Association of Longitudinal Nutrient Patterns with Body Composition in Black Middle-Aged South African Women: A Five-Year Follow-Up Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Setting
2.2. Dietary Intake
2.3. Body Composition Measurements
2.4. Socio-Demographics and Physical Activity
2.5. Statistical Analysis: Analysis of Longitudinal Data
3. Results
3.1. Descriptive Characteristics
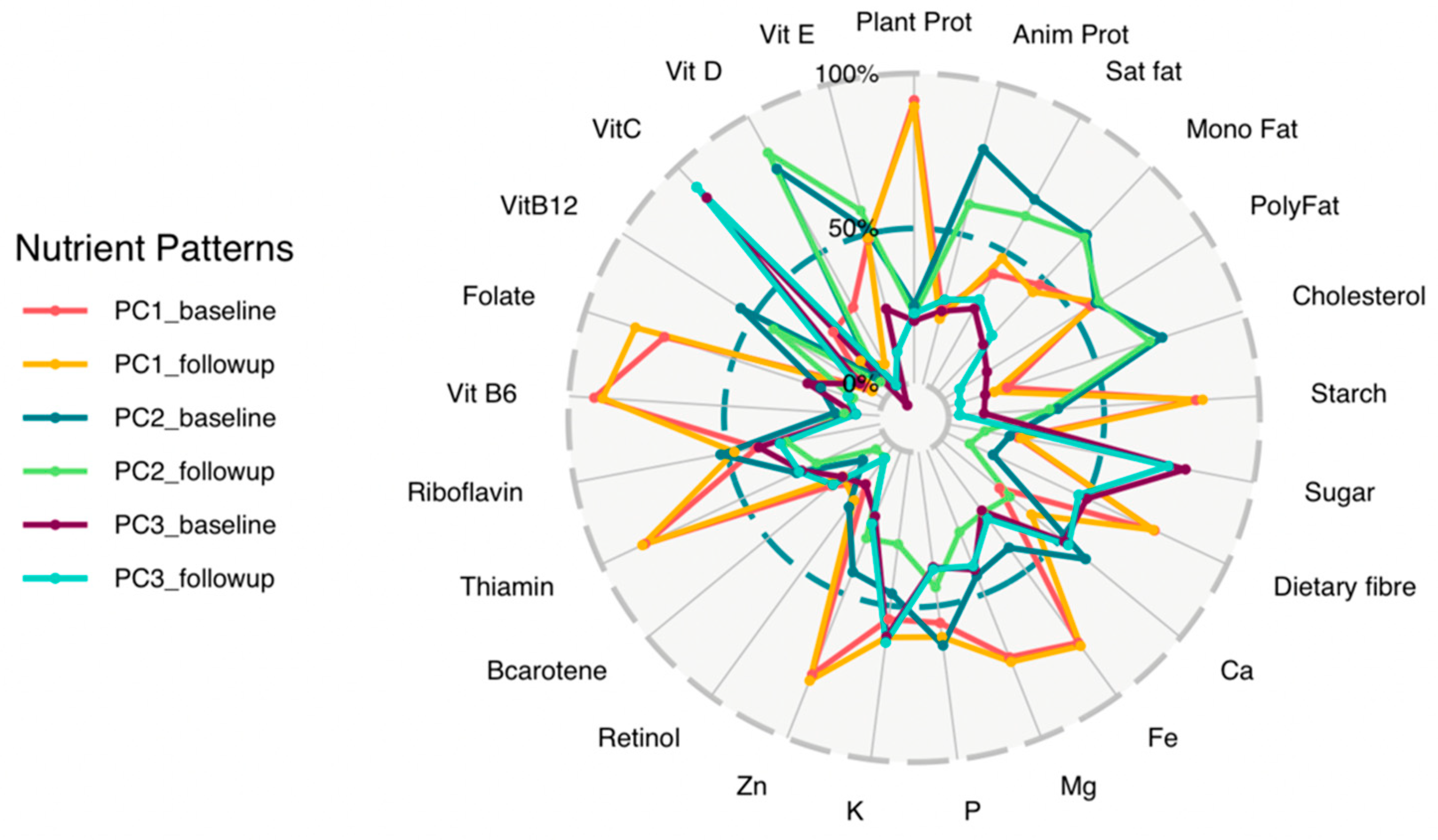
3.2. Nutrient Patterns
3.3. Longitudinal Association between Nutrient Patterns and Body Composition Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peer, N.; Steyn, K.; Lombard, C.; Gwebushe, N.; Levitt, N. A high burden of hypertension in the urban black population of Cape Town: The Cardiovascular Risk in Black South Africans (CRIBSA) study. PLoS ONE 2013, 8, e78567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, J.R.; Mabhida, S.E.; Myers, B.; Apalata, T.; Nicol, E.; Benjeddou, M.; Muller, C.; Johnson, R. Prevalence of hypertension and its associated risk factors in a rural black population of Mthatha town, South Africa. Int. J. Environ. Res. Public Health 2021, 18, 1215. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Noncommunicable Disease Country Profile 2018; Heart of Africa: Clinical Profile of an Evolving Burden of Heart Disease in Africa; World Health Organization: Geneva, Switzerland, 2018; p. 188.
- Chang, E.; Varghese, M.; Singer, K. Gender and Sex Differences in Adipose Tissue. Curr. Diabetes Rep. 2018, 18, 69. [Google Scholar] [CrossRef]
- Stelmach-Mardas, M.; Rodacki, T.; Dobrowolska-Iwanek, J.; Brzozowska, A.; Walkowiak, J.; Wojtanowska-Krosniak, A.; Zagrodzki, P.; Bechthold, A.; Mardas, M.; Boeing, H. Link between food energy density and body weight changes in obese adults. Nutrients 2016, 8, 229. [Google Scholar] [CrossRef]
- Vergnaud, A.C.; Norat, T.; Romaguera, D.; Mouw, T.; May, A.M.; Travier, N.; Luan, J.A.; Wareham, N.; Slimani, N.; Rinaldi, S.; et al. Meat consumption and prospective weight change in participants of the EPIC-PANACEA study. Am. J. Clin. Nutr. 2010, 92, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Veldhorst, M.; Smeets, A.; Soenen, S.; Hochstenbach-Waelen, A.; Hursel, R.; Diepvens, K.; Lejeune, M.; Luscombe-Marsh, N.; Westerterp-Plantenga, M. Protein-induced satiety: Effects and mechanisms of different proteins. Physiol. Behav. 2008, 94, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Kengne, A.P. Nutrient patterns and their relationship with general and central obesity in US adults. Eur. J. Clin. Investig. 2017. [Google Scholar] [CrossRef]
- Finer, N. Medical consequences of obesity. Obes. Metab. Complicat. 2015, 43, 88–93. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO); World Health Organization (WHO). Preparation and Use of Food-Based Dietary Guidelines: A Report of a Joint FAO/WHO Expert Consultation; Food and Agriculture Organization (FAO): Nicosia, Cyprus, 1996.
- Makura-Kankwende, C.B.T.; Gradidge, P.J.-L.; Crowther, N.J.; Norris, S.A.; Chikowore, T. Nutrient Patterns and Body Composition Parameters of Black South African Women. Nutrients 2021, 13, 6. [Google Scholar] [CrossRef]
- Conradie, C.; Baumgartner, J.; Malan, L.; Symington, E.A.; Cockeran, M.; Smuts, C.M.; Faber, M. A priori and a posteriori dietary patterns among pregnant women in johannesburg, south africa: The nuped study. Nutrients 2021, 13, 565. [Google Scholar] [CrossRef]
- Chikowore, T.; Pisa, P.; van Zyl, T.; Feskens, E.; Wentzel-Viljoen, E.; Conradie, K. Nutrient Patterns Associated with Fasting Glucose and Glycated Haemoglobin Levels in a Black South African Population. Nutrients 2017, 9, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisa, P.T.; Pedro, T.M.; Kahn, K.; Tollman, S.M.; Pettifor, J.M.; Norris, S.A.S. Nutrient patterns and their association with socio-demographic, lifestyle factors and obesity risk in rural South African adolescents. Nutrients 2015, 7, 3464–3482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratshikombo, T.; Goedecke, J.H.; Soboyisi, M.; Kufe, C.; Makura-Kankwende, C.B.; Masemola, M.; Micklesfield, L.K.; Chikowore, T. Sex Differences in the Associations of Nutrient Patterns with Total and Regional Adiposity: A Study of Middle-Aged Black South African Men and Women. Nutrients 2021, 13, 4558. [Google Scholar] [CrossRef]
- Jaff, N.G.; Norris, S.A.; Snyman, T.; Toman, M.; Crowther, N.J. Body composition in the Study of Women Entering and in Endocrine Transition (SWEET): A perspective of African women who have a high prevalence of obesity and HIV infection. Metabolism 2015, 64, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Richter, L.; Norris, S.; Pettifor, J.; Yach, D.; Cameron, N. Cohort Profile: Mandela’s children: The 1990 birth to twenty study in South Africa. Int. J. Epidemiol. 2007, 36, 504–511. [Google Scholar] [CrossRef] [Green Version]
- Goedecke, J.H.; Nguyen, K.A.; Kufe, C.; Masemola, M.; Chikowore, T.; Mendham, A.E.; Norris, S.A.; Crowther, N.J.; Karpe, F.; Olsson, T.; et al. Waist circumference thresholds predicting incident dysglycemia and type 2 diabetes in Black African men and women. Obes. Metab. 2022, 24, 918–927. [Google Scholar] [CrossRef]
- Zingoni, C.; Norris, S.A.; Griffiths, P.L.; Cameron, N. Studying a population undergoing nutrition transition: A practical case study of dietary assessment in urban South African adolescents. Ecol. Food Nutr. 2009, 48, 178–198. [Google Scholar] [CrossRef] [Green Version]
- Wrottesley, S.V.; Pisa, P.T.; Norris, S.A. The Influence of Maternal Dietary Patterns on Body Mass Index and Gestational Weight Gain in Urban Black South African Women. Nutrients 2017, 9, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrottesley, S.V.; Micklesfield, L.K.; Hamill, M.M.; Goldberg, G.R.; Prentice, A.; Pettifor, J.M.; Norris, S.A.; Feeley, A.B. Dietary intake and body composition in HIV-positive and -negative South African women. Public Health Nutr. 2014, 17, 1603–1613. [Google Scholar] [CrossRef]
- Feely, A.B.; Musenge, E.; Pettifor, J.M.; Norris, S.A. Investigation into longitudinal dietary behaviours and household socio-economic indicators and their association with BMI Z-score and fat mass in South African adolescents: The Birth to Twenty (Bt20) cohort. Public Health Nutr. 2012, 16, 693–703. [Google Scholar] [CrossRef]
- Steyn, N.P.; Senekal, M.; Norris, S.A.; Whati, L.; MacKeown, J.M.; Nel, J.H. How well do adolescents determine portion sizes of foods and beverages? Asia Pac. J. Clin. Nutr. 2006, 15, 35–42. [Google Scholar] [PubMed]
- South African Medical Research Council (SAMRC). FoodFinder3. Dietary Analysis Software; South African Medical Research Council: Cape Town, South Africa, 2002. [Google Scholar]
- Vorster, H.H.; Kruger, A.; Wentzel-Viljoen, E.; Kruger, H.S.; Margetts, B.M. Added sugar intake in South Africa: Findings from the Adult Prospective Urban and Rural Epidemiology cohort study. Am. J. Clin. Nutr. 2014, 99, 1479–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessri, M.; Lou, W.Y.; L’Abbé, M.R. Evaluation of different methods to handle misreporting in obesity research: Evidence from the Canadian national nutrition survey. Br. J. Nutr. 2016, 115, 147–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyeyemi, A.L.; Moss, S.J.; Monyeki, M.A.; Kruger, H.S. Measurement of physical activity in urban and rural South African adults: A comparison of two self-report methods. BMC Public Health 2016, 16, 1004. [Google Scholar] [CrossRef] [Green Version]
- Imboden, M.T.; Swartz, A.M.; Finch, H.W.; Harber, M.P.; Kaminsky, L.A. Reference standards for lean mass measures using GE dual energy x-ray absorptiometry in Caucasian adults. PLoS ONE 2017, 12, e0176161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micklesfield, L.K.; Goedecke, J.H.; Punyanitya, M.; Wilson, K.E.; Kelly, T.L. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity 2012, 20, 1109–1114. [Google Scholar] [CrossRef]
- Bull, F.; Maslin, T.; Armstrong, T. Global physical activity questionnaire (GPAQ): Nine country reliability and validity study. J. Phys. Act. Health 2009, 6, 790–804. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). The WHO STEP Wise Approach to Noncommunicable Disease Risk Factor Surveillance; World Health Organization: Geneva, Switzerland, 2017.
- StataCorp. Stata Statistical Software: Release 14; StataCorp LP.: College Station, TX, USA, 2015. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 25.0; IBM Corp: Armonk, NY, USA, 2017. [Google Scholar]
- Mundfrom, D.J.; Shaw, D.G.; Ke, T.L. Minimum Sample Size Recommendations for Conducting Factor Analyses. Int. J. Test. 2005, 5, 159–168. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Twisk, J.W.R. Applied Longitudinal Data Analysis for Epidemiology: A Practical Guide, 2nd ed.; Cambridge University Press: Cambridge, UK, 2013; pp. 57–66. Available online: https://books.google.co.za/books?hl=en&lr=&id=DRSAfYDnZqUC&oi=fnd&pg=PR13&ots=UR_IQdAwAW&sig=hZ2G4pDbrD5jV3zJJAQdh5h5LZc&redir_esc=y#v=onepage&q&f=false (accessed on 26 March 2022).
- Wang, M. Generalized estimating equations in longitudinal data analysis: A review and recent developments. Adv. Stat. 2014, 2014, 6438–6441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosqvist, F.; Iggman, D.; Kullberg, J.; Cedernaes, J.; Johansson, H.E.; Larsson, A.; Johansson, L.; Ahlström, H.; Arner, P.; Dahlman, I.; et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes 2014, 63, 2356–2368. [Google Scholar] [CrossRef] [Green Version]
- Fischer, K.; Moewes, D.; Koch, M.; Müller, H.P.; Jacobs, G.; Kassubek, J.; Lieb, W.; Nöthlings, U. MRI-determined total volumes of visceral and subcutaneous abdominal and trunk adipose tissue are differentially and sex-dependently associated with patterns of estimated usual nutrient intake in a northern German population. Am. J. Clin. Nutr. 2015, 101, 794–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bales, C.W.; Porter Starr, K.N.; Orenduff, M.C.; McDonald, S.R.; Molnar, K.; Jarman, A.K.; Onyenwoke, A.; Mulder, H.; Payne, M.E.; Pieper, C.F. Influence of protein intake, race, and age on responses to a weight-reduction intervention in obese women. Curr. Dev. Nutr. 2017, 1, e000703. [Google Scholar] [CrossRef] [Green Version]
- Isanejad, M.; Mursu, J.; Sirola, J.; Kröger, H.; Rikkonen, T.; Tuppurainen, M.; Erkkilä, A.T. Association of protein intake with the change of lean mass among elderly women: The Osteoporosis Risk Factor and Prevention—Fracture Prevention Study (OSTPRE-FPS). J. Nutr. Sci. 2015, 4, e41. [Google Scholar] [CrossRef] [Green Version]
- Lim, M.T.; Pan, B.J.; Toh, D.W.K.; Sutanto, C.N.; Kim, J.E. Animal protein versus plant protein in supporting lean mass and muscle strength: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2021, 13, 661. [Google Scholar] [CrossRef] [PubMed]
- Dominik, P.; Varman, S. A high-protein diet for reducing body fat: Mechanisms and possible caveats. Nutr. Metab. 2014, 11, 53. [Google Scholar]
- School of Public Health Harvard. The Nutrition Source. 2022. Available online: https://www.hsph.harvard.edu/nutritionsource/potassium/ (accessed on 10 August 2021).
- Gunn, C.A.; Weber, J.L.; McGill, A.T.; Kruger, M.C. Increased intake of selected vegetables, herbs and fruit may reduce bone turnover in post-menopausal women. Nutrients 2015, 7, 2499–2517. [Google Scholar] [CrossRef] [PubMed]
- Charlton, K.; Kowal, P.; Soriano, M.M.; Williams, S.; Banks, E.; Vo, K.; Byles, J. Fruit and vegetable intake and body mass index in a large sample of middle-aged Australian men and women. Nutrients 2014, 6, 2305–2319. [Google Scholar] [CrossRef] [Green Version]
- Dawson-Hughes, B.; Harris, S.S.; Ceglia, L. Alkaline diets favor lean tissue mass in older adults. Am. J. Clin. Nutr. 2008, 87, 662–665. [Google Scholar] [CrossRef] [Green Version]
- Siervo, M.; Montagnese, C.; Mathers, J.C.; Soroka, K.R.; Stephan, B.C.M.; Wells, J.C.K. Sugar consumption and global prevalence of obesity and hypertension: An ecological analysis. Public Health Nutr. 2014, 17, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Naja, F.; Hwalla, N.; Itani, L.; Karam, S.; Mehio Sibai, A.; Nasreddine, L. A Western dietary pattern is associated with overweight and obesity in a national sample of Lebanese adolescents (13–19 years): A cross-sectional study. Br. J. Nutr. 2015, 114, 1909–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Baseline | Follow-Up | p Value | |
|---|---|---|---|
| Demographics | |||
| Age, years | 48 (44; 52) | 53 (50; 58) | <0.001 |
| Socio-economic status (a score out of 9) | 5 (4; 6) | 5 (3; 5) | 0.572 |
| Physically active (%) | 94 (71.2%) | 93 (70.5%) | 0.882 |
| Body composition | |||
| BMI (kg m−2) | 33.7 (28.1; 39.0) | 33.7 (28.1; 39.2) | 0.767 |
| Whole-body fat mass (kg) | 60.5 (47.1; 73.3) | 63.4 (49.1; 76.3) | 0.015 |
| Fat mass index (FMI) (kg m−2) | 23.5 (20.5; 30.3) | 26.6 (20.7; 31.8) | <0.001 |
| Whole body lean mass (kg) | 41.9 (36.9; 45.1) | 41.0 (34.4; 52.4) | 0.029 |
| Lean mass index (LMI) (kg m−2) | 16.6 (14.7; 18.2) | 16.4 (14.5; 20.8) | 0.055 |
| Gynoid fat mass (kg) | 3.2. (2.6; 4.2) | 3.6 (3.0; 4.6) | <0.001 |
| Subcutaneous Adipose Tissue (cm2) | 452.7 (360.2; 574.5) | 457.6 (349.1; 576.4) | 0.547 |
| Visceral Adipose Tissue (cm2) | 102.6 (75.2; 128.9) | 107.8 (70.4; 137.3) | 0.001 |
| Nutritional intake data | |||
| Energy intake (mJ) | 9.3 (7.9; 12.0) | 6.4 (5.1; 8.4) | <0.001 |
| % Carbohydrates (of total energy) | 54.5 (49.7; 58.1) | 52.6 (48.6; 56.5) | 0.052 |
| % Protein (of total energy) | 11.4 (10.1; 12.8) | 11.2 (9.7; 12.7) | 0.871 |
| % Fat (of total energy) | 30.1 (26.0; 34.3) | 31.7 (27.0; 35.5) | 0.035 |
| BMI | VAT | SAT | FMI | LMI | GYNOID | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Plant Protein Driven NP | 0.138 | 0.290 | 0.952 | −4.040 | −1.432 | −2.135 | 0.461 ** | 0.361 | −0.890 ** | 0.150 | 0.127 | −0.787 |
| Animal Protein Driven NP | 0.307 | 0.243 | 10.115 *** | 5.788 ** | 1.451 | −7.103 | 0.449 *** | 0.469 ** | 0.466 * | 0.498 ** | 0.724 ** | 0.002 |
| Vit C, Sugar, and Potassium Driven NP | 0.157 | 0.404 | 3.525 | 0.010 | 6.096 | −2.785 | 0.425 *** | 0.502 *** | 0.499 ** | 0.584 ** | 0.308 | −0.622 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makura-Kankwende, C.B.T.; Gradidge, P.J.; Crowther, N.J.; Ratshikombo, T.; Goedecke, J.H.; Micklesfield, L.K.; Norris, S.A.; Chikowore, T. Association of Longitudinal Nutrient Patterns with Body Composition in Black Middle-Aged South African Women: A Five-Year Follow-Up Study. Int. J. Environ. Res. Public Health 2022, 19, 12792. https://doi.org/10.3390/ijerph191912792
Makura-Kankwende CBT, Gradidge PJ, Crowther NJ, Ratshikombo T, Goedecke JH, Micklesfield LK, Norris SA, Chikowore T. Association of Longitudinal Nutrient Patterns with Body Composition in Black Middle-Aged South African Women: A Five-Year Follow-Up Study. International Journal of Environmental Research and Public Health. 2022; 19(19):12792. https://doi.org/10.3390/ijerph191912792
Chicago/Turabian StyleMakura-Kankwende, Caroline B. T., Philippe J. Gradidge, Nigel J. Crowther, Tshifhiwa Ratshikombo, Julia H. Goedecke, Lisa K. Micklesfield, Shane A. Norris, and Tinashe Chikowore. 2022. "Association of Longitudinal Nutrient Patterns with Body Composition in Black Middle-Aged South African Women: A Five-Year Follow-Up Study" International Journal of Environmental Research and Public Health 19, no. 19: 12792. https://doi.org/10.3390/ijerph191912792
APA StyleMakura-Kankwende, C. B. T., Gradidge, P. J., Crowther, N. J., Ratshikombo, T., Goedecke, J. H., Micklesfield, L. K., Norris, S. A., & Chikowore, T. (2022). Association of Longitudinal Nutrient Patterns with Body Composition in Black Middle-Aged South African Women: A Five-Year Follow-Up Study. International Journal of Environmental Research and Public Health, 19(19), 12792. https://doi.org/10.3390/ijerph191912792







