Quantification of the Influence of Citrate/Fe(II) Molar Ratio on Hydroxyl Radical Production and Pollutant Degradation during Fe(II)-Catalyzed O2 and H2O2 Oxidation Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Oxic Experiments
2.3. Anoxic Experiments
2.4. Analysis
2.5. Kinetic Modeling and Speciation Calculation
3. Results and Discussion
3.1. Effect of Citrate/Fe(II) Molar Ratio on •OH Production during Fe(II)-Catalyzed O2 Oxidation Process
3.2. Effect of Citrate/Fe(II) Molar Ratio on •OH Yield during Fe(II)-Catalyzed H2O2 Oxidation Process
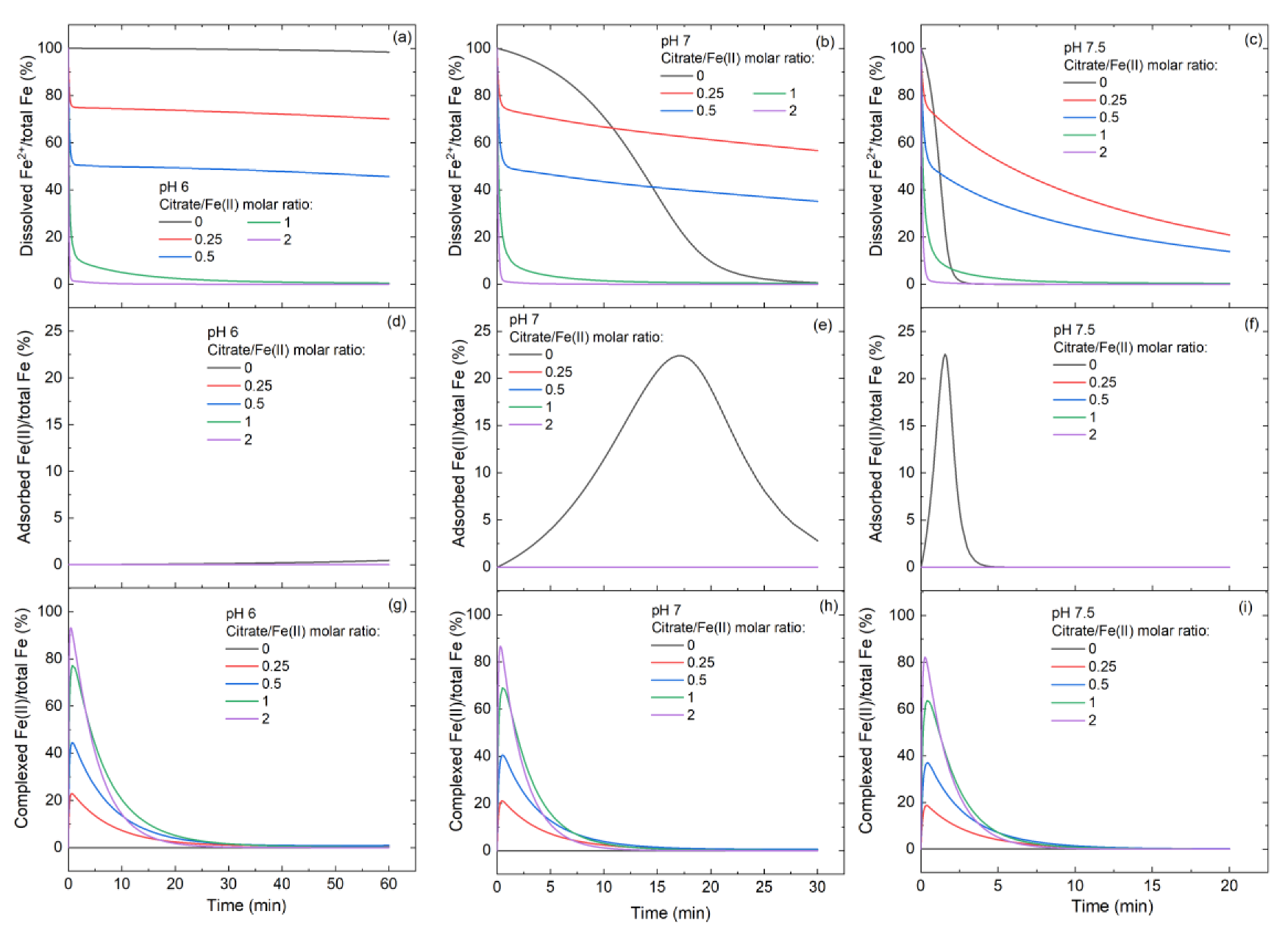
3.3. Variation of Fe(II)/Fe(III) Species at Different Citrate/Fe(II) Ratios during Oxidation Process
3.4. Controlling Mechanisms of Citrate/Fe(II) Molar Ratio on •OH Production
3.5. Effect of Citrate/Fe(II) Molar Ratio on Phenol Degradation during Fe(II)-Catalyzed O2 Oxidation Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; Jones, A.; Waite, T.D.; Chen, Y.; Huang, X.; Rosso, K.M.; Kappler, A.; Mansor, M.; Tratnyek, P.G.; Zhang, H. Fe(II) redox chemistry in the environment. Chem. Rev. 2021, 121, 8161–8233. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, S.; Zhan, S. Superior photocatalytic disinfection effect of Ag-3D ordered mesoporous CeO2 under visible light. Appl. Catal. B Environ. 2018, 224, 27–37. [Google Scholar] [CrossRef]
- Tsydenova, O.; Batoev, V.; Batoeva, A. Solar-enhanced advanced oxidation processes for water treatment: Simultaneous removal of pathogens and chemical pollutants. Int. J. Environ. Res. Public Health 2015, 12, 9542–9561. [Google Scholar] [CrossRef] [PubMed]
- Aseev, D.; Batoeva, A.; Sizykh, M.; Olennikov, D.; Matafonova, G. Degradation of bisphenol A in an aqueous solution by a photo-Fenton-like process using a UV KrCl excilamp. Int. J. Environ. Res. Public Health 2021, 18, 1152. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yuan, S.; Tong, M.; Liu, D. Oxidation of trichloroethylene by the hydroxyl radicals produced from oxygenation of reduced nontronite. Water Res. 2017, 113, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, C.E.; Ho, P.; Berns, E.; Werth, C. Mechanisms for abiotic dechlorination of trichloroethene by ferrous minerals under oxic and anoxic conditions in natural sediments. Environ. Sci. Technol. 2018, 52, 13747–13755. [Google Scholar] [CrossRef]
- Tong, M.; Yuan, S.; Ma, S.; Jin, M.; Liu, D.; Cheng, D.; Liu, X.; Gan, Y.; Wang, Y. Production of abundant hydroxyl radicals from oxygenation of subsurface sediments. Environ. Sci. Technol. 2016, 50, 214–221. [Google Scholar] [CrossRef]
- Chen, N.; Huang, D.; Liu, G.; Chu, L.; Fang, G.; Zhu, C.; Zhou, D.; Gao, J. Active iron species driven hydroxyl radicals formation in oxygenation of different paddy soils: Implications to polycyclic aromatic hydrocarbons degradation. Water Res. 2021, 203, 117484. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Yuan, S.; Tong, M.; Ma, S.; Liao, W.; Zhang, N.; Chen, C. Contaminant degradation by •OH during sediment oxygenation: Dependence on Fe(II) species. Environ. Sci. Technol. 2020, 54, 2975–2984. [Google Scholar] [CrossRef]
- Cheng, D.; Neumann, A.; Yuan, S.H.; Liao, W.J.; Qian, A. Oxidative degradation of organic contaminants by FeS in the presence of O2. Environ. Sci. Technol. 2020, 54, 4091–4101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, M. A critical review of the application of chelating agents to enable Fenton and Fenton-like reactions at high pH values. J. Hazard. Mater. 2019, 362, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Remucal, C.K.; Sedlak, D.L. The role of iron coordination in the production of reactive oxidants from ferrous iron oxidation by oxygen and hydrogen peroxide. In Aquatic Redox Chemistry; ACS Publications: Washington, DC, USA, 2011; pp. 177–197. [Google Scholar]
- Cheng, D.; Yuan, S.; Liao, P.; Zhang, P. Oxidizing impact induced by mackinawite (FeS) nanoparticles at oxic conditions due to production of hydroxyl radicals. Environ. Sci. Technol. 2016, 50, 11646–11653. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, P.; Yuan, S.; Liao, P.; Qian, A.; Liu, X.; Tong, M.; Li, L. Production of Hydroxyl radicals from oxygenation of simulated AMD due to CaCO3-induced pH increase. Water Res. 2017, 111, 118–126. [Google Scholar] [CrossRef]
- Liu, X.X.; Yuan, S.H.; Zhang, P.; Zhu, J.; Tong, M. Reduced nontronite-activated H2O2 for contaminants degradation: The beneficial role of clayed fractions in ISCO treatments. J. Hazard. Mater. 2020, 386, 121945. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, P.; Liao, W.; Tong, M.; Yuan, S. Ligand-enhanced electron utilization for trichloroethylene degradation by •OH during sediment oxygenation. Environ. Sci. Technol. 2021, 55, 7044–7051. [Google Scholar] [CrossRef]
- Keenan, C.R.; Sedlak, D.L. Ligand-enhanced reactive oxidant generation by nanoparticulate zero-valent iron and oxygen. Environ. Sci. Technol. 2008, 42, 6936–6941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yuan, S. Production of hydroxyl radicals from abiotic oxidation of pyrite by oxygen under circumneutral conditions in the presence of low-molecular-weight organic acids. Geochim. Cosmochim. Acta 2017, 218, 153–166. [Google Scholar] [CrossRef]
- Zeng, Q.; Dong, H.; Wang, X. Effect of ligands on the production of oxidants from oxygenation of reduced Fe-bearing clay mineral nontronite. Geochim. Cosmochim. Acta 2019, 251, 136–156. [Google Scholar] [CrossRef]
- Lewis, S.; Lynch, A.; Bachas, L.; Hampson, S.; Ormsbee, L.; Bhattacharyya, D. Chelate-modified Fenton reaction for the degradation of trichloroethylene in aqueous and two-phase systems. Environ. Eng. Sci. 2009, 26, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.J.; Rose, A.L.; Waite, T.D. Importance of iron complexation for Fenton-mediated hydroxyl radical production at circumneutral pH. Front. Mar. Sci. 2016, 3, 134. [Google Scholar] [CrossRef]
- Miller, C.J.; Rose, A.L.; Waite, T.D. Hydroxyl radical production by H2O2-mediated oxidation of Fe(II) complexed by Suwannee River fulvic acid under circumneutral freshwater conditions. Environ. Sci. Technol. 2013, 47, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Choi, W. Oxidation of aquatic pollutants by ferrous-oxalate complexes under dark aerobic conditions. J. Hazard. Mater. 2014, 274, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Griffin, P.J.; Waite, T.D. Ferrous iron oxidation by molecular oxygen under acidic conditions: The effect of citrate, EDTA and fulvic acid. Geochim. Cosmochim. Acta 2015, 160, 117–131. [Google Scholar] [CrossRef]
- Mopper, K.; Zhou, X.L. Hydroxyl radical photoproduction in the sea and its potential impact on marine processes. Science 1990, 250, 661–664. [Google Scholar] [CrossRef]
- Joo, S.H.; Feitz, A.J.; Sedlak, D.L.; Waite, T.D. Quantification of the oxidizing capacity of nanoparticulate zero-valent iron. Environ. Sci. Technol. 2005, 39, 1263–1268. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical-review of rate constants for reactions of hydrated electrons, hydrogen-atoms and hydroxyl radicals (•OH/•O−) in aqueous-solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Zhang, P.; Yuan, S.; Liao, P. Mechanisms of hydroxyl radical production from abiotic oxidation of pyrite under acidic conditions. Geochim. Cosmochim. Acta 2016, 172, 444–457. [Google Scholar] [CrossRef]
- Viollier, E.; Inglett, P.; Hunter, K.; Roychoudhury, A.; Van Cappellen, P. The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl. Geochem. 2000, 15, 785–790. [Google Scholar] [CrossRef]
- Ianni, J.C. Kintecus. Windows Version 6.51. 2018. Available online: www.kintecus.com (accessed on 10 July 2022).
- Rose, A.L.; Waite, T.D. Kinetic model for Fe(II) oxidation in seawater in the absence and presence of natural organic matter. Environ. Sci. Technol. 2002, 36, 433–444. [Google Scholar] [CrossRef]
- Pham, A.N.; Waite, T.D. Oxygenation of Fe(II) in natural waters revisited: Kinetic modeling approaches, rate constant estimation and the importance of various reaction pathways. Geochim. Cosmochim. Acta 2008, 72, 3616–3630. [Google Scholar] [CrossRef]
- Pham, A.N.; Rose, A.L.; Feitz, A.J.; Waite, T.D. Kinetics of Fe(III) precipitation in aqueous solutions at pH 6.0–9.5 and 25 °C. Geochim. Cosmochim. Acta 2006, 70, 640–650. [Google Scholar] [CrossRef]
- Kinsela, A.S.; Jones, A.M.; Bligh, M.W.; Pham, A.N.; Collins, R.N.; Harrison, J.J.; Wilsher, K.L.; Payne, T.E.; Waite, T.D. Influence of dissolved silicate on rates of Fe(II) oxidation. Environ. Sci. Technol. 2016, 50, 11663–11671. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-S.; Gurol, M.D. Catalytic decomposition of hydrogen peroxide on iron oxide: Kinetics, mechanism, and implications. Environ. Sci. Technol. 1998, 32, 1417–1423. [Google Scholar] [CrossRef]
- Rose, A.L.; Waite, T.D. Reduction of organically complexed ferric iron by superoxide in a simulated natural water. Environ. Sci. Technol. 2005, 39, 2645–2650. [Google Scholar] [CrossRef]
- Fujii, M.; Rose, A.L.; Omura, T.; Waite, T.D. Effect of Fe(II) and Fe(III) transformation kinetics on iron acquisition by a toxic strain of Microcystis aeruginosa. Environ. Sci. Technol. 2010, 44, 1980–1986. [Google Scholar] [CrossRef]
- Garg, S.; Rose, A.L.; Waite, T.D. Superoxide mediated reduction of organically complexed Iron(III): Comparison of non-dissociative and dissociative reduction pathways. Environ. Sci. Technol. 2007, 41, 3205–3212. [Google Scholar] [CrossRef]
- Gustafsson, J. Visual MINTEQ, Version 3.1 Division of Land and Water Resources; Royal Institute of Technology: Stockholm, Sweden, 2013. [Google Scholar]
- Qian, A.; Yuan, S.; Xie, S.; Tong, M.; Zhang, P.; Zheng, Y. Oxidizing capacity of iron electrocoagulation systems for refractory organic contaminant transformation. Environ. Sci. Technol. 2019, 53, 12629–12638. [Google Scholar] [CrossRef]
- Jones, A.M.; Griffin, P.J.; Collins, R.N.; Waite, T.D. Ferrous iron oxidation under acidic conditions–The effect of ferric oxide surfaces. Geochim. Cosmochim. Acta 2014, 145, 1–12. [Google Scholar] [CrossRef]
- King, D.W.; Lounsbury, H.A.; Millero, F.J. Rates and mechanism of Fe(II) oxidation at nanomolar total iron concentrations. Environ. Sci. Technol. 1995, 29, 818–824. [Google Scholar] [CrossRef]
- Tamura, H.; Kawamura, S.; Hagayama, M. Acceleration of the oxidation of Fe2+ ions by Fe(III)-oxyhydroxides. Corrosion Sci. 1980, 20, 963–971. [Google Scholar] [CrossRef]
- Zhang, P.; Van Cappellen, P.; Pi, K.; Yuan, S. Effects of riboflavin and desferrioxamine B on Fe(II) oxidation by O2. Fundam. Res. 2022, 2, 208–217. [Google Scholar] [CrossRef]
- Krishnamurti, G.; Huang, P. Influence of citrate on the kinetics of Fe(II) oxidation and the formation of iron oxyhydroxides. Clays Clay Min. 1991, 39, 28–34. [Google Scholar] [CrossRef]





| No. | Reactions | Rate Constant | Source | ||
|---|---|---|---|---|---|
| pH 6 | pH 7 | pH 7.5 | |||
| Reactions in Inorganic Fe(II)dis System | |||||
| A1 | Fe(II) + O2 → Fe(III) + •O2− | 1 × 10−3 M−1·s−1 | 1.3 × 10−1 M−1·s−1 | 1.8 M−1·s−1 | Fitting |
| A2 | Fe(II) + •O2− → Fe(III) + H2O2 | 1 × 107 M−1·s−1 | [31] | ||
| A3 | Fe(II) + H2O2 → Fe(III) + (0.052, 0.018, 0.007) •OH + OH− | 5.5 × 101 M−1·s−1 | 4.79 × 103 M−1·s−1 | 1.33 × 104 M−1·s−1 | [32] |
| A4 | Fe(II) + •OH → Fe(III) + OH− | 5 × 108 M−1·s−1 | [32] | ||
| A5 | Fe(III) + •O2− → Fe(II) + O2 | 1.5 × 108 M−1·s−1 | [31] | ||
| A6 a | Fe(III) + Fe(III) → LEP + LEP | 3.2 × 105 M−1·s−1 | 3.4 × 106 M−1·s−1 | 5.0 × 106 M−1·s−1 | [33] |
| A7 a | Fe(III) + LEP → LEP + LEP | 3.2 × 105 M−1·s−1 | 3.4 × 106 M−1·s−1 | 5.0 × 106 M−1·s−1 | [33] |
| A8 | Fe(II) + LEP → Fe(II)-LEP | 1.1 × 106 M−1·s−1 | 1.0 × 108 M−1·s−1 | 1.0 × 108 M−1·s−1 | [34] |
| A9 | Fe(II)-LEP → Fe(II) + LEP | 2.3 × 103 M−1·s−1 | [34] | ||
| A10 | Fe(II)-LEP + O2 → LEP + LEPi + •O2− | 2 M−1·s−1 | 6 M−1·s−1 | 60 M−1·s−1 | Fitting |
| A11 | Fe(II)-LEP + •O2− → LEP + LEPi + H2O2 | 1 × 107 M−1·s−1 | [31] | ||
| A12 | Fe(II)-LEP + H2O2 → LEP + LEPi + (0.052, 0.018, 0.007) •OH + OH− | 5.5 × 101 M−1·s−1 | 4.79 × 103 M−1·s−1 | 1.33 × 104 M−1·s−1 | [32] |
| A13 | Fe(II)-LEP + •OH → LEP + LEPi + +OH− | 5 × 108 M−1·s−1 | [32] | ||
| A14 | LEP + H2O2 → LEP + H2O + 0.5O2 | 3.1 × 10−2 M−1·s−1 | [35] | ||
| A15 | LEP + •O2− → Fe(II) + LEP + O2 | 6.5 × 10−2 M−1·s−1 | [36] | ||
| Trapping of •OH by benzoate | |||||
| B1 | benzoate + •OH → HBA + •O2− | 5.9 × 109 M−1·s−1 | [27] | ||
| Extended reactions in Fe(II)-citrate systems | |||||
| C1 | Fe(II) + citrate → Fe(II)-citrate− | 5.0 × 102 M−1·s−1 | [37] | ||
| C2 | Fe(II)-citrate− → Fe(II) + citrate | 2.0 × 10−3 s−1 | [37] | ||
| C3 | Fe(III) + citrate → Fe(III)-citrate | 2.1 × 105 M−1·s−1 | [37] | ||
| C4 | Fe(III)-citrate → Fe(III) + citrate | 1.1 × 10−4 s−1 | [37] | ||
| C5 | Fe(II)-citrate− + O2 → Fe(III)-citrate + •O2− | 2.9 M−1·s−1 | 8 M−1·s−1 | 12 M−1·s−1 | Fitting |
| C6 | Fe(II)-citrate− + •O2− → Fe(III)-citrate + H2O2 | 1 × 107 M−1·s−1 | [31] | ||
| C7 | Fe(II)-citrate− + H2O2 → Fe(III)-citrate + (0.522, 0.315, 0.176) •OH + OH− | 1.3 × 102 M−1·s−1 | 8 × 102 M−1·s−1 | 5 × 104 M−1·s−1 | Fitting |
| C8 | Fe(II)-citrate + •OH → Fe(III)-citrate + OH− | 5 × 108 M−1·s−1 | [32] | ||
| C9 | Fe(III)-citrate + H2O2 → Fe(II)-citrate + •O2− + 2H+ | 2.5 × 10−3 M−1·s−1 | [24] | ||
| C10 | Fe(III)-citrate + •O2− → Fe(II)-citrate + O2 | 5.6 × 102 M−1·s−1 | [38] | ||
| C11 | Fe(III)-citrate + •OH → Fe(III)-citrateox + •O2− | 1.2 × 108 M−1·s−1 | [21] | ||
| C12 | citrate + •OH → citrateox + •O2− | 5.0 × 107 M−1·s−1 | [27] | ||
| Experimental Conditions | Yield of •OH Relative to H2O2 Decomposition | R2 | |
|---|---|---|---|
| pH 6 | 250 μM Fe(II)dis | 5.2 ± 0.03% | 0.99 |
| 250 μM Fe(II)dis + 62.5 μM citrate | 6.3 ± 0.02% | 0.99 | |
| 250 μM Fe(II)dis + 125 μM citrate | 7.6 ± 0.2% | 0.89 | |
| 250 μM Fe(II)dis + 250 μM citrate | 26.0 ± 0.6% | 0.99 | |
| 250 μM Fe(II)dis + 500 μM citrate | 52.2 ± 0.01% | 0.99 | |
| pH 7 | 250 μM Fe(II)dis | 1.8 ± 0.04% | 0.99 |
| 250 μM Fe(II)dis + 62.5 μM citrate | 2.9 ± 0.01% | 0.99 | |
| 250 μM Fe(II)dis + 125 μM citrate | 4.5 ± 0.4% | 0.97 | |
| 250 μM Fe(II)dis + 250 μM citrate | 12.8 ± 0.3% | 0.99 | |
| 250 μM Fe(II)dis + 500 μM citrate | 31.5 ± 0.5% | 0.99 | |
| pH 7.5 | 250 μM Fe(II)dis | 0.7 ± 0.02% | 0.95 |
| 250 μM Fe(II)dis + 62.5 μM citrate | 2.9 ± 0.01% | 0.99 | |
| 250 μM Fe(II)dis + 125 μM citrate | 3.7 ± 0.3% | 0.97 | |
| 250 μM Fe(II)dis + 250 μM citrate | 9.1 ± 0.5% | 0.99 | |
| 250 μM Fe(II)dis + 500 μM citrate | 17.6 ± 0.7% | 0.99 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, B.; Zhang, P.; Liu, H.; Yuan, S. Quantification of the Influence of Citrate/Fe(II) Molar Ratio on Hydroxyl Radical Production and Pollutant Degradation during Fe(II)-Catalyzed O2 and H2O2 Oxidation Processes. Int. J. Environ. Res. Public Health 2022, 19, 12977. https://doi.org/10.3390/ijerph191912977
Hu B, Zhang P, Liu H, Yuan S. Quantification of the Influence of Citrate/Fe(II) Molar Ratio on Hydroxyl Radical Production and Pollutant Degradation during Fe(II)-Catalyzed O2 and H2O2 Oxidation Processes. International Journal of Environmental Research and Public Health. 2022; 19(19):12977. https://doi.org/10.3390/ijerph191912977
Chicago/Turabian StyleHu, Bingbing, Peng Zhang, Hui Liu, and Songhu Yuan. 2022. "Quantification of the Influence of Citrate/Fe(II) Molar Ratio on Hydroxyl Radical Production and Pollutant Degradation during Fe(II)-Catalyzed O2 and H2O2 Oxidation Processes" International Journal of Environmental Research and Public Health 19, no. 19: 12977. https://doi.org/10.3390/ijerph191912977
APA StyleHu, B., Zhang, P., Liu, H., & Yuan, S. (2022). Quantification of the Influence of Citrate/Fe(II) Molar Ratio on Hydroxyl Radical Production and Pollutant Degradation during Fe(II)-Catalyzed O2 and H2O2 Oxidation Processes. International Journal of Environmental Research and Public Health, 19(19), 12977. https://doi.org/10.3390/ijerph191912977






