 0.81]) ↔ Total work (set 3): +2.5% (29.1 ± 5.38 vs. 28.4 ± 4.75 J; d = 0.14 [−0.71 to 0.98]) ↔ Total work (set 4): +0.2% (28.9 ± 5.62 vs. 28.9 ± 4.39 J; d = 0.00 [−0.85 to 0.85]) ↔ Work decrement (set 1): −47.1% (11.9 ± 6.9 vs. 17.5 ± 11.7%; d = 0.48 [−1.43 to 0.30]) ↔ Work decrement (set 2): −21.7% (12.9 ± 9.1 vs. 15.7 ± 14.5%; d = 0.47 [−1.07 to 0.63]) ↔ Work decrement (set 3): +4.5% (13.9 ± 8.4 vs. 13.3 ± 11%; d = 0.09 [−0.79 to 0.91]) ↔ Work decrement (set 4): +2.5% (12.2 ± 6.3 vs. 11.9 ± 7.1%; d = 0.05 [−0.80 to 0.89]) |
| Kokkinoplitis et al. [56] | 7 healthy males | 3 h prior to exercise acute BR ingestion (NO3− 6.45 mmol) | 5 × 6 s running sprints on treadmill, 30 s rest | ↔ Ppeak (mean): +4.9% (4133.5 ± 674.4 vs. 3938.3 ± 603.1 W; d = 0.33 [−0.89 to 1.46]) |
| Thompson et al. [60] | 36 team sport players | 2.5 h prior to exercise and 5 d of BR supplementation (12.8 mmol NO3− per day) | 5 × 20 m running sprints in running lanes, 30 s rest | ↓ Total time: −1.2% (3.98 ± 0.18 vs. 4.03 ± 0.19 s; d = 0.27 [−0.71 to 0.20]) ↓ Time (5 m): −2.3% (1.73 ± 0.09 vs. 1.77 ± 0.09 s; d = 0.45 [−0.92 to 0.04]) ↓ Time (10 m): −1.6% (2.53 ± 0.2 vs. 2.57 ± 0.12 s; d = 0.25 [−0.71 to 0.23]) ↓ Time (5–10 m): −1.2% (0.80 ± 0.04 vs. 0.81 ± 0.04 s; d = 0.25 [−0.72 to 0.22]) ↔ Time (10–20 m): −0.7% (1.45 ± 0.07 vs. 1.46 ± 0.09 s; d = 0.13 [−0.59 to 0.35]) |
| Clifford et al. [64] | 20 male team sport players | 4 d of BR supplementation (2.31 mmol NO3− per day) | 20 × 30 m sprints, 30 s rest | ↔ Best sprint time: BR −0.7% (4.38 ± 0.17 vs. 4.41 ± 0.23 s; d = 0.15 [−0.79 to 0.50]) and PL +1.1% (4.53 ± 0.15 vs. 4.48 ± 0.14 s; d = 0.35 [−0.31 to 0.98]). |
| López-Samanes et al. [57] | 13 trained male tennis players | 3 h prior to exercise of acute BR ingestion (6.4 mmol NO3−) | 5 tennis serves, 2 × 10 m sprints, 2 × agility test (5–0–5), 1 min rest | ↔ Serve speed: −2.7% (160.6 ± 10.4 vs. 165.0 ± 10.8 km/h; d = 0.15 [−1.22 to 0.42]) ↔ Best sprint time (10 m): +1.1% (1.86 ± 0.07 vs. 1.88 ± 0.05 s; d = 0.39 [−1.13 to 0.50]) ↔ Best sprint time (5–0–5): +2.0% (2.60 ± 0.10 vs. 2.64 ± 0.10 s; d = 0.69 [−1.21 to 0.43]) |
| Reference | Subjects | Supplementation | Exercise Protocol | Results |
|---|---|---|---|---|
| Ranchal-Sánchez et al. [47] | 12 resistance-trained male athletes | 2 h prior to exercise acute BR ingestion (NO3− 6.4 mmol) | 2 × concentric Smith-machine back squats and bench press at 60%, 70%, and 80% 1RM, 2 min rest | ↔ Pmax (60% 1RM) squat: +1.8% (389 ± 117 vs. 382 ± 111 W; d = 0.06 [−0.79 to 0.91]) ↔ Pmax (70% 1RM) squat: −0.5% (393 ± 116 vs. 395 ± 107 W; d = 0.02 [−0.83 to 0.83]) ↔ Pmax (80% 1RM) squat: −0.3% (377 ± 108 vs. 378 ± 96 W; d = 0.01 [−0.86 to 0.84]) ↔ Pmax (60% 1RM) bench press: −1.0% (289 ± 88 vs. 292 ± 94 W; d = 0.03 [−0.88 to 0.81]) ↔ Pmax (70% 1RM) bench press: +1.7% (242 ± 81 vs. 238 ± 81 W; d = 0.05 [−0.80 to 0.89]) ↔ Pmax (80% 1RM) bench press: −8.5% (176 ± 66 vs. 191 ± 55 W; d = 0.26 [−1.09 to 0.61]) ↔ Vmax (60% 1RM) squat: +1.8% (0.70 ± 0.09 vs. 0.69 ± 0.09 m/s; d = 0.14 [−0.74 to 0.95]) ↔ Vmax (70% 1RM) squat: +0.0% (0.61 ± 0.08 vs. 0.61 ± 0.08 m/s; d = 0.08 [−0.85 to 0.85]) ↔ Vmax (80% 1RM) squat: +0.0% (0.51 ± 0.09 vs. 0.51 ± 0.06 m/s; d = 0.01 [−0.85 to 0.85]) ↔ Vmax (60% 1RM) bench press: +0.0% (0.61 ± 0.08 vs. 0.61 ± 0.08 m/s; d = 0.04 [−0.85 to 0.85]) ↔ Vmax (70% 1RM) bench press: +0.0% (0.43 ± 0.06 vs. 0.43± 0.08 m/s; d = 0.03 [−0.85 to 0.85]) ↔ Vmax (80% 1RM) bench press: −9.7% (0.28 ± 0.05 vs. 0.31 ± 0.05 m/s; d = 0.62 [−1.45 to 0.29]) |
| Rodríguez-Fernández et al. [65] | 18 trained male athletes | 2.5 h prior to exercise acute BR ingestion (NO3− 12.9 mmol) | 4 × 8 half squat in a flywheel device (0.025, 0.05 and 0.100 kg/m2) with 3 min of rest | ↑ Ppeak CON (0.025 kg/m2): +16.4% (1251 ± 249 vs. 1075 ± 205 W; d = 0.79 [0.05 to 1.46]) ↑ Ppeak ECC (0.025 kg/m2): +18.9% (1195 ± 265 vs. 1005 ± 176 W; d = 0.87 [0.12 to 1.53]) ↑ Ppeak CON (0.050 kg/m2): +15.3% (1182 ± 226 vs. 1025 ± 181 W; d = 0.79 [0.05 to 1.45]) ↑ Ppeak ECC (0.050 kg/m2): +12.9% (1168 ± 261 vs. 1034 ± 172 W; d = 0.62 [−0.10 to 1.29]) ↑ Ppeak CON (0.075 kg/m2): +20.8% (1132 ± 239 vs. 937 ± 158 W; d = 0.99 [0.23 to 1.66]) ↑ Ppeak ECC (0.075 kg/m2): +19.7% (1201 ± 261 vs. 1003 ± 187 W; d = 0.90 [0.19 to 1.20]) ↑ Ppeak CON (0.100 kg/m2): +18.4% (1008 ± 197 vs. 851 ± 161 W; d = 0.90 [0.14 to 1.56]) ↑ Ppeak ECC (0.100 kg/m2): +12.0% (1070 ± 230 vs. 955 ± 191 W; d = 0.56 [−0.16 to 1.22]) ↑ Pmean CON (0.025 kg/m2): +16.4% (750 ± 173 vs. 644 ± 153 W; d = 0.67 [−0.06 to 1.33]) ↑ Pmean ECC (0.025 kg/m2): +19.6% (684 ± 154 vs. 572 ± 131 W; d = 0.81 [0.06 to 1.47]) ↑ Pmean CON (0.050 kg/m2): +18.6% (709 ± 146 vs. 598 ± 140 W; d = 0.80 [0.06 to 1.46]) ↑ Pmean ECC (0.050 kg/m2): +17.8% (687 ± 150 vs. 583 ± 162 W; d = 0.69 [−0.04 to 1.35]) ↑ Pmean CON (0.075 kg/m2): +21.9% (672 ± 157 vs. 551 ± 120 W; d = 0.89 [0.14 to 1.56]) ↑ Pmean ECC (0.075 kg/m2): +22.2% (709 ± 177 vs. 580 ± 145 W; d = 0.82 [0.08 to 1.48]) ↑ Pmean CON (0.100 kg/m2): +21.7% (600 ± 127 vs. 493 ± 120 W; d = 0.89 [0.14 to 1.56]) ↑ Pmean ECC (0.010 kg/m2): +13.9% (615 ± 150 vs. 540 ± 139 W; d = 0.53 [−0.18 to 1.20]) |
| Tillin et al. [66] | 17 male recreationally active athletes | 2.5 h prior to exercise and 7 d of BR supplementation (NO3− 12.9 mmol per day) | 10 × MIVC leg extensions, 1 min rest | ↔ Fmax: +0.27% (741 ± 136 vs. 739 ± 135 N; d = 0.02 [−0.68 to 0.71]) |
| Williams et al. [46] | 11 resistance-trained male athletes | 2 h prior to exercise of BR ingestion (NO3− 6.4 mmol) | 2 × 2 at 70% 1RM free-weight bench press, 3 min rest | ↑ Pmean: +19.5% (607± 112 vs. 508 ± 118 W; d = 0.19 [−0.10 to 1.76]) ↑ Vmean: +6.5% (0.66 ± 0.08 vs. 0.62 ± 0.08 m/s; d = 0.52 [−0.42 to 1.38]) |
| Kramer et al. [67] | 12 trained male CrossFit athletes | 6 d of KNO3 supplementation (NO3− 8 mmol per day) | 2 sets × 5 isometric knee extensions/flexions, 60° flexion, 5 s rest, interspersed with 1 min rest 2 × 5 isokinetic knee extensions and flexions at 60°/s and 180°/s, 1 min rest | ↔ Tpeak (isometric extension): KNO3 +10.2% (186 ± 49 vs. 169 ± 37 N; d = 0.42 [−0.48 to 1.23]) and PL +6.1% (185 ± 43 vs. 174 ± 28 N; d = 0.31 [−0.56 to 1.14]) ↔ Tpeak (isometric flexion): KNO3 +1.8% (119 ± 27 vs. 117 ± 21 N; d = 0.09 [−0.77 to 0.93]) and PL +4.8% (126 ± 20 vs. 120 ± 17 N; d = 0.33 [−0.54 to 1.16]). ↔ Tpeak (extension at 60°/s): KNO3 −4.1% (168 ± 50 vs. 175 ± 41 N; d = 0.16 [−1.00 to 0.70]) and PL −2.7% (179 ± 44 vs. 184 ± 48.53 N; d = 0.11 [−0.95 to 0.74]) ↔ Tpeak (flexion at 60°/s): KNO3 −1.5% (102 ± 26 vs. 104 ± 21 N; d = 0.07 [−0.93 to 0.77]) and PL −2.7% (104 ± 25 vs. 106 ± 25 N; d = 0.12 [−0.92 to 0.77]) ↔ Tpeak (extension at 180°/s): KNO3 +6.5% (128 ± 32 vs. 120 ± 36 N; d = 0.24 [−0.62 to 1.08]) and PL +2.6% (123 ± 35 vs. 120 ± 42 N; d = 0.09 [−0.77 to 0.92]) ↔ Tpeak (flexion at 180°/s): KNO3 +0.4% (80 ± 16 vs. 79 ± 14 N; d = 0.02 [−0.78 to 0.91]) and PL +0.8% (76 ± 20 vs. 76 ± 28 N; d = 0.02 [−0.85 to 0.85]) |
| Jonvik et al. [40] | 14 male recreationally active athletes | 3 h prior to exercise and 6 d of BR supplementation (NO3− 15.8 mmol per day) | 5 × CMJ, 1 min rest 5 isokinetic knee extensions and flexions at 60°/s, 120°/s, 180°/s, and 300°/s. 3 × 4 s MIVC leg extension with 30° and 60° of flexion, 1 min rest | ↔ Pmax (extension at 60°/s): +0.9% (220 ± 45 vs. 218 ± 40 W; d = 0.05 [−0.73 to 0.82]) ↔ Pmax (extension at 120°/s): +1.3% (392 ± 74 vs. 387 ± 62 W; d = 0.08 [−0.71 to 0.85]) ↔ Pmax (extension at 180°/s): +2.7% (500 ± 86 vs. 487 ± 67 W; d = 0.18 [−0.61 to 0.94]) ↔ Pmax (extension at 300°/s): +1.8% (554 ± 102 vs. 544 ± 81 W; d = 0.11 [−0.67 to 0.88]) ↑ Pmax (flexion at 60°/s): +2.0% (151 vs. 148 W; d = unknown) ↔ Pmax (flexion at 120°/s): +1.3% (392 ± 74 vs. 387 ± 62 W; d = 0.08 [−0.71 to 0.85]) ↔ Pmax (flexion at 180°/s): +2.9% (391 ± 57 vs. 380 ± 58 W; d = 0.20 [−0.59 to 0.96]) ↔ Pmax (flexion at 300°/s): +1.6% (493 ± 73 vs. 485 ± 81 W; d = 0.11 [−0.68 to 0.88]) ↔ Smax (flexion of 30°): +2.0% (204 ± 39 vs. 200 ± 37 Nm; d = 0.11 [−0.68 to 0.88]) ↔ Smax (flexion of 60°): +0.4% (286 ± 43 vs. 285 ± 47 Nm; d = 0.02 [−0.76 to 0.80]) ↔ CMJ height: −0.7% (39.3 ± 6.3 vs. 39.6 ± 6.3 cm; d = 0.05 [−0.82 to 0.73]) ↔ GRFmax: −0.5% (3.04 vs. 3.06 N; d = unknown) |
| Coggan et al. [37] | 12 active athletes (female, n = 5, male, n = 7) | 2 h prior to exercise acute BR ingestion (NO3− 11.2 mmol) | 3–4 isokinetic knee extensions at 0 rad/s, 1.57 rad/s, 3.14 rad/s, 4.17 rad/s, and 6.28 rad/s, 2 min rest | ↔ Ppeak (extension at 1.57 rad/s): −2.1% (3.31 ± 0.16 vs. 3.38 ± 0.21 W/; d = 0.39 [−1.22 to 0.49]) ↔ Ppeak (extension at 3.14 rad/s): −1.9% (5.38 ± 0.32 vs. 5.48 ± 0.38 W/kg; d = 0.30 [−1.13 to 0.58]) ↔ Ppeak (extension at 4.17 rad/s): +0.0% (6.67 ± 0.46 vs. 6.67 ± 0.50 W/kg; d = 0.00 [−0.85 to 0.85]) ↑ Ppeak (extension at 6.28 rad/s): +4.1% (7.64 ± 0.52 vs. 7.34 ± 0.54 W/kg; d = 0.59 [−0.32 to 1.41]) ↔ Tpeak (extension at 1.57 rad/s): −1.9% (2.11 ± 0.10 vs. 2.15 ± 0.11 Nm/kg; d = 0.40 [−1.22 to 0.49]) ↔ Tpeak (extension at 3.14 rad/s): −1.8% (1.71 ± 0.10 vs. 1.74 ± 0.12 Nm/kg; d = 0.28 [−1.11 to 0.59]) ↔ Tpeak (extension at 4.17 rad/s): +0.0% (1.42 ± 0.10 vs. 1.42 ± 0.11 Nm/kg; d = 0.00 [−0.85 to 0.85]) ↔ Tpeak (extension at 6.28 rad/s): +4.3% (1.22 ± 0.08 vs. 1.17 ± 0.08 Nm/kg; d = 0.65 [−0.26 to 1.47]) ↔ Tmax (0 rad/s): −1.5% (2.6 ± 0.13 vs. 2.64 ± 0.13 Nm/kg; d = 0.32 [−1.15 to 0.55]) |
| Kokkinoplitis et al. [56] | 7 healthy males | 3 h prior to exercise acute BR ingestion (NO3− 6.45 mmol) | Isokinetic knee extension and flexion at 60°/s and 240°/s | ↔ Tpeak (extension at 60°/s): −2.6% (200.2 ± 25.8 vs. 207.4 ± 37.5 Nm; d = 0.24 [−1.38 to 0.96]) ↔ Tpeak (extension at 240°/s): −5.9% (124.1 ± 9.2 vs. 131.4 ± 17.1 Nm; d = 0.57 [−1.68 to 0.69]) ↔ Tpeak (flexion at 60°/s): −7.4% (103.3 ± 27.7 vs. 110.9 ± 29.9 Nm; d = 0.28 [−1.42 to 0.92]) ↔ Tpeak (flexion at 240°/s): −16.1% (59.8 ± 29.5 vs. 69.4 ± 21.5 Nm; d = 0.40 [−1.52 to 0.83]) |
| López-Samanes et al. [57] | 13 trained male tennis players | 3 h prior to exercise acute BR ingestion (NO3− 6.4 mmol) | 2 MIVC handgrip 3 CMJ with 45 s of rest | ↔ Smax (handgrip): +3.9% (47.8 ± 9.3 vs. 46.0 ± 7.9 kg; d = 0.26 [−0.61 to 1.01]) ↔ CMJ height: + 2.5% (33.0 ± 4.9 vs. 32.2 ± 5.1 cm; d = 0.143 [−0.66 to 0.97]) |
| Haider et al. [68] | 19 healthy males | 2.5 h prior to exercise and 7 d of BR supplementation (NO3− ~9.7 mmol per day) | 4 × 3 s MIVC leg extension with 110° of flexion with ≥30 s rest 15 × 1 s isometric knee extensions with ≥15 s rest | ↔ Fmax: (value not specified; d = unknown) |
| Reference | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | Item 9 | Item 10 | Item 11 | Item 12 | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buck et al. [61] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10/11 |
| Rimer et al. [44] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10/11 |
| Smith et al. [62] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10/11 |
| Thompson et al. [60] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | 9/11 |
| Wylie et al. [49] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | 9/11 |
| Kent et al. [63] | No | No | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | No | 7/11 |
| Kokkinoplitis et al. [56] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | 9/11 |
| Thompson et al. [59] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | 9/11 |
| Clifford et al. [64] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10/11 |
| López-Samanes et al. [57] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | 9/11 |
| Ranchal-Sánchez et al. [47] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10/11 |
| Rodríguez-Fernández et al. [65] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | 9/11 |
| Tillin et al. [66] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10/11 |
| Williams et al. [46] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10/11 |
| Kramer et al. [67] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10/11 |
| Jonvik et al. [40] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10/11 |
| Coggan et al. [37] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | 9/11 |
| Haider et al. [68] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10/11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, R.; Cano, L.; Lago-Rodríguez, Á.; Domínguez, R. The Effects of Dietary Nitrate Supplementation on Explosive Exercise Performance: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 762. https://doi.org/10.3390/ijerph19020762
Tan R, Cano L, Lago-Rodríguez Á, Domínguez R. The Effects of Dietary Nitrate Supplementation on Explosive Exercise Performance: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(2):762. https://doi.org/10.3390/ijerph19020762
Chicago/Turabian StyleTan, Rachel, Leire Cano, Ángel Lago-Rodríguez, and Raúl Domínguez. 2022. "The Effects of Dietary Nitrate Supplementation on Explosive Exercise Performance: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 2: 762. https://doi.org/10.3390/ijerph19020762
APA StyleTan, R., Cano, L., Lago-Rodríguez, Á., & Domínguez, R. (2022). The Effects of Dietary Nitrate Supplementation on Explosive Exercise Performance: A Systematic Review. International Journal of Environmental Research and Public Health, 19(2), 762. https://doi.org/10.3390/ijerph19020762









