Dynamic of Glucose Homeostasis in Virtual Patients: A Comparison between Different Behaviors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mathematical Representation
2.1.1. Glucose Homeostasis Representation
2.1.2. Effect of Physical Activity on the Dynamics of Glucose Homeostasis
2.1.3. System Inputs and Disturbances
2.2. Experimentation Definition
2.2.1. Personal Factor Measurements
2.2.2. Instrumentation
2.2.3. Sectioning
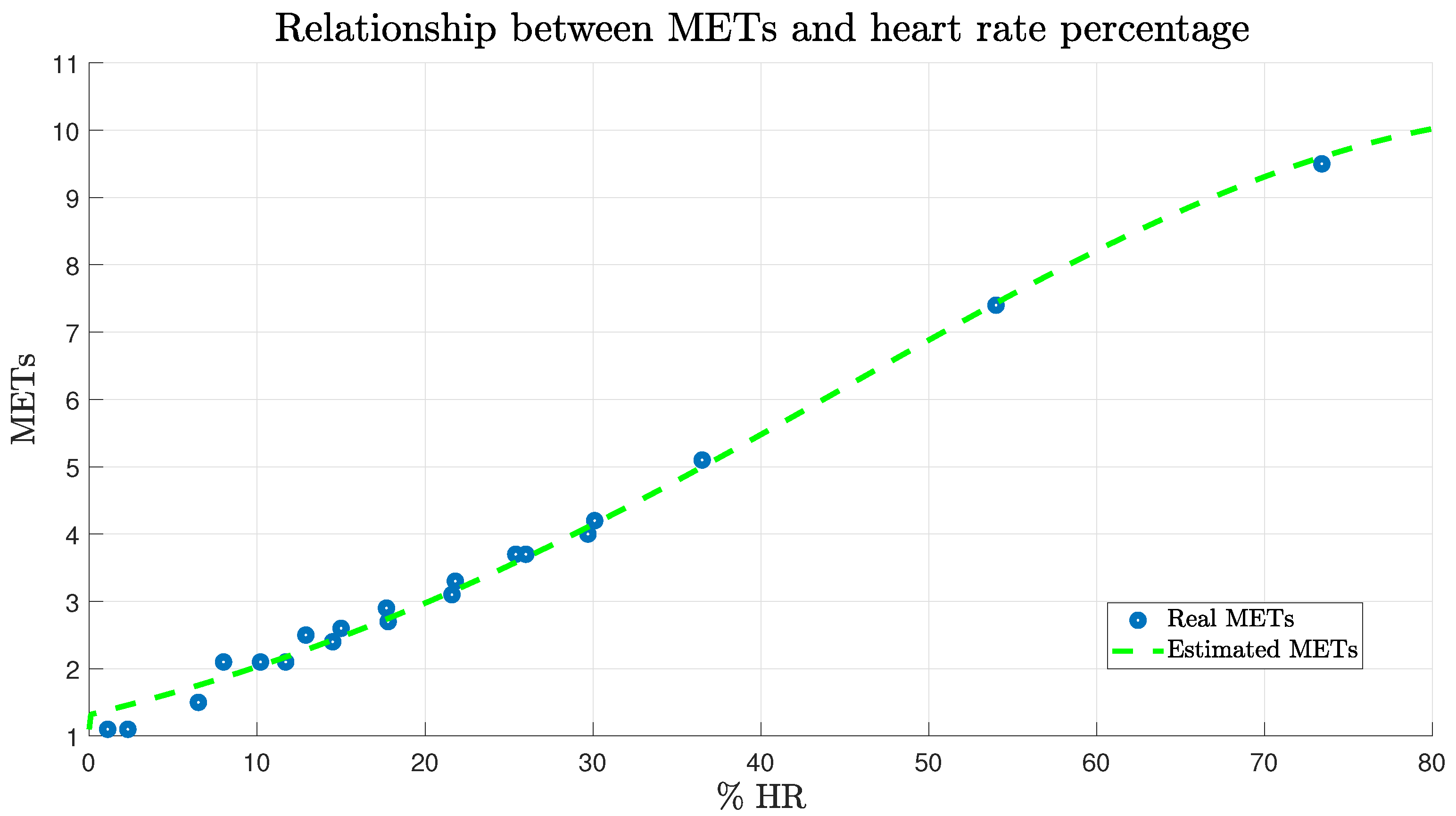
2.2.4. Metabolic Equivalent of Task
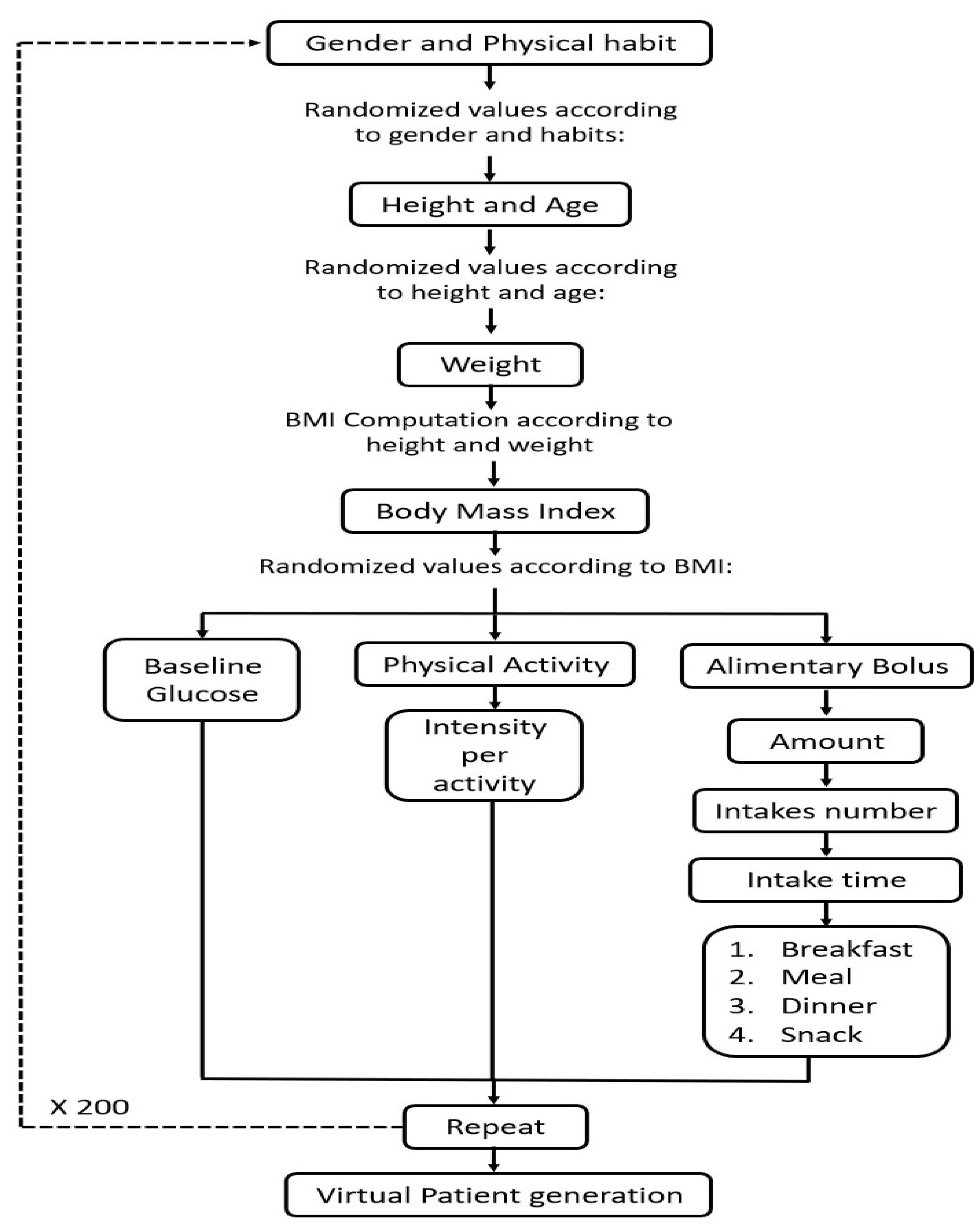
2.2.5. Generation of a Population Sample based on the Monte Carlo Approach
3. Results
3.1. Effect of Primary and Secondary Factors on the Behavior of Glucose Homeostasis
3.2. Dynamic Behavior of Glucose Homeostasis
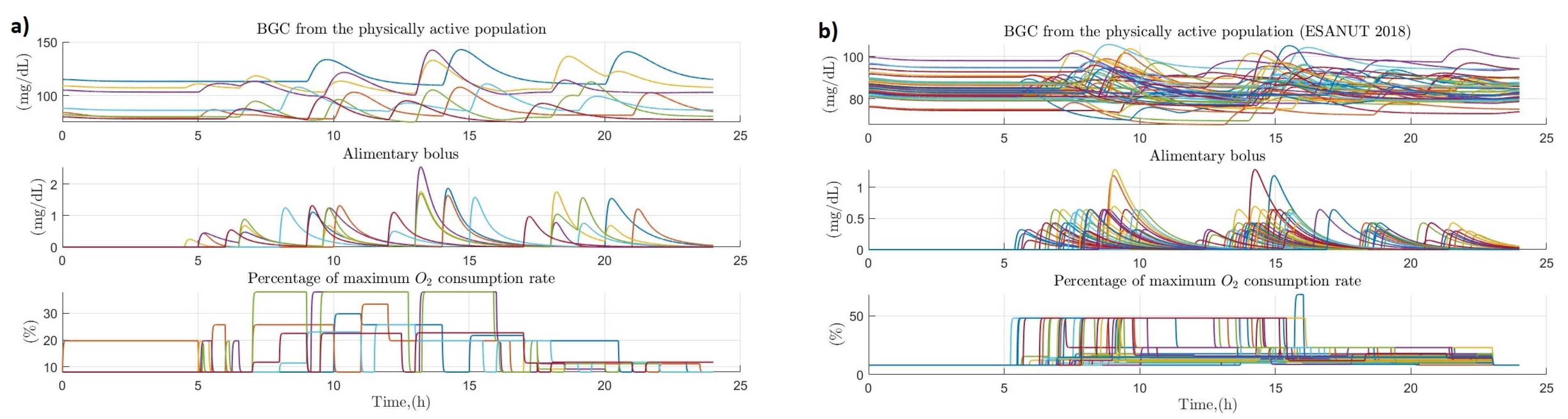
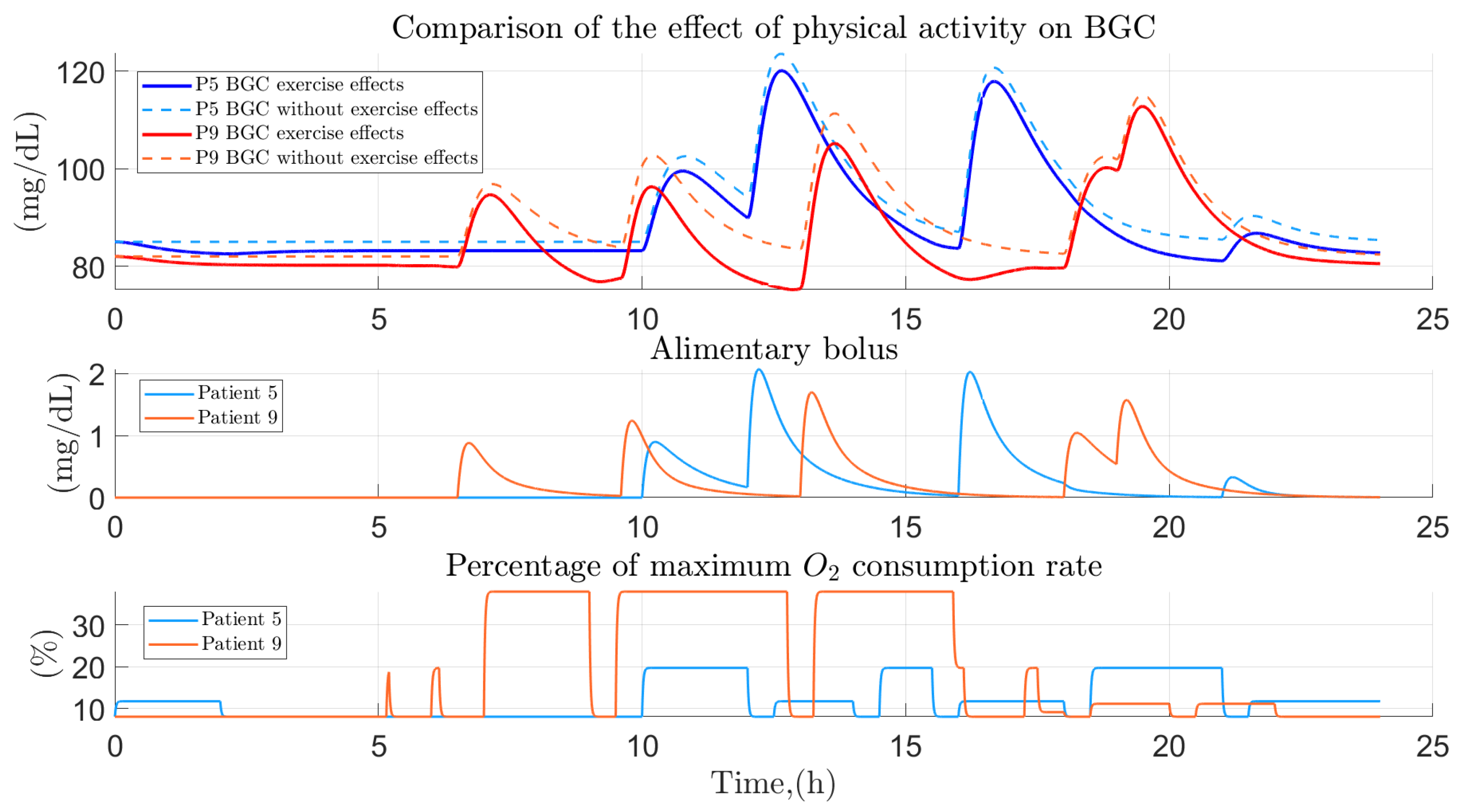
3.3. People with Regular PHYSICAL activity
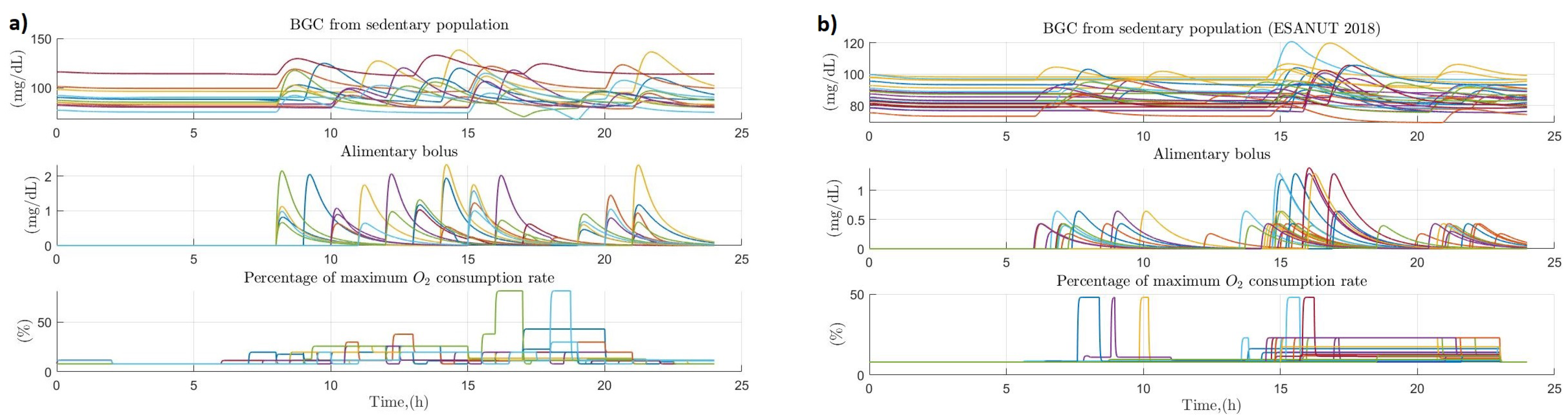
3.4. People with Sedentary Habits
3.5. Comparison between the Variation of Glucose Homeostasis of the Analyzed Profiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- INEGI. Estadísticas a Propósito del Día Mundial de la Diabetes (14 de Noviembre); Datos Nacionales; INEGI: Aguascalientes, Mexico, 2021. [Google Scholar]
- Williams, R.; Karuranga, S.; Malanda, B.; Saeedi, P.; Basit, A.; Besançon, S.; Bommer, C.; Esteghamati, A.; Ogurtsova, K.; Zhang, P.; et al. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2020, 162, 108072. [Google Scholar] [CrossRef] [Green Version]
- Dendup, T.; Feng, X.; Clingan, S.; Astell-Burt, T. Environmental risk factors for developing type 2 diabetes mellitus: A systematic review. Int. J. Environ. Res. Public Health 2018, 15, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88. [Google Scholar] [CrossRef]
- Sattar, N.; Rawshani, A.; Franzén, S.; Rawshani, A.; Svensson, A.M.; Rosengren, A.; McGuire, D.K.; Eliasson, B.; Gudbjörnsdottir, S. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks: Findings from the Swedish National Diabetes Registry. Circulation 2019, 139, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lou, X. Type 2 diabetes mellitus-related environmental factors and the gut microbiota: Emerging evidence and challenges. Clinics 2020, 75. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Tarlatzis, B.C.; Kauffman, R.P. Polycystic ovarian syndrome (PCOS): Long-term metabolic consequences. Metabolism 2018, 86, 33–43. [Google Scholar] [CrossRef]
- Mortada, I. Hyperuricemia, type 2 diabetes mellitus, and hypertension: An emerging association. Curr. Hypertens. Rep. 2017, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sozen, E.; Ozer, N.K. Impact of high cholesterol and endoplasmic reticulum stress on metabolic diseases: An updated mini-review. Redox Biol. 2017, 12, 456–461. [Google Scholar] [CrossRef]
- Athyros, V.G.; Doumas, M.; Imprialos, K.P.; Stavropoulos, K.; Georgianou, E.; Katsimardou, A.; Karagiannis, A. Diabetes and lipid metabolism. Hormones 2018, 17, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Forouhi, N.G.; Wareham, N.J. Epidemiology of diabetes. Medicine 2019, 47, 22–27. [Google Scholar] [CrossRef]
- Dalla Man, C.; Rizza, R.A.; Cobelli, C. Meal simulation model of the glucose-insulin system. IEEE Trans. Biomed. Eng. 2007, 54, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Kovatchev, B.P.; Breton, M.; Dalla Man, C.; Cobelli, C. In silico preclinical trials: A proof of concept in closed-loop control of type 1 diabetes. J. Diabetes Sci. Technol. 2009, 3, 44–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, C.D.; Micheletto, F.; Lv, D.; Breton, M.; Kovatchev, B.; Cobelli, C. The UVA/PADOVA type 1 diabetes simulator: New features. J. Diabetes Sci. Technol. 2014, 8, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orozco-López, J.O.; Castañeda, C.E.; Rodríguez-Herrero, A.; García-Sáez, G.; Hernando, E. Linear time-varying luenberger observer applied to diabetes. IEEE Access 2018, 6, 23612–23625. [Google Scholar] [CrossRef]
- Visentin, R.; Campos-Náñez, E.; Schiavon, M.; Lv, D.; Vettoretti, M.; Breton, M.; Kovatchev, B.P.; Dalla Man, C.; Cobelli, C. The UVA/Padova type 1 diabetes simulator goes from single meal to single day. J. Diabetes Sci. Technol. 2018, 12, 273–281. [Google Scholar] [CrossRef]
- Visentin, R.; Cobelli, C.; Dalla Man, C. The Padova type 2 diabetes simulator from triple-tracer single-meal studies: In silico trials also possible in rare but not-so-rare individuals. Diabetes Technol. Ther. 2020, 22, 892–903. [Google Scholar] [CrossRef]
- Garcia-Tirado, J.; Colmegna, P.; Corbett, J.P.; Ozaslan, B.; Breton, M.D. In silico analysis of an exercise-safe artificial pancreas with multistage model predictive control and insulin safety system. J. Diabetes Sci. Technol. 2019, 13, 1054–1064. [Google Scholar] [CrossRef]
- Rahmanian, F.; Dehghani, M.; Karimaghaee, P.; Mohammadi, M.; Abolpour, R. Hardware-in-the-loop control of glucose in diabetic patients based on nonlinear time-varying blood glucose model. Biomed. Signal Process. Control 2021, 66, 102467. [Google Scholar] [CrossRef]
- Nath, A.; Dey, R.; Aguilar-Avelar, C. Observer based nonlinear control design for glucose regulation in type 1 diabetic patients: An LMI approach. Biomed. Signal Process. Control 2019, 47, 7–15. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Easwaran, A.; Leow, M.K.S.; Cho, N. Evaluation of an artificial pancreas in in silico patients with online-tuned internal model control. Biomed. Signal Process. Control 2018, 41, 198–209. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Park, S.W.; Jin, S.M.; Park, S.M. Toward a fully automated artificial pancreas system Using a bioinspired reinforcement learning design: In silico validation. IEEE J. Biomed. Health Inform. 2020, 25, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.M.; Samadi, S.; Sevil, M.; Hobbs, N.; Park, M.; Quinn, L.; Cinar, A. Virtual Patients: An Enabling Technology for Multivariable Control of Biomedical Systems. IFAC-PapersOnLine 2020, 53, 16233–16238. [Google Scholar] [CrossRef]
- Toffanin, C.; Kozak, M.; Sumnik, Z.; Cobelli, C.; Petruzelkova, L. In silico trials of an open-source Android-based artificial pancreas: A new paradigm to test safety and efficacy of do-it-yourself systems. Diabetes Technol. Ther. 2020, 22, 112–120. [Google Scholar] [CrossRef]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Alkhateeb, H.; El Fathi, A.; Ghanbari, M.; Haidar, A. Modelling glucose dynamics during moderate exercise in individuals with type 1 diabetes. PLoS ONE 2021, 16, e0248280. [Google Scholar] [CrossRef]
- Kartono, A.; Sari, M.; Wahyudi, S. Simulation of physical activities effect and treatment exogenous insulin for managing plasma glucose concentration in type 1 diabetes mellitus. J. Phys. Conf. Ser. 2020, 1572, 012060. [Google Scholar] [CrossRef]
- Resalat, N.; El Youssef, J.; Tyler, N.; Castle, J.; Jacobs, P.G. A statistical virtual patient population for the glucoregulatory system in type 1 diabetes with integrated exercise model. PLoS ONE 2019, 14, e0217301. [Google Scholar] [CrossRef] [PubMed]
- Moser, O.; Mueller, A.; Eckstein, M.L.; Ziko, H.; Aberer, F.; Treiber, G.; Unteregger, C.; Kojzar, H.; Mader, J.K.; Sourij, C.; et al. Improved glycaemic variability and basal insulin dose reduction during a running competition in recreationally active adults with type 1 diabetes—A single-centre, prospective, controlled observational study. PLoS ONE 2020, 15, e0239091. [Google Scholar] [CrossRef]
- Roy, A.; Parker, R.S. Dynamic Modeling of Exercise Effects on Plasma Glucose and Insulin Levels. J. Diabetes Sci. Technol. 2007, 1, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Herrero, A.; Pérez-Gandía, C.; Rigla, M.; de Leiva, A.; Gómez, E.J.; Hernando, M.E. A simulation study of an inverse controller for closed-and semiclosed-loop control in type 1 diabetes. Diabetes Technol. Ther. 2010, 12, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Caballero, Y.; Ando, T.J.; Nakae, S.; Usui, C.; Aoyama, T.; Nakanishi, M.; Nagayoshi, S.; Fujiwara, Y.; Tanaka, S. Simple Prediction of Metabolic Equivalents of Daily Activities Using Heart Rate Monitor without Calibration of Individuals. Int. J. Environ. Res. Public Health 2020, 17, 216. [Google Scholar] [CrossRef] [Green Version]
- Gilgen-Ammann, R.; Schweizer, T.; Wyss, T. Accuracy of the Multisensory Wristwatch Polar Vantage’s Estimation of Energy Expenditure in Various Activities: Instrument Validation Study. JMIR mHealth uHealth 2019, 7, e14534. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.E.; Patriquin, M.A.; Nguyen, H.; Oh, H.; Rufino, K.A.; Storch, E.A.; Schanzer, B.; Mathew, S.J.; Salas, R.; Najafi, B. Objective measurement of sleep, heart rate, heart rate variability, and physical activity in suicidality: A systematic review. J. Affect. Disord. 2020, 273, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Landau, D.; Binder, K. A Guide to Monte Carlo Simulations in Statistical Physics; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Ceron, J.D.; Lopez, D.M.; Ramirez, G.A. A mobile system for sedentary behaviors classification based on accelerometer and location data. Comput. Ind. 2017, 92, 25–31. [Google Scholar] [CrossRef]
- Bequette, B.W.; Cameron, F.; Buckingham, B.A.; Maahs, D.M.; Lum, J. Overnight hypoglycemia and hyperglycemia mitigation for individuals with type 1 diabetes: How risks can be reduced. IEEE Control Syst. Mag. 2018, 38, 125–134. [Google Scholar] [CrossRef]
- Lewis, S.J.; Mueller, B.A. Evaluation and Development of Vancomycin Dosing Schemes to Meet New AUC/MIC Targets in Intermittent Hemodialysis Using Monte Carlo Simulation Techniques. J. Clin. Pharmacol. 2021, 61, 211–223. [Google Scholar] [CrossRef]
- Bekisz, S.; Holder-Pearson, L.; Chase, J.G.; Desaive, T. In silico validation of a new model-based oral-subcutaneous insulin sensitivity testing through Monte Carlo sensitivity analyses. Biomed. Signal Process. Control 2020, 61, 102030. [Google Scholar] [CrossRef]
- Ahmad, S.; Ramkissoon, C.M.; Beneyto, A.; Conget, I.; Giménez, M.; Vehi, J. Generation of Virtual Patient Populations That Represent Real Type 1 Diabetes Cohorts. Mathematics 2021, 9, 1200. [Google Scholar] [CrossRef]
- Krishnamoorthy, D.; Boiroux, D.; Aradóttir, T.B.; Engell, S.E.; Jørgensen, J.B. A Model-free Approach to Automatic Dose Guidance in Long Acting Insulin Treatment of Type 2 Diabetes. IEEE Control Syst. Lett. 2020, 5, 2030–2035. [Google Scholar] [CrossRef]
- Smaoui, M.R.; Rabasa-Lhoret, R.; Haidar, A. Development platform for artificial pancreas algorithms. PLoS ONE 2020, 15, e0243139. [Google Scholar] [CrossRef]
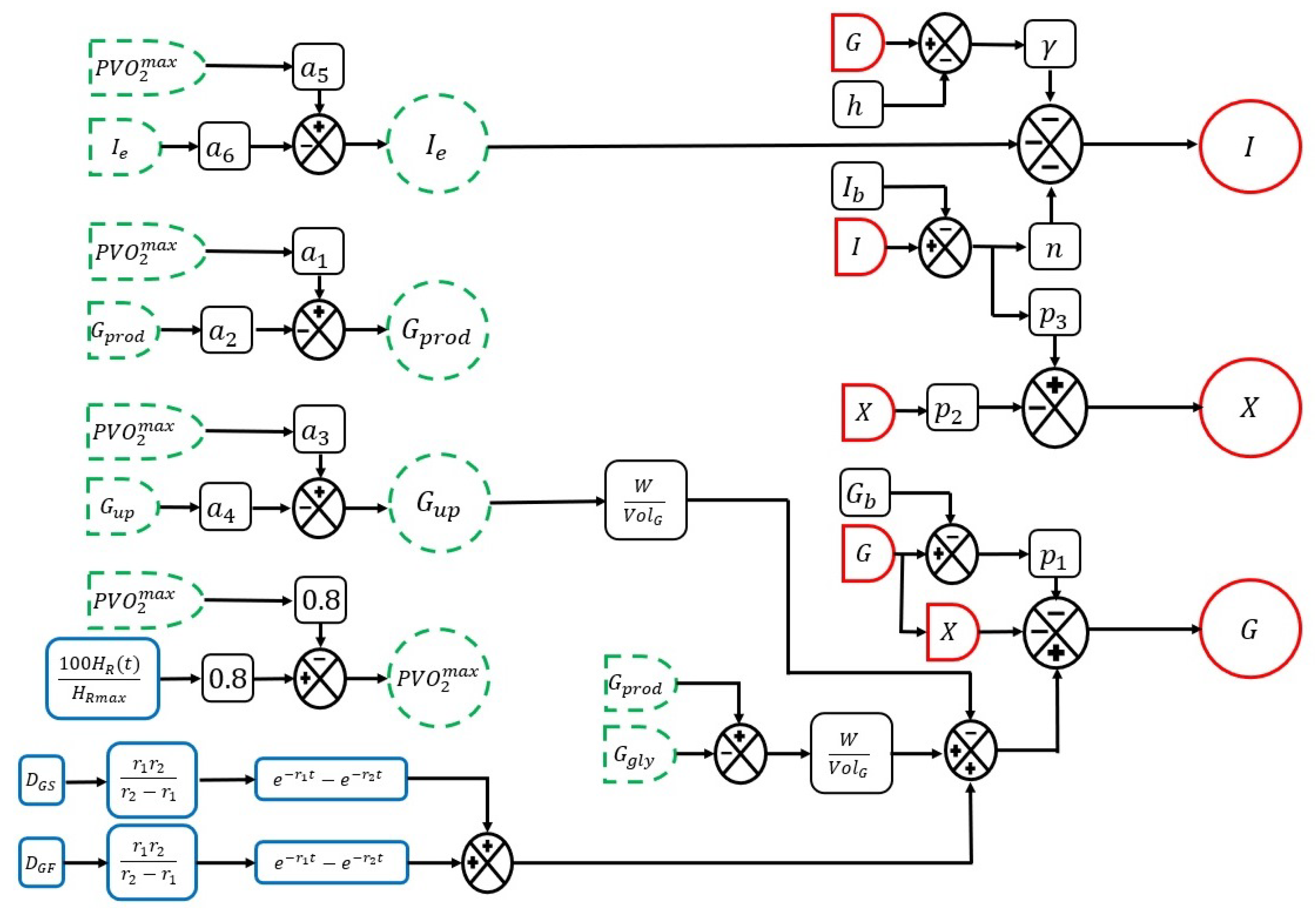





| Symbol | Units | Description |
|---|---|---|
| (1/min) | Insulin independent rate constant | |
| (1/min) | Rate for decrease in tissues glucose uptake ability | |
| [(U/mL)/min2] | Insulin-dependent increase | |
| (1/min) | First-order decay rate for insulin in blood | |
| h | (mg/dL) | Glucose threshold above cells release insulin |
| [(U/mL)/(min2 (mg/dL))] | Insulin release rate from pancreatic cells | |
| W | (kg) | Weight of the subject |
| (dL) | Glucose distribution volume | |
| (mg/kg min2) | dynamic constant | |
| (1/min) | dynamic constant | |
| (mg/kg min2) | dynamic constant | |
| (1/min) | dynamic constant | |
| (U/mL min) | dynamic constant | |
| (1/min) | dynamic constant | |
| (min) | Glycogenesis time constant | |
| (1/min) | CH slow absorption parameter | |
| (1/min) | CH slow absorption parameter | |
| (1/min) | CH fast absorption parameter | |
| (1/min) | CH fast absorption parameter |
| Own Sample | ENSANUT, 2018 | ||
|---|---|---|---|
| Demographic Variable | Unit | Mean ± SD | Mean ± SD |
| Age | Years | 42.75 ± 16.35 | 36.10 ± 11.35 |
| Height | m | 1.68 ± 0.09 | - |
| Weight | kg | 70.00 ± 13.24 | 71.86 ± 10.97 |
| Body mass index | Kg/m2 | 24.58 ± 4.05 | - |
| Fat percentage | - | 27.88 ± 9.04 | - |
| Muscle percentage | - | 32.10 ± 6.74 | - |
| Visceral fat percentage | - | 6.6 ± 2.85 | - |
| Heart rate (rest) | bpm | 75.4 ± 7.32 | - |
| Glucose concentration | mg/dL | 91.55 ± 11.78 | 85.37 ± 5.06 |
| # Participant | Occupation | Time (h) | # Participant | Occupation | Time (h) |
|---|---|---|---|---|---|
| 1 | Retired | 5.10 | 11 | Student | 0.76 |
| 2 | House keeper | 9.69 | 12 | Professional | 6.63 |
| 3 | Office worker | 2.80 | 13 | House keeper | 7.65 |
| 4 | Student | 0.51 | 14 | Office worker | 6.12 |
| 5 | House keeper | 5.61 | 15 | Retired | 8.16 |
| 6 | House keeper | 14.78 | 16 | Retired | 7.14 |
| 7 | Factory worker | 9.93 | 17 | Office worker | 3.57 |
| 8 | Factory worker | 9.42 | 18 | Professional | 5.81 |
| 9 | Factory worker | 8.72 | 19 | Office worker | 5.61 |
| 10 | Student | 1.53 | 20 | Professional | 8.16 |
| Mean ± SD | |||||
|---|---|---|---|---|---|
| Factor | Unit | Physically Active | Sedentary | ||
| Men | Women | Men | Women | ||
| Age | years | 42 ± 14 | 41 ± 12 | 43 ± 14 | 40 ± 12 |
| Basal glucose | mg/dL | 88.06 ± 5.25 | 88.63 ± 7.35 | 89.90 ± 7.81 | 90.02 ± 7.05 |
| BMI | kg/m2 | 23.59 ± 2.4 | 23.79 ± 3.12 | 25.67 ± 3.4 | 25.03 ± 3.22 |
| CHOs quantity | g | 300 ± 27 | 279 ± 34 | 305 ± 35 | 288 ± 32 |
| Height | m | 1.69 ± 0.06 | 1.59 ± 0.07 | 1.69 ± 0.06 | 1.60 ± 0.06 |
| Weight | Kg | 67.39 ± 6 | 63.02 ± 7 | 73.02 ± 9 | 64.68 ± 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-Bastida, A.; Adam-Medina, M.; Posada-Gómez, R.; Salazar-Piña, D.A.; Osorio-Gordillo, G.-L.; Vela-Valdés, L.G. Dynamic of Glucose Homeostasis in Virtual Patients: A Comparison between Different Behaviors. Int. J. Environ. Res. Public Health 2022, 19, 716. https://doi.org/10.3390/ijerph19020716
Alonso-Bastida A, Adam-Medina M, Posada-Gómez R, Salazar-Piña DA, Osorio-Gordillo G-L, Vela-Valdés LG. Dynamic of Glucose Homeostasis in Virtual Patients: A Comparison between Different Behaviors. International Journal of Environmental Research and Public Health. 2022; 19(2):716. https://doi.org/10.3390/ijerph19020716
Chicago/Turabian StyleAlonso-Bastida, Alexis, Manuel Adam-Medina, Rubén Posada-Gómez, Dolores Azucena Salazar-Piña, Gloria-Lilia Osorio-Gordillo, and Luis Gerardo Vela-Valdés. 2022. "Dynamic of Glucose Homeostasis in Virtual Patients: A Comparison between Different Behaviors" International Journal of Environmental Research and Public Health 19, no. 2: 716. https://doi.org/10.3390/ijerph19020716
APA StyleAlonso-Bastida, A., Adam-Medina, M., Posada-Gómez, R., Salazar-Piña, D. A., Osorio-Gordillo, G.-L., & Vela-Valdés, L. G. (2022). Dynamic of Glucose Homeostasis in Virtual Patients: A Comparison between Different Behaviors. International Journal of Environmental Research and Public Health, 19(2), 716. https://doi.org/10.3390/ijerph19020716







