Blue-Light-Blocking Lenses Ameliorate Structural Alterations in the Rodent Hippocampus
Abstract
1. Introduction
2. Methodology
2.1. Ethical Statement
2.2. Standardization of Light Exposure and Laboratory Setup
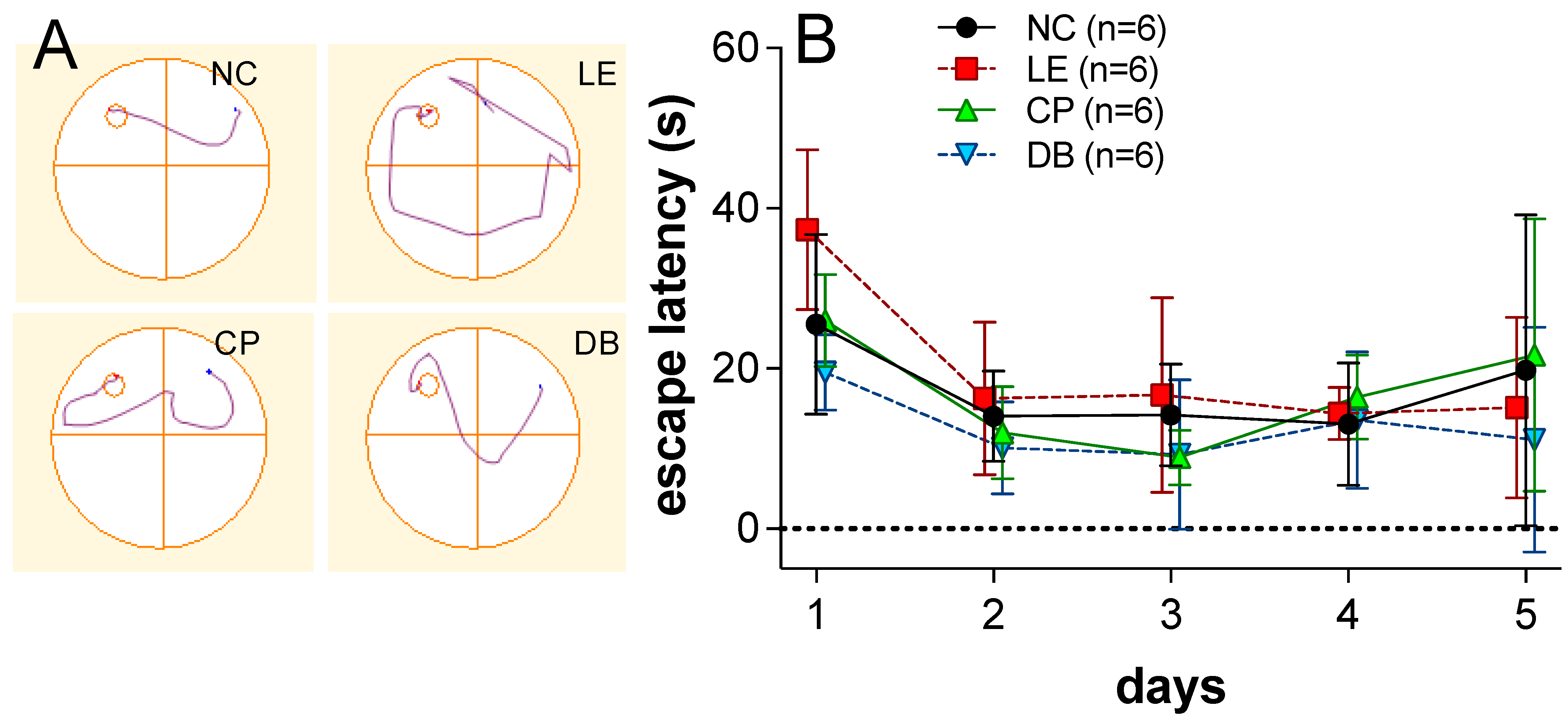
2.3. Behavioral Assessment Post-Light Exposure
2.4. Structural Assessment
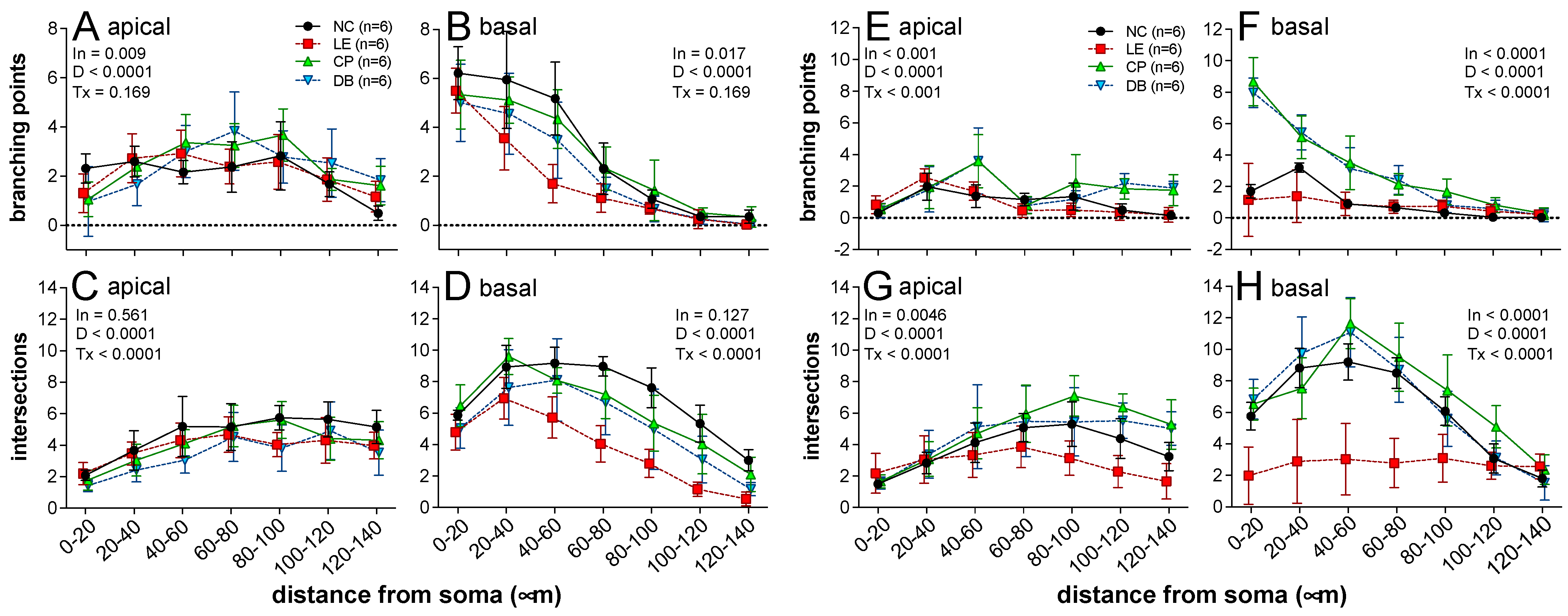
2.4.1. Dendritic Arborization Assessment Using Golgi Stain
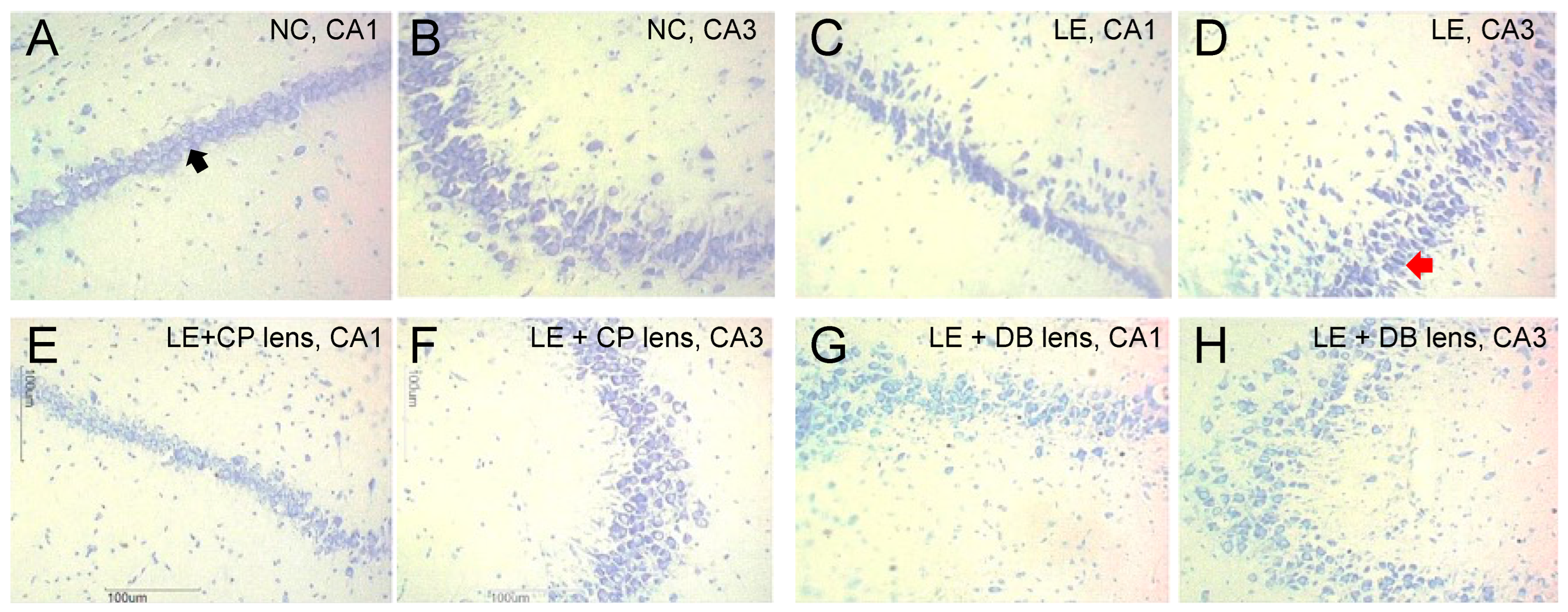
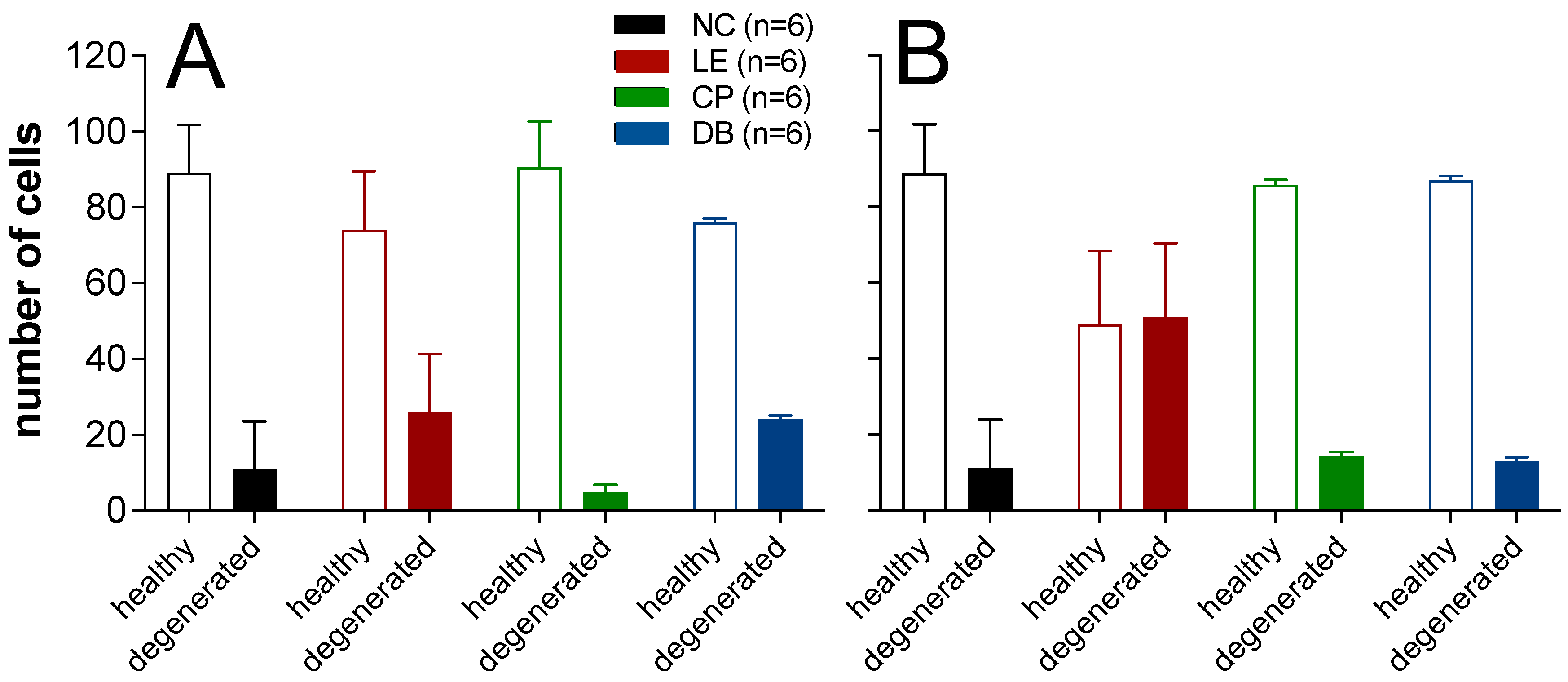
2.4.2. Histopathological Assessment Using Cresyl Violet Stain
2.4.3. Statistical Analysis
3. Results
3.1. Behavioral Analysis
3.2. Structural Analysis
3.2.1. Morphological Assessment Using Golgi Stain
3.2.2. Histopathological Analysis Using Cresyl Violet Stain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef]
- Dijk, D.J.; Archer, S.N. Light, sleep, and circadian rhythms: Together again. PLoS Biol. 2009, 7, e1000145. [Google Scholar] [CrossRef]
- Nash, T.R.; Chow, E.S.; Law, A.D.; Fu, S.D.; Fuszara, E.; Bilska, A.; Bebas, P.; Kretzschmar, D.; Giebultowicz, J.M. Daily blue-light exposure shortens lifespan and causes brain neurodegeneration in Drosophila. npj Aging Mech. Dis. 2019, 5, 8. [Google Scholar] [CrossRef]
- Emmer, K.M.; Russart, G.K.L.; Walker, W.H.; Nelson, R.J.; Courtney DeVries, A. Effects of light at night on laboratory animals and research outcomes. Behav. Neurosci. 2018, 132, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Behar-Cohen, F.; Martinsons, C.; Viénot, F.; Zissis, G.; Barlier-Salsi, A.; Cesarini, J.P.; Enouf, O.; Garcia, M.; Picaud, S.; Attia, D. Light-emitting diodes (LED) for domestic lighting: Any risks for the eye? Prog. Retin. Eye Res. 2011, 30, 239–257. [Google Scholar] [CrossRef]
- Schubert, E.F.; Kim, J.K. Solid-state light sources getting smart. Science 2005, 308, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Giannos, S.A.; Kraft, E.R.; Lyons, L.J.; Gupta, P.K. Spectral Evaluation of Eyeglass Blocking Efficiency of Ultraviolet/High-energy Visible Blue Light for Ocular Protection. Optom. Vis. Sci. 2019, 96, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Van Norren, D.; Vos, J.J. Light damage to the retina: An historical approach. Eye (Lond) 2016, 30, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-C.; Zhou, Y.; Tan, G.; Li, J. Research progress about the effect and prevention of blue light on eyes. Int. J. Ophthalmol. 2018, 11, 1999–2003. [Google Scholar] [CrossRef]
- Gabel, V.; Reichert, C.F.; Maire, M.; Schmidt, C.; Schlangen, L.J.M.; Kolodyazhniy, V.; Garbazza, C.; Cajochen, C.; Viola, A.U. Differential impact in young and older individuals of blue-enriched white light on circadian physiology and alertness during sustained wakefulness. Sci. Rep. 2017, 7, 7620. [Google Scholar] [CrossRef]
- Grimm, C.; Wenzel, A.; Williams, T.P.; Rol, P.O.; Hafezi, F.; Reme, C.E. Rhodopsin-Mediated Blue-Light Damage to the Rat Retina: Effect of Photoreversal of Bleaching. Invest. Ophthalmol. Vis. Sci. 2001, 42, 497–505. [Google Scholar] [PubMed]
- Noell, W.K.; Walker, V.S.; Kang, B.S.; Berman, S. Retinal damage by light in rats. Invest. Ophthalmol. 1966, 5, 450–473. [Google Scholar]
- Ham, W.T.; Mueller, H.A.; Ruffolo, J.J.; Clarke, A.M. Sensitivity of the Retina To Radiation Damage As a Function of Wavelength. Photochem. Photobiol. 1979, 29, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Youssef, P.N.; Sheibani, N.; Albert, D.M. Retinal light toxicity. Eye 2011, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.N.; Núñez-Álvarez, C.; del Olmo-Aguado, S. The effect of visual blue light on mitochondrial function associated with retinal ganglions cells. Exp. Eye Res. 2014, 128, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Del Olmo-Aguado, S.; Núñez-Álvarez, C.; Osborne, N.N. Blue Light Action on Mitochondria Leads to Cell Death by Necroptosis. Neurochem. Res. 2016, 41, 2324–2335. [Google Scholar] [CrossRef] [PubMed]
- Berson, D.M. Strange vision: Ganglion cells as circadian photoreceptors. Trends Neurosci. 2003, 26, 314–320. [Google Scholar] [CrossRef]
- Lucas, R.J.; Freedman, M.S.; Muñoz, M.; Garcia-Fernández, J.M.; Foster, R.G. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science 1999, 284, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Cassone, V.M.; Speh, J.C.; Card, J.P.; Moore, R.Y. Comparative Anatomy of the Mammalian Hypothalamic Suprachiasmatic Nucleus. J. Biol. Rhythms 1988, 3, 71–91. [Google Scholar] [CrossRef]
- Mikkelsen, J.D.; Vrang, N. A direct pretectosuprachiasmatic projection in the rat. Neuroscience 1994, 62, 497–505. [Google Scholar] [CrossRef]
- Sabbah, S.; Worden, M.S.; Laniado, D.D.; Berson, D.M.; Sanes, J.N. Luxotonic signals in human prefrontal cortex as a possible substrate for effects of light on mood and cognition. Proc. Natl. Acad. Sci. USA 2022, 119, e2118192119. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, H. Prefrontal—Hippocampal interactions in episodic memory. Nat. Publ. Gr. 2017, 18, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.W.; Lee, H.; Jeong, Y.; Lee, J.W.; Lee, I. Remembering rewarding futures: A simulation-selection model of the hippocampus. Hippocampus 2018, 28, 913–930. [Google Scholar] [CrossRef] [PubMed]
- Alkozei, A.; Smith, R.; Pisner, D.A.; Vanuk, J.R.; Berryhill, S.M.; Fridman, A.; Shane, B.R.; Knight, S.A.; Killgore, W.D.S. Exposure to Blue Light Increases Subsequent Functional Activation of the Prefrontal Cortex During Performance of a Working Memory Task. Sleep 2016, 39, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Bedrosian, T.A.; Nelson, R.J. Timing of light exposure affects mood and brain circuits. Transl. Psychiatry 2017, 7, e1017. [Google Scholar] [CrossRef]
- Green, A.; Cohen-Zion, M.; Haim, A.; Dagan, Y. Evening light exposure to computer screens disrupts human sleep, biological rhythms, and attention abilities. Chronobiol. Int. 2017, 34, 855–865. [Google Scholar] [CrossRef]
- Tähkämö, L.; Partonen, T.; Pesonen, A.K. Systematic review of light exposure impact on human circadian rhythm. Chronobiol. Int. 2019, 36, 151–170. [Google Scholar] [CrossRef]
- Al-Karawi, D.; Jubair, L. Bright light therapy for nonseasonal depression: Meta-analysis of clinical trials. J. Affect. Disord. 2016, 198, 64–71. [Google Scholar] [CrossRef]
- Alzahrani, H.S.; Khuu, S.K.; Roy, M. Modelling the effect of commercially available blue-blocking lenses on visual and non-visual functions. Clin. Exp. Optom. 2019, 103, 339–346. [Google Scholar] [CrossRef]
- Casparis, H.; Lindsley, K.; Kuo, I.C.; Sikder, S.; Bressler, N.M. Surgery for cataracts in people with age-related macular degeneration. Cochrane Database Syst. Rev. 2017, CD006757. [Google Scholar] [CrossRef]
- Theruveethi, N.; Bui, B.V.; Joshi, M.B.; Valiathan, M.; Ganeshrao, S.B.; Gopalakrishnan, S.; Kabekkodu, S.P.; Bhat, S.S.; Surendran, S. Blue Light-Induced Retinal Neuronal Injury and Amelioration by Commercially Available Blue Light-Blocking Lenses. Life 2022, 12, 243. [Google Scholar] [CrossRef] [PubMed]
- Thangarajan, R.; Tantradi, R.R.; Rai, K.S.; Gopalakrishnan, S.; Perumal, V. Inflammation During Gestation Induced Spatial Memory and Learning Deficits: Attenuated By Physical Exercise in Juvenile Rats. J. Clin. Diagnostic Res. 2015, 9, CF01–CF04. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, T.N.; Joshi, M.B.; Ballae Ganeshrao, S.; Valiathan, M.; Surendran, S. Blue LED light exposure induces metabolic rewiring in vitreous tissues in rat models. J. King Saud Univ.-Sci. 2022, 34, 101986. [Google Scholar] [CrossRef]
- Van Norren, D.; Gorgels, T.G.M.F. The Action Spectrum of Photochemical Damage to the Retina: A Review of Monochromatic Threshold Data. Photochem. Photobiol. 2011, 87, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Illouz, T.; Madar, R.; Louzon, Y.; Griffioen, K.J.; Okun, E. Unraveling cognitive traits using the Morris water maze unbiased strategy classification (MUST-C) algorithm. Brain. Behav. Immun. 2016, 52, 132–144. [Google Scholar] [CrossRef]
- Zhou, T.; Ming, Y.; Perry, S.F.; Tatic-Lucic, S. Estimation of the physical properties of neurons and glial cells using dielectrophoresis crossover frequency. J. Biol. Phys. 2016, 42, 571–586. [Google Scholar] [CrossRef]
- Mavroudis, I.; Petrides, F.; Kazis, D.; Baloyannis, S.J. Golgi method: A 140 years old, yet unique and powerful method for the study of the central nervous system. Caj. Ann. Ser. Biol. Sci. 2017, 6, 44–54. [Google Scholar]
- McAllister, A.K.; Lo, D.C.; Katz, L.C. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron 1995, 15, 791–803. [Google Scholar] [CrossRef]
- Hausser, M.; Spruston, N.; Stuart, G.J. Diversity and Dynamics of Dendritic Signaling. Science 2000, 290, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Arikkath, J. Molecular mechanisms of dendrite morphogenesis. Front. Cell. Neurosci. 2012, 6, 61. [Google Scholar] [CrossRef]
- Spruston, N. Pyramidal neurons: Dendritic structure and synaptic integration. Nat. Rev. Neurosci. 2008, 9, 206–221. [Google Scholar] [CrossRef]
- Orooji, Y.; Ghasali, E.; Emami, N.; Noorisafa, F.; Razmjou, A. ANOVA design for the optimization of TiO2 coating on polyether sulfone membranes. Molecules 2019, 24, 2924. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, U.; Pandey, C.M. Application of Student’s t-test, Analysis of Variance, and Covariance. Ann. Card. Anaesth. 2019, 22, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Theruveethi, N.N.; Sudarshan, S.; Joshi Manjunath, M.; Valiathan, M.; Ganeshrao, S.B. Blue light-induced alterations in visual cortex layer5pyramidal neuron and amelioration by Blue light blocking lenses(BBLs) in-Wistar rats. Investig. Ophthalmol. Vis. Sci. 2021, 62, 621. [Google Scholar]
- Fonken, L.K.; Kitsmiller, E.; Smale, L.; Nelson, R.J. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J. Biol. Rhythms 2012, 27, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Bedrosian, T.A.; Fonken, L.K.; Walton, J.C.; Haim, A.; Nelson, R.J. Dim light at night provokes depression-like behaviors and reduces CA1 dendritic spine density in female hamsters. Psychoneuroendocrinology 2011, 36, 1062–1069. [Google Scholar] [CrossRef]
- Bedrosian, T.A.; Vaughn, C.A.; Galan, A.; Daye, G.; Weil, Z.M.; Nelson, R.J. Nocturnal light exposure impairs affective responses in a wavelength-dependent manner. J. Neurosci. 2013, 33, 13081–13087. [Google Scholar] [CrossRef]
- Fujioka, A.; Fujioka, T.; Tsuruta, R.; Izumi, T.; Kasaoka, S.; Maekawa, T. Effects of a constant light environment on hippocampal neurogenesis and memory in mice. Neurosci. Lett. 2011, 488, 41–44. [Google Scholar] [CrossRef]
- Shang, Y.M.; Wang, G.S.; Sliney, D.H.; Yang, C.H.; Lee, L.L. Light-emitting-diode induced retinal damage and its wavelength dependency in vivo. Int. J. Ophthalmol. 2017, 10, 191–202. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akansha, E.O.; Bui, B.V.; Ganeshrao, S.B.; Bakthavatchalam, P.; Gopalakrishnan, S.; Mattam, S.; Poojary, R.R.; Jathanna, J.S.; Jose, J.; Theruveethi, N.N. Blue-Light-Blocking Lenses Ameliorate Structural Alterations in the Rodent Hippocampus. Int. J. Environ. Res. Public Health 2022, 19, 12922. https://doi.org/10.3390/ijerph191912922
Akansha EO, Bui BV, Ganeshrao SB, Bakthavatchalam P, Gopalakrishnan S, Mattam S, Poojary RR, Jathanna JS, Jose J, Theruveethi NN. Blue-Light-Blocking Lenses Ameliorate Structural Alterations in the Rodent Hippocampus. International Journal of Environmental Research and Public Health. 2022; 19(19):12922. https://doi.org/10.3390/ijerph191912922
Chicago/Turabian StyleAkansha, Elizebeth O., Bang V. Bui, Shonraj B. Ganeshrao, Pugazhandhi Bakthavatchalam, Sivakumar Gopalakrishnan, Susmitha Mattam, Radhika R. Poojary, Judith S. Jathanna, Judy Jose, and Nagarajan N. Theruveethi. 2022. "Blue-Light-Blocking Lenses Ameliorate Structural Alterations in the Rodent Hippocampus" International Journal of Environmental Research and Public Health 19, no. 19: 12922. https://doi.org/10.3390/ijerph191912922
APA StyleAkansha, E. O., Bui, B. V., Ganeshrao, S. B., Bakthavatchalam, P., Gopalakrishnan, S., Mattam, S., Poojary, R. R., Jathanna, J. S., Jose, J., & Theruveethi, N. N. (2022). Blue-Light-Blocking Lenses Ameliorate Structural Alterations in the Rodent Hippocampus. International Journal of Environmental Research and Public Health, 19(19), 12922. https://doi.org/10.3390/ijerph191912922




