Potential Impact of FDA Flavor Enforcement Policy on Vaping Behavior on Twitter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Preprocessing
2.2. Tweets Related to Quitting Vaping
2.3. Demographic Inference of Twitter Users
3. Results
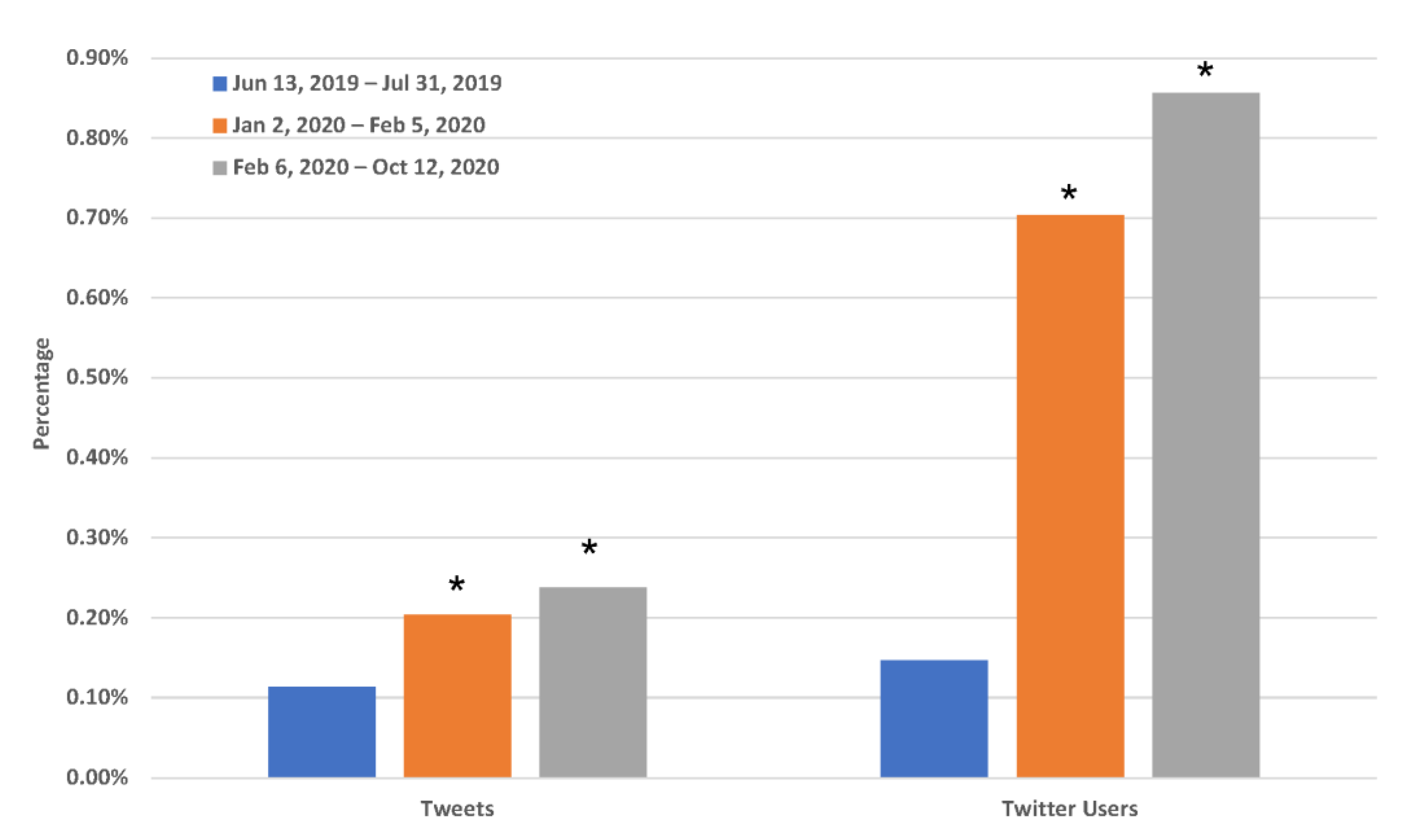
3.1. Potential Impact of FDA Flavor Enforcement Policy on One Vaping Behavior Change on Twitter
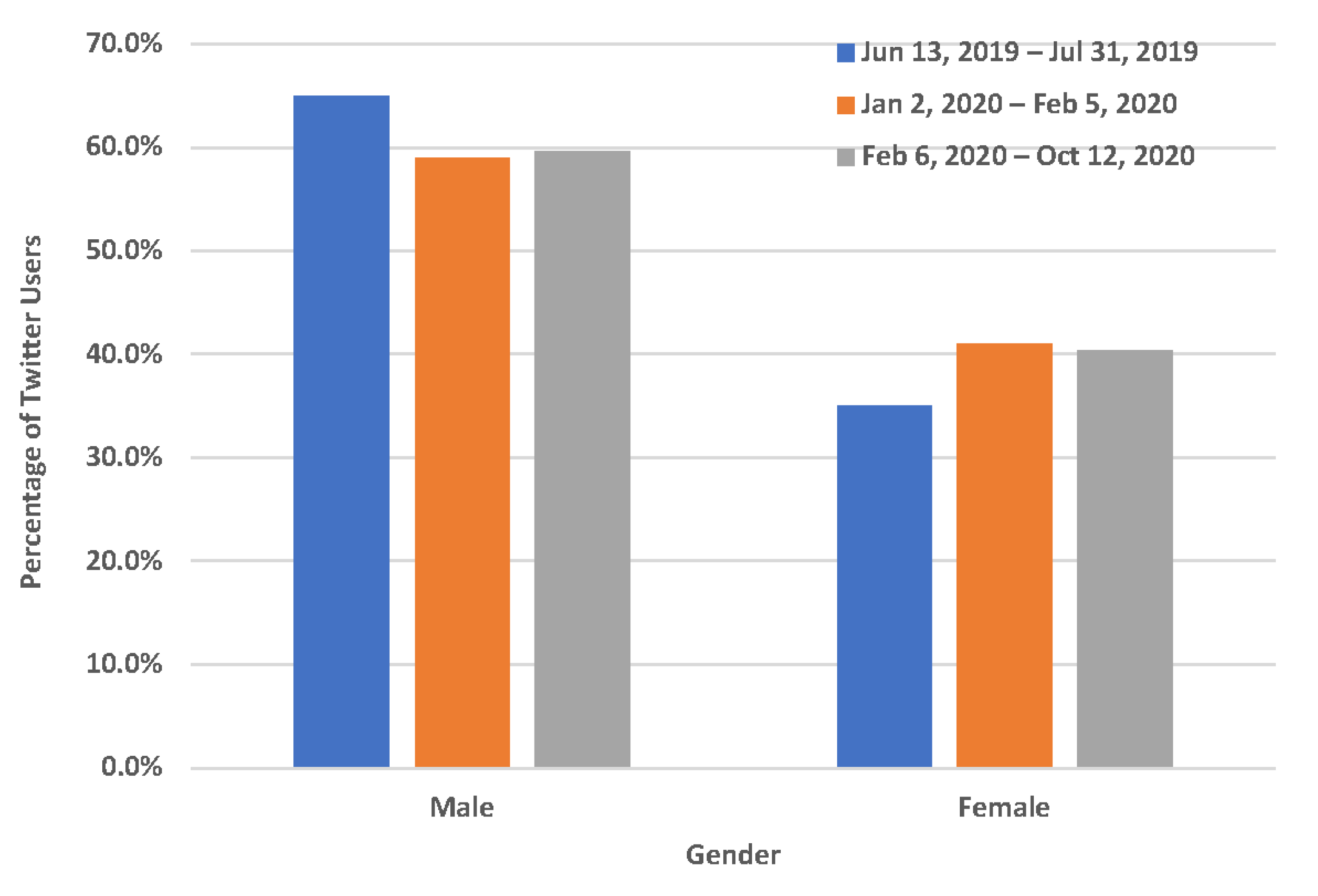
3.2. Demographic Characteristics of Twitter Users Who Mentioned Quitting Vaping
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grana, R.; Benowitz, N.; Glantz, S.A. E-cigarettes: A scientific review. Circulation 2014, 129, 1972–1986. [Google Scholar] [CrossRef] [Green Version]
- Rom, O.; Pecorelli, A.; Valacchi, G.; Reznick, A.Z. Are E-cigarettes a safe and good alternative to cigarette smoking? Ann. N. Y. Acad. Sci. 2015, 1340, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.W.; Neff, L.J.; Park-Lee, E.; Ren, C.F.; Cullen, K.A.; King, B.A. E-cigarette Use Among Middle and High School Students-United States, 2020. Mmwr-Morbid. Mortal. W 2020, 69, 1310–1312. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.H. E-Cigarette Use Among Youth and Young Adults A Major Public Health Concern. Jama Pediatr. 2017, 171, 209–210. [Google Scholar] [CrossRef]
- Park-Lee, E.; Ren, C.; Sawdey, M.D.; Gentzke, A.S.; Cornelius, M.; Jamal, A.; Cullen, K.A. Notes from the Field: E-Cigarette Use Among Middle and High School Students-National Youth Tobacco Survey, United States, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1387–1389. [Google Scholar] [CrossRef]
- Lee, W.H.; Ong, S.-G.; Zhou, Y.; Tian, L.; Bae, H.R.; Baker, N.; Whitlatch, A.; Mohammadi, L.; Guo, H.; Nadeau, K.C. Modeling cardiovascular risks of e-cigarettes with human-induced pluripotent stem cell–derived endothelial cells. J. Am. Coll. Cardiol. 2019, 73, 2722–2737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthumalage, T.; Lamb, T.; Friedman, M.R.; Rahman, I. E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci. Rep. 2019, 9, 19035. [Google Scholar] [CrossRef] [Green Version]
- Epstein, M.; Bailey, J.A.; Kosterman, R.; Rhew, I.C.; Furlong, M.; Oesterle, S.; McCabe, S.E. E-cigarette use is associated with subsequent cigarette use among young adult non-smokers, over and above a range of antecedent risk factors: A propensity score analysis. Addiction 2020, 116, 1224–1232. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, K.P.; Maremanda, K.P.; Li, D.; Chand, H.S.; Rahman, I. Differential plasma exosomal long non-coding RNAs expression profiles and their emerging role in E-cig users, cigarette, waterpipe, and dual smokers. PLoS ONE 2020, 15, e0243065. [Google Scholar] [CrossRef]
- Li, D.; Sundar, I.K.; McIntosh, S.; Ossip, D.J.; Goniewicz, M.L.; O’Connor, R.J.; Rahman, I. Association of smoking and electronic cigarette use with wheezing and related respiratory symptoms in adults: Cross-sectional results from the Population Assessment of Tobacco and Health (PATH) study, wave 2. Tob. Control 2020, 29, 140–147. [Google Scholar] [CrossRef]
- Shi, H.; Tavarez, Z.Q.; Xie, Z.; Schneller, L.M.; Croft, D.P.; Goniewicz, M.L.; McIntosh, S.; O’Connor, R.J.; Ossip, D.J.; Rahman, I.; et al. Association of flavored electronic nicotine delivery system (ENDS) use with self-reported chronic obstructive pulmonary disease (COPD): Results from the Population Assessment of Tobacco and Health (PATH) study, Wave 4. Tob. Induc. Dis. 2020, 18, 82. [Google Scholar] [CrossRef]
- Xie, C.; Xie, Z.; Li, D. Association of electronic cigarette use with self-reported difficulty concentrating, remembering, or making decisions in US youth. Tob. Induc. Dis. 2020, 18, 106. [Google Scholar] [CrossRef]
- Xie, Z.; Ossip, D.J.; Rahman, I.; Li, D. Use of Electronic Cigarettes and Self-Reported Chronic Obstructive Pulmonary Disease Diagnosis in Adults. Nicotine Tob. Res. 2020, 22, 1155–1161. [Google Scholar] [CrossRef]
- Xie, Z.; Ossip, D.J.; Rahman, I.; O’Connor, R.J.; Li, D. Electronic cigarette use and subjective cognitive complaints in adults. PLoS ONE 2020, 15, e0241599. [Google Scholar] [CrossRef]
- Navon, L.; Jones, C.M.; Ghinai, I.; King, B.A.; Briss, P.A.; Hacker, K.A.; Layden, J.E. Risk factors for e-cigarette, or vaping, product use–associated lung injury (EVALI) among adults who use e-cigarette, or vaping, products—Illinois, July–October 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 1034. [Google Scholar] [CrossRef] [Green Version]
- Salzman, G.A.; Alqawasma, M.; Asad, H. Vaping Associated Lung Injury (EVALI): An Explosive United States Epidemic. Mo. Med. 2019, 116, 492–496. [Google Scholar]
- The Lancet Respiratory, M. The EVALI outbreak and vaping in the COVID-19 era. Lancet Respir. Med. 2020, 8, 831. [Google Scholar] [CrossRef]
- Sapru, S.; Vardhan, M.; Li, Q.; Guo, Y.; Li, X.; Saxena, D. E-cigarettes use in the United States: Reasons for use, perceptions, and effects on health. BMC Public Health 2020, 20, 1518. [Google Scholar] [CrossRef]
- Zavala-Arciniega, L.; Lozano, P.; Kollath-Cattano, C.; Gutierrez-Torres, D.S.; Arillo-Santillan, E.; Barrientos-Gutierrez, I.; Hardin, J.W.; Thrasher, J.F. E-cigarette use frequency and motivations among current users in middle school. Drug Alcohol Depend. 2019, 204, 107585. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Finalizes Enforcement Policy on Unauthorized Flavored Cartridge-Based E-Cigarettes That Appeal to Children, Including Fruit and Mint; FDA news release. Available online: https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children (accessed on 2 January 2020).
- Cole-Lewis, H.; Pugatch, J.; Sanders, A.; Varghese, A.; Posada, S.; Yun, C.; Schwarz, M.; Augustson, E. Social Listening: A Content Analysis of E-Cigarette Discussions on Twitter. J. Med. Internet Res. 2015, 17, e243. [Google Scholar] [CrossRef] [Green Version]
- Lazard, A.J.; Wilcox, G.B.; Tuttle, H.M.; Glowacki, E.M.; Pikowski, J. Public reactions to e-cigarette regulations on Twitter: A text mining analysis. Tob. Control 2017, 26, e112–e116. [Google Scholar] [CrossRef]
- Lu, X.; Chen, L.; Yuan, J.; Luo, J.; Luo, J.; Xie, Z.; Li, D. User Perceptions of Different Electronic Cigarette Flavors on Social Media: Observational Study. J. Med. Internet Res. 2020, 22, e17280. [Google Scholar] [CrossRef]
- McCausland, K.; Maycock, B.; Leaver, T.; Wolf, K.; Freeman, B.; Jancey, J. E-Cigarette Advocates on Twitter: Content Analysis of Vaping-Related Tweets. JMIR Public Health Surveill. 2020, 6, e17543. [Google Scholar] [CrossRef]
- Sun, L.; Lu, X.; Xie, Z.; Li, D. Public Reactions to the New York State Policy on Flavored Electronic Cigarettes on Twitter: Observational Study. JMIR Public Health Surveill. 2022, 8, e25216. [Google Scholar] [CrossRef]
- Harris, J.K.; Moreland-Russell, S.; Choucair, B.; Mansour, R.; Staub, M.; Simmons, K. Tweeting for and against public health policy: Response to the Chicago Department of Public Health’s electronic cigarette Twitter campaign. J. Med. Internet Res. 2014, 16, e238. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Sun, L.; Xie, Z.; Li, D. Perception of the Food and Drug Administration Electronic Cigarette Flavor Enforcement Policy on Twitter: Observational Study. JMIR Public Health Surveill. 2022, 8, e25697. [Google Scholar] [CrossRef]
- Lienemann, B.A.; Unger, J.B.; Cruz, T.B.; Chu, K.-H. Methods for coding tobacco-related Twitter data: A systematic review. J. Med. Internet Res. 2017, 19, e91. [Google Scholar] [CrossRef]
- Zou, C.; Wang, X.; Xie, Z.; Li, D. Public Reactions towards the COVID-19 Pandemic on Twitter in the United Kingdom and the United States. medRxiv 2020. [Google Scholar] [CrossRef]
- Chen, L.; Lu, X.; Yuan, J.; Luo, J.; Luo, J.; Xie, Z.; Li, D. A Social Media Study on the Associations of Flavored Electronic Cigarettes With Health Symptoms: Observational Study. J. Med. Internet Res. 2020, 22, e17496. [Google Scholar] [CrossRef]
- Cadham, C.J.; Sanchez-Romero, L.M.; Fleischer, N.L.; Mistry, R.; Hirschtick, J.L.; Meza, R.; Levy, D.T. The actual and anticipated effects of a menthol cigarette ban: A scoping review. BMC Public Health 2020, 20, 1055. [Google Scholar] [CrossRef]
- Rossheim, M.E.; Livingston, M.D.; Krall, J.R.; Barnett, T.E.; Thombs, D.L.; McDonald, K.K.; Gimm, G.W. Cigarette Use Before and After the 2009 Flavored Cigarette Ban. J. Adolesc. Health 2020, 67, 432–437. [Google Scholar] [CrossRef]
- Yang, Y.; Lindblom, E.N.; Salloum, R.G.; Ward, K.D. The impact of a comprehensive tobacco product flavor ban in San Francisco among young adults. Addict. Behav. Rep. 2020, 11, 100273. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, X.; Zhu, X.W.; Yang, D.C.; Chen, R.; Jiang, Y.; Xu, T. Long non-coding RNAs in Oral squamous cell carcinoma: Biologic function, mechanisms and clinical implications. Mol. Cancer 2019, 18, 102. [Google Scholar] [CrossRef]
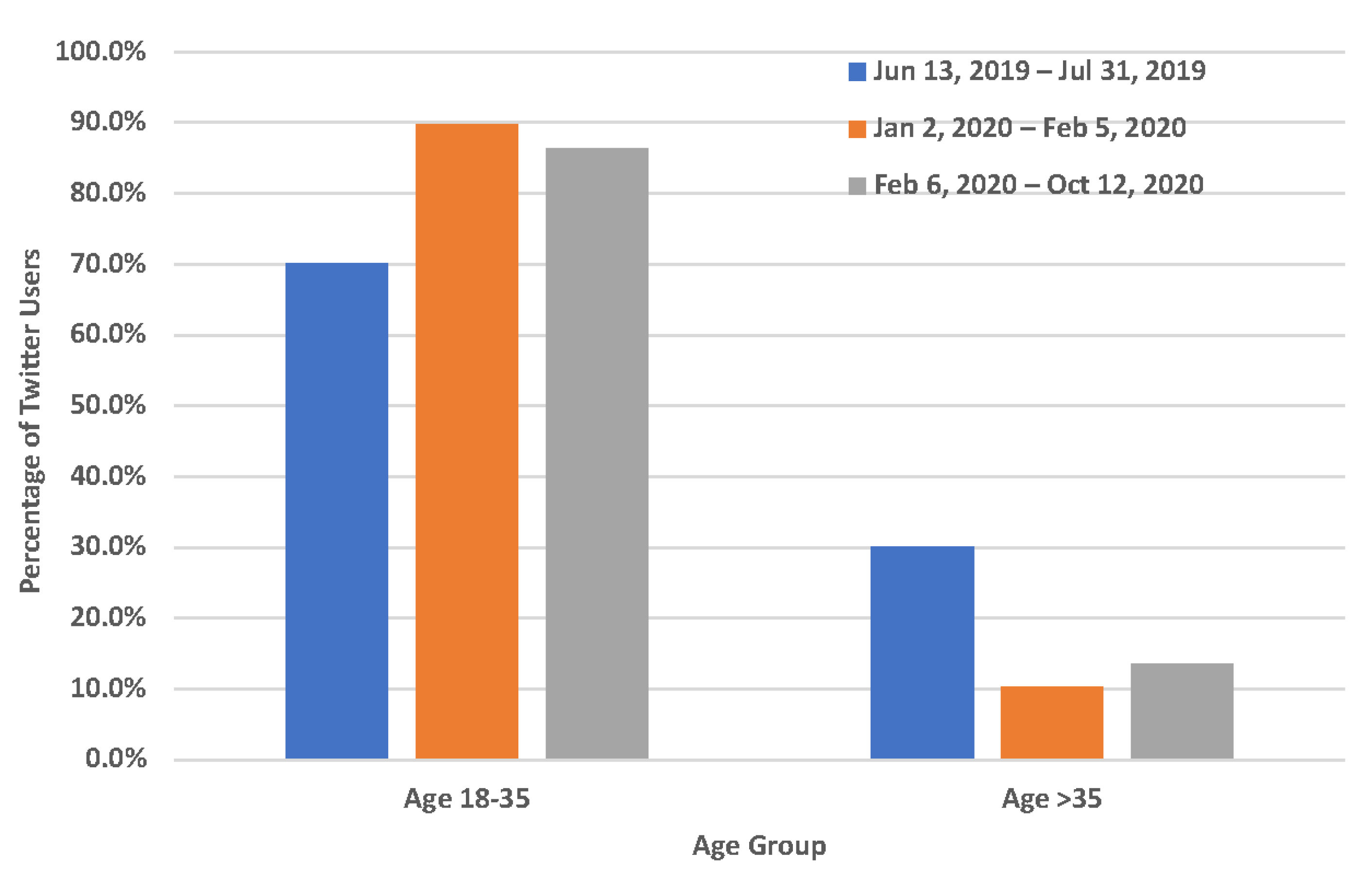
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Ruan, J.; Jiang, Y.; Zhang, B.; Chen, T.; Luo, J.; Li, D. Potential Impact of FDA Flavor Enforcement Policy on Vaping Behavior on Twitter. Int. J. Environ. Res. Public Health 2022, 19, 12836. https://doi.org/10.3390/ijerph191912836
Xie Z, Ruan J, Jiang Y, Zhang B, Chen T, Luo J, Li D. Potential Impact of FDA Flavor Enforcement Policy on Vaping Behavior on Twitter. International Journal of Environmental Research and Public Health. 2022; 19(19):12836. https://doi.org/10.3390/ijerph191912836
Chicago/Turabian StyleXie, Zidian, Jinlong Ruan, Yifan Jiang, Bokai Zhang, Tianlang Chen, Jiebo Luo, and Dongmei Li. 2022. "Potential Impact of FDA Flavor Enforcement Policy on Vaping Behavior on Twitter" International Journal of Environmental Research and Public Health 19, no. 19: 12836. https://doi.org/10.3390/ijerph191912836
APA StyleXie, Z., Ruan, J., Jiang, Y., Zhang, B., Chen, T., Luo, J., & Li, D. (2022). Potential Impact of FDA Flavor Enforcement Policy on Vaping Behavior on Twitter. International Journal of Environmental Research and Public Health, 19(19), 12836. https://doi.org/10.3390/ijerph191912836






