Assessment of the Chronic Toxicity and Interactions between Arsenic and Riverbed Biofilms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Characteristics of Water and Sediments
- -
- Eguas (43°13′24.26″ N, 8°45′44.61″ W): not affected by mining activities, so As concentration in sediments was expected to be low.
- -
- Xavarido (43°13′48.82″ N, 8°49′54.29″ W): located 8 km downstream Eguas, just a little downstream from the area affected by mining, and known to have high As concentrations.
2.2. Experimental
2.2.1. Chronic Toxicity Experiment
2.2.2. Natural Tolerance Evaluation
2.3. Analytical Methods
2.3.1. Biofilm Growth and Characteristics
Phytoplankton Pulse-Amplitude-Modulated (Phyto-PAM) Measurements
Live/Dead Analyses
2.3.2. Arsenic
Arsenic in Water
Arsenic in Biofilm
Arsenic in EPS
2.4. Statistics
3. Results
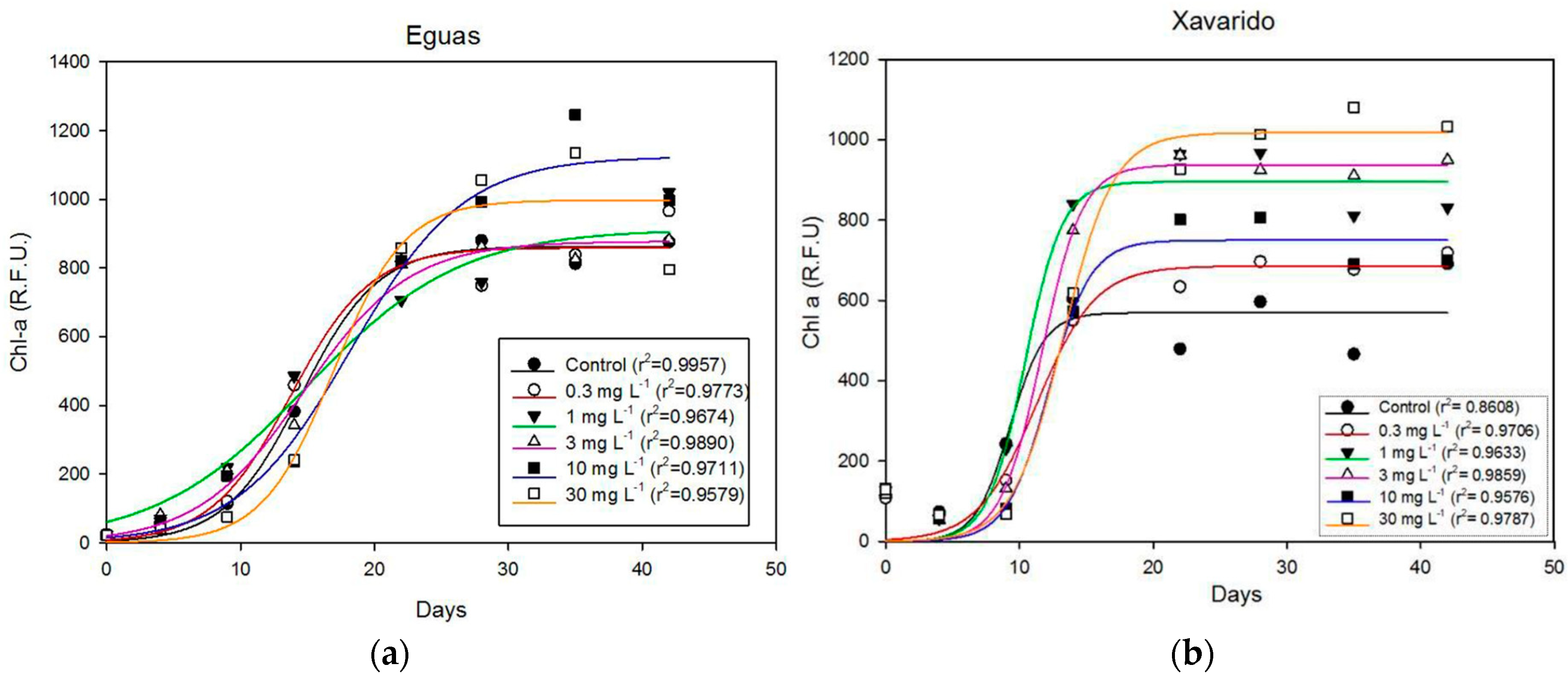
3.1. Effect of As Concentration on Biofilm Growth
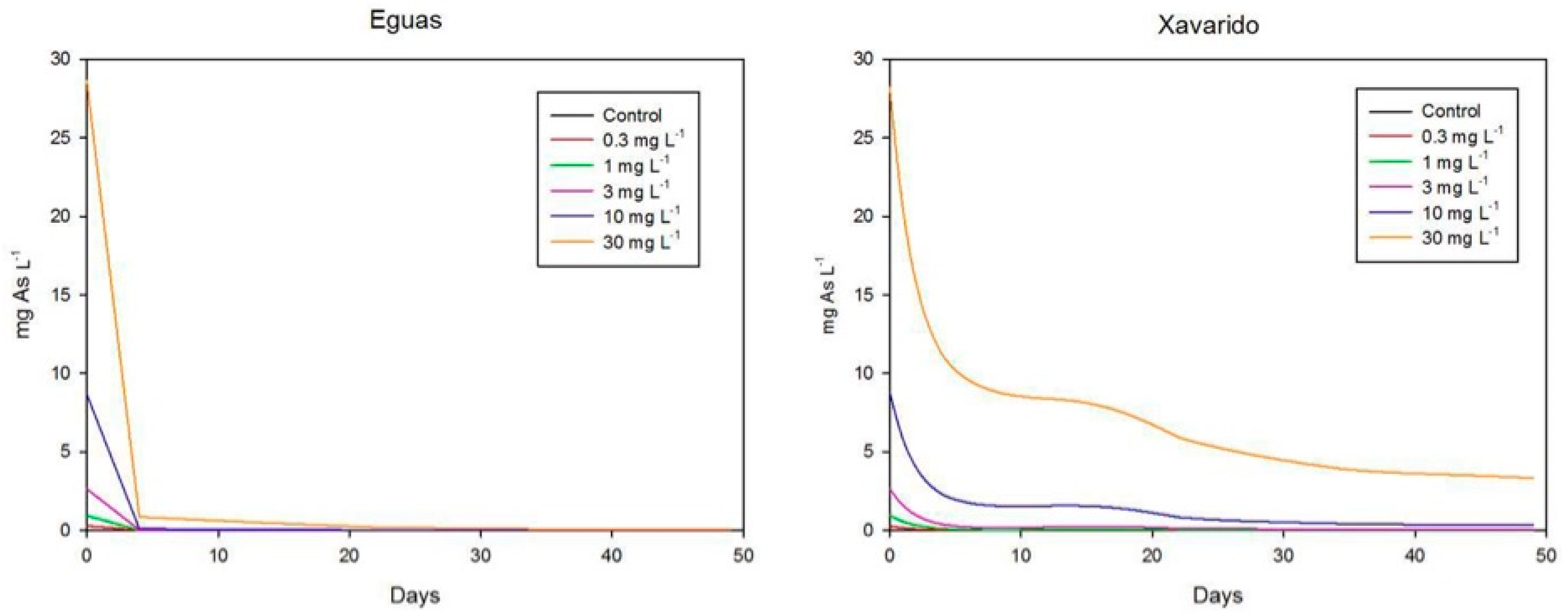
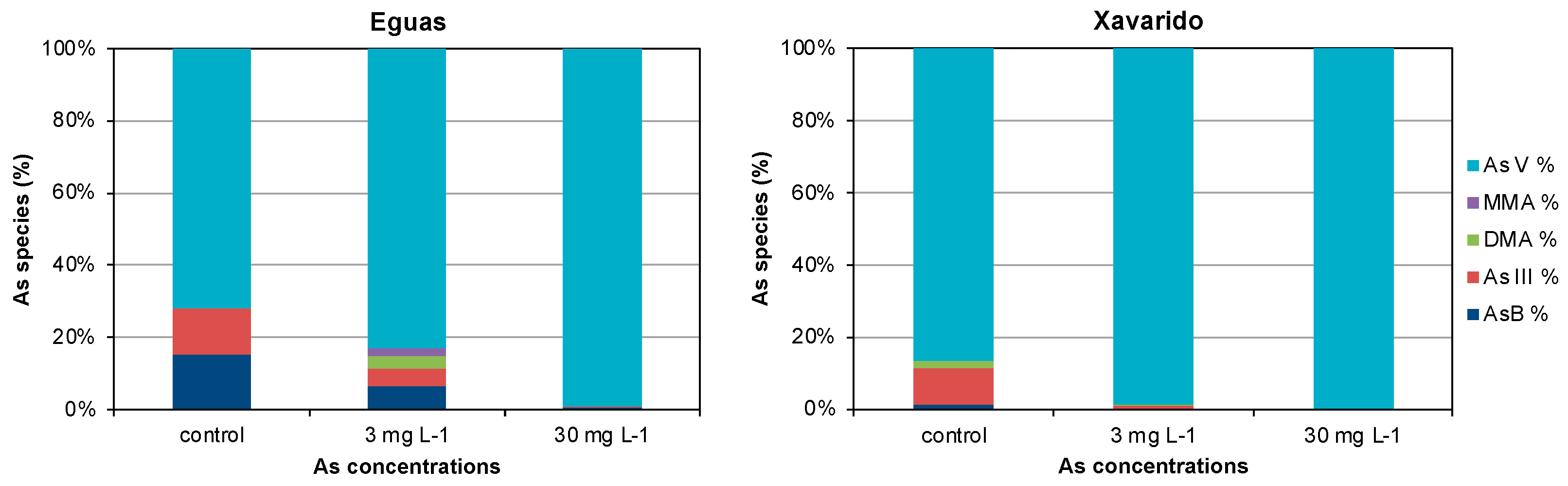
3.2. Arsenic in Water and Biofilm
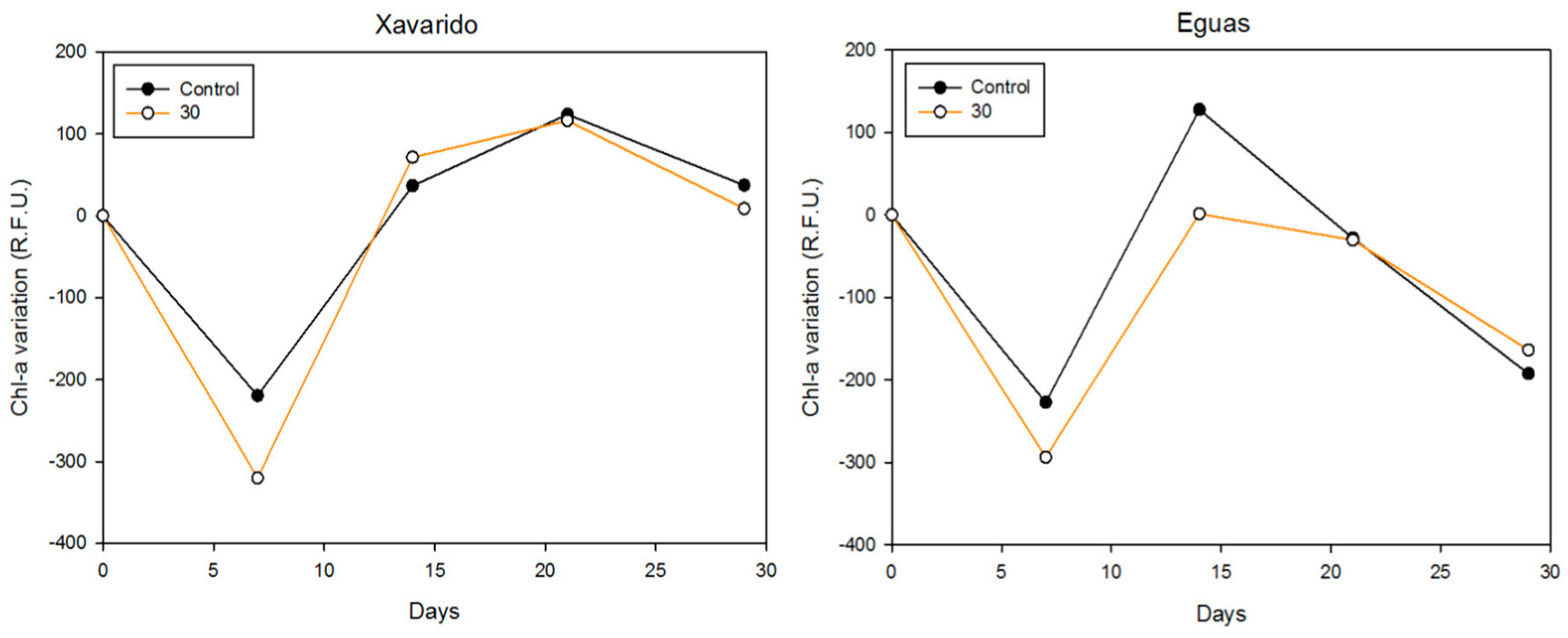
3.3. Development of Tolerance to As
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Fang, H.H. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 2002, 95, 249–256. [Google Scholar] [CrossRef]
- Denkhaus, E.; Meisen, S.; Telgheder, U.; Wingender, J. Chemical and physical methods for characterisation of biofilms. Microchim. Acta 2007, 158, 1–27. [Google Scholar] [CrossRef]
- Proia, L. Biofilm Responses to Multiple Stressors Associated to Global Change in River Ecosystems. Ph.D. Thesis, University of Girona, Girona, Spain, 2012. [Google Scholar]
- Van Hullebusch, E.D.; Zandvoort, M.H.; Lens, P.N.L. Metal immobilisation by biofilms, Mechanisms and analytical tools. Environ. Sci. Bio/Technol. 2003, 2, 9–33. [Google Scholar] [CrossRef]
- Sabater, S.; Guasch, H.; Ricart, M.; Romaní, A.; Vidal, G.; Klünder, C.; Schmitt-Jansen, M. Monitoring the effect of chemicals on biological communities: The biofilm as an interface. Anal. Bioanal. Chem. 2007, 387, 1425–1434. [Google Scholar] [CrossRef]
- Corcoll, N.; Bonet, B.; Leira, M.; Guasch, H. Chl-a fluorescence parameters as biomarkers of metal toxicity in fluvial biofilms, an experimental study. Hydrobiologia 2011, 673, 119–136. [Google Scholar] [CrossRef]
- Villeneuve, A.; Montuelle, B.; Pesce, S.; Bouchez, A. Environmental River Biofilms as Biological Indicators of the Impact of Chemical Contaminants. In Encyclopedia of Aquatic Ecotoxicology; Férard, J.F., Blaise, C., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 443–456. [Google Scholar] [CrossRef]
- Yang, S.I.; Lawrence, J.R.; Swerhone, G.D.W.; Pickering, I. Biotransformation of selenium and arsenic in multi-species biofilm. Environ. Chem. 2011, 8, 543–551. [Google Scholar] [CrossRef]
- Drahota, P.; Filippi, M. Secondary Arsenic Minerals in the Environment: A Review. Environ. Int. 2009, 35, 1243–1255. [Google Scholar] [CrossRef]
- Barral-Fraga, L.; Prieto, D.M.; Barral, M.T.; Morin, S.; Guasch, H. Mutual interaction between arsenic and biofilm in a mining impacted river. Sci. Total Environ. 2018, 636, 985–998. [Google Scholar] [CrossRef]
- Prieto, D.M.; Devesa-Rey, R.; Rubinos, D.A.; Díaz-Fierros, F.; Barral, M.T. Arsenate retention by episammic biofilms developed on streambed sediments: Influence of phosphate. BioMed. Res. Int. 2013, 2013, 591634. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Effect of natural organic matter on arsenic release from soils and sediments into groundwater. Environ. Geochem. Health 2006, 28, 197–214. [Google Scholar] [CrossRef]
- Prieto, D.M.; Martín-Liñares, V.; Piñeiro, V.; Barral, M.T. Arsenic Transfer from As-Rich Sediments to River Water in the Presence of Biofilms. J. Chem. 2016, 2016, 6092047. [Google Scholar] [CrossRef] [Green Version]
- Tuulaikhuu, B.-A.; Romaní, A.M.; Guasch, H. Arsenic toxicity effects on microbial communities and nutrient cycling in indoor experimental channels mimicking a fluvial system. Aquat. Toxicol. 2015, 166, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Barral-Fraga, L.; Barral, M.T.; MacNeill, K.L.; Martiñá-Prieto, D.; Morin, S.; Rodríguez-Castro, M.C.; Tuulaikhuu, B.-A.; Guasch, H. Biotic and Abiotic Factors Influencing Arsenic Biogeochemistry and Toxicity in Fluvial Ecosystems: A Review. Int. J. Environ. Res. Public Health 2020, 17, 2331. [Google Scholar] [CrossRef] [Green Version]
- Levy, J.L.; Stauber, J.L.; Adams, M.S.; Maher, W.A.; Kirby, J.K.; Jolley, D.F. Toxicity, biotransformation, and mode of action of arsenic in two freshwater microalgae (Chlorella sp. and Monoraphidium arcuatum). Environ. Toxicol Chem. 2005, 10, 2630–2639. [Google Scholar] [CrossRef]
- Rodríguez-Castro, M.C.; Urrea, G.; Guasch, H. Influence of the interaction between phosphate and arsenate on periphyton’s growth and its nutrient uptake capacity. Sci. Tot. Environ. 2015, 503, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.X.; Li, Y.; Deng, X.H.; Miao, A.J.; Ji, R.; Yang, L.Y. Toxicity and bioaccumulation kinetics of arsenate in two freshwater green algae under different phosphate regimes. Water Res. 2013, 47, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Prieto, D.M.; Rubinos, D.A.; Piñeiro, V.; Díaz-Fierros, F.; Barral, M.T. Influence of epipsammic biofilm on the biogeochemistry of arsenic in freshwater environments. Biogeochemistry 2016, 129, 291–306. [Google Scholar] [CrossRef]
- Blank, H. A critical review of procedures and approaches used for assesing pollution-induced community tolerance (PICT) in biotic communities. Hum. Ecol. Risk Assess. 2002, 8, 1003–1034. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Paradelo, R.; Díaz-Fierros, F.; Barral, M.T. Fractionation and bioavailability of arsenic in the bed sediments of the Anllóns River (NW Spain). Water Air Soil Pollut. 2008, 195, 189–199. [Google Scholar] [CrossRef]
- Nespereira, X. Síntesis sobre los yacimientos auríferos gallegos. Braña 1978, 1, 18–49. [Google Scholar]
- Boixet, L.; Gleeson, C.F.; García, J. The Corcoesto Gold Deposit. In Proceedings of the 23rd Intrtnational Int Applied Geochem Symp (IAGS 2007), Oviedo, España, 14–19 June 2007. [Google Scholar]
- Rubinos, D.A.; Iglesias, L.; Devesa-Rey, R.; Díaz-Fierros, F.; Barral, M.T. Arsenic release from river sediments in a gold-mining area (Anllóns River basin, Spain), Effect of time, pH and phosphorous concentration. Eur. J. Miner. 2010, 22, 665–678. [Google Scholar] [CrossRef]
- Costas, M.; Prego, R.; Filgueiras, A.V.; Bendicho, C. Land–ocean contributions of arsenic through a river–estuary–ria system (SW Europe) under the influence of arsenopyrite deposits in the fluvial basin. Sci. Total Environ. 2011, 412–413, 304–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubinos, D.; Iglesias, L.; Díaz-Fierros, F.; Barral, M.T. Interacting Effect of pH, Phosphate and Time on the Release of Arsenic from Polluted River Sediments (Anllóns River, Spain). Aquat. Geochem. 2011, 17, 281–306. [Google Scholar] [CrossRef]
- Iglesias, M.L.; Devesa-Rey, R.; Pérez-Moreira, R.; Díaz-Fierros, F.; Barral, M.T. Phosphorus transfer across boundaries, from basin soils to riverbed sediments. J. Soils Sediments 2011, 11, 1125–1134. [Google Scholar] [CrossRef]
- Barral, M.T.; Devesa-Rey, R.; Ruiz, B.; Díaz-Fierros, F. Evaluation of phosphorous species in the bed sediments of an Atlantic Basin. Bioavailability and relation with surface active components of the sediment. Soil Sed. Contam. 2012, 21, 1–18. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Moldes, A.B.; Díaz-Fierros, F.; Barral, M.T. Study of phytopigments in riverbed sediments, effects of the organic matter, nutrients and metal composition. Environ. Monit. Assess. 2009, 153, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Prieto, D.M.; Devesa-Rey, R.; Paradelo, R.; Díaz-Fierros, F.; Barral, M.T. Monitoring benthic microflora in river bed sediments, a case study in the Anllóns River (Spain). J. Soil Sediment 2016, 16, 1825–1839. [Google Scholar] [CrossRef]
- U.S. EPA. Quality Criteria for Water; Technical report 440/5-86-001; Office of Water Regulations and Standards: Washington, DC, USA, 1986. [Google Scholar]
- U.S. EPA. National Recommended Water Quality Criteria: Aquatic Life Criteria; EPA: Washington, DC, USA, 2014. [Google Scholar]
- WHO. WHO Guidelines for Drinking Water Quality, Arsenic in Drinking Water; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Lamb, A.L.; Wilson, G.P.; Leng, M.J. A review of coastal palaeoclimate and relative sea-level reconstructions using δ 13 C and C/N ratios in organic material. Earth-Sci. Rev. 2006, 75, 29–57. [Google Scholar] [CrossRef]
- Macías, F.; Calvo de Anta, R. Niveles Genéricos de Referencia de Metales Pesados y Otros Elementos Traza en Suelos de Galicia; Xunta de Galicia: Santiago de Compostela, Spain, 2009. [Google Scholar]
- European Union. Directive 2000/60/EC of the European Council, October the 23rd, establishing a framework for the Community action in the field of water policy. Off. J. 2000, 327, 1–73. [Google Scholar]
- Corcoll, N.; Bonet, B.; Morin, S.; Tlili, A.; Leira, M.; Guasch, H. The effect of metals on photosynthesis processes and diatom metrics of biofilm from a metal-contaminated river, a translocation experiment. Ecol. Indic. 2012, 18, 620–631. [Google Scholar] [CrossRef]
- Romaní, A.M.; Artigas, J.; Camacho, A.; Graça, M.; Pascoal, C. La biota de los ríos, los microorganismos heterotróficos. In Conceptos y Técnicas en Ecología Fluvial; Elosegi, A., Sabater, S., Eds.; Fundación BBVA: Bilbao, Spain, 2009; pp. 169–218. [Google Scholar]
- Underwood, G.J.C.; Paterson, D.M.; Parkes, R.J. The measurement of microbial carbohydrate exopolymers from intertidal sediments. Limnol. Oceanogr. 1995, 40, 1243–1253. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Mueller, M.; Pander, J.; Wild, R.; Lueders, T.; Geist, J. The effects of stream substratum texture on interstitial conditions and bacterial biofilms: Methodological strategies. Limnologica 2013, 43, 106–113. [Google Scholar] [CrossRef]
- Liñares, V. Influencia del biofilm episámico sobre la movilidad y toxicidad de arsénico en aguas y sedimentos fluviales contaminados. Master’s Thesis, University of Santiago de Compostela, Santiago de Compostela, Spain, 2016. [Google Scholar]
- Barral-Fraga, L.; Morin, S.; Rovira, M.D.M.; Urrea, G.; Magellan, K.; Guasch, H. Short-term arsenic exposure reduces diatom cell size in biofilm communities. Environ. Sci. Pollut. Res. Int. 2016, 23, 4257–4270. [Google Scholar] [CrossRef] [PubMed]
- Blanck, H.; Wangberg, S.A. Validity of an ecotoxicological test system, Short-term and long-term effects of arsenate on marine periphyton communities in laboratory systems. Can. J. Fish Aquat. Sci. 1988, 45, 1807–1815. [Google Scholar] [CrossRef]
- Genter, R.B.; Cherry, D.S.; Smith, E.P.; Cairns, J., Jr. Algal-periphyton population and community changes from zinc stress in stream mesocosms. Hydrobiologia 1987, 153, 261–275. [Google Scholar] [CrossRef]
- Barranguet, C.; Charantoni, E.; Plans, M.; Admiraal, W. Short-term response of monospecific and natural algal biofilms to copper exposure. Eur. J. Phycol. 2000, 35, 397–406. [Google Scholar] [CrossRef]
- Barranguet, C.; van den Ende, F.P.; Rutgers, M.; Breure, A.M.; Greijdanus, M.; Sinke, J.J.; Admiraal, W. Copper-induced modifications of the trophic relations in riverine algal-bacterial biofilms. Environ. Toxicol. Chem. 2003, 22, 1340–1349. [Google Scholar]
- Serra, A.; Guasch, H.; Admiraal, W.; Van der Geest, H.G.; Van Beusekom, S.A.M. Influence of phosphorus on copper sensitivity of fluvial periphyton, the role of chemical, physiological and community-related factors. Ecotoxicology 2010, 19, 770–780. [Google Scholar] [CrossRef] [Green Version]
- Bonet, B. Antioxidant enzyme activities in fluvial biofilms as biomarkers of metal pollution. Ph.D. Thesis, University of Girona, Girona, Spain, 2012. [Google Scholar]
- Devesa-Rey, R.; Barral, M.T.; Jouanneau, J.M.; Díaz-Fierros, F. Analysis of the degree of contamination and evolution in the last 100 years of the composition of the bed sediments of the Anllóns Basin. Environ. Earth Sci. 2010, 61, 1401–1417. [Google Scholar] [CrossRef]
- Karadjova, I.B.; Slaveykova, V.I.; Tsalev, D.L. The biouptake and toxicity of arsenic species on the green microalga Chlorella salina in seawater. Aquat. Toxicol. 2008, 87, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Oremland, R.S.; Stolz, J.F. The ecology of arsenic. Science 2003, 300, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Sohn, M. Aquatic arsenic, Toxicity, speciation, transformations and remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Duker, A.A.; Carranza, E.J.M.; Hale, M. Arsenic geochemistry and health. Environ. Int. 2005, 31, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Li, X.F.; Cullen, W.R.; Weinfeld, M.; Le, X.C. Arsenic binding to proteins. Chem. Rev. 2013, 113, 7769–7792. [Google Scholar] [CrossRef]
- Styblo, M.; Del Razo, L.M.; Vega, L.; Germolec, D.R.; LeCluyse, E.L.; Hamilton, G.A.; Reed, W.; Wang, C.; Cullen, W.R.; Thomas, D.J. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch. Toxicol. 2000, 74, 289–299. [Google Scholar] [CrossRef]
- Mass, M.J.; Tennant, A.; Roop, B.C.; Cullen, W.R.; Styblo, M.; Thomas, D.J.; Kligerman, A.D. Methylated trivalent arsenic species are genotoxic. Chem. Res. Toxicol. 2001, 14, 355–361. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, J.F.; Meharg, A.A.; McGrath, S.P. Arsenic uptake and metabolism in plants. New Phytol. 2009, 181, 777–794. [Google Scholar] [CrossRef]
- Yin, X.; Chen, J.; Qin, J.; Sun, G.; Rosen, B.P.; Zhu, Y. Biotransformation and volatilization of arsenic by three photosynthetic cyanobacteria. Plant Physiol. 2011, 156, 1631–1638. [Google Scholar] [CrossRef] [Green Version]
- Hellweger, F.L.; Farley, K.J.; Lall, U.; Di Toro, D.M. Greedy algae reduce arsenate. Limnol. Oceanogr. 2003, 48, 2275–2288. [Google Scholar] [CrossRef]



| pH | Electrical Conductivity (µS cm−1) | Alkalinity (mg L−1) | Total N (mg L−1) | Total P (mg L−1) | |
|---|---|---|---|---|---|
| Eguas | 7.39 | 151 | 27.1 | 1.64 | 0.045 |
| Xavarido | 7.40 | 146 | 26.6 | 1.52 | 0.026 |
| Na | K | Mg | Ca | As | |
| (mg L−1) | (µg L−1) | ||||
| Eguas | 11.4 | 1.6 | 3.6 | 7.0 | 0.85 |
| Xavarido | 10.9 | 1.5 | 3.6 | 8.5 | 1.33 |
| Particle Size (%) | >2 mm | 2–1 mm | 1–0.5 mm | 0.5–0.2 mm | 0.25–0.1 mm | 0.1–0.05 mm | <0.05 mm |
|---|---|---|---|---|---|---|---|
| Eguas | 0.64 | 5.11 | 9.26 | 6.28 | 20.00 | 0.11 | 58.62 |
| Xavarido | 17.25 | 25.64 | 30.95 | 18.19 | 6.14 | 0.09 | 1.75 |
| N% | C% | S% | C/N | Al% | Fe% | Ti% | |
| Eguas | 0.60 | 9.19 | 0.13 | 15 | 6.5 | 6.1 | 0.8 |
| Xavarido | 0.06 | 0.44 | 0.01 | 7 | 6.3 | 4.7 | 1.4 |
| P (mg kg−1) | Mn (mg kg−1) | Ni (mg kg−1) | Zn (mg kg−1) | Cu (mg kg−1) | As (mg kg−1) | ||
| Eguas | 1349 | 1126 | 39 | 257 | 36 | 31 | |
| Xavarido | n.d. 1 | 994 | 16 | 65 | 11 | 85 |
| Day 0 | Day 9 | Day 42 | |||
|---|---|---|---|---|---|
| Ymax | Eguas | 0 mg L−1 | 0.02 ± 0.02 | 0.59 ± 0.02 | 0.49 ± 0.18 |
| 0.3 mg L−1 | 0.02 ± 0.02 | 0.57 ± 0.05 | 0.44 ± 0.21 | ||
| 1 mg L−1 | 0.01 ± 0.02 | 0.61 ± 0.02 | 0.43 ± 0.13 | ||
| 3 mg L−1 | 0.04 ± 0.04 | 0.59 ± 0.05 | 0.47 ± 0.09 | ||
| 10 mg L−1 | 0.04 ± 0.04 | 0.59 ± 0.04 | 0.44 ± 0.14 | ||
| 30 mg L−1 | 0.05 ± 0.05 | 0.50 ± 0.04 | 0.47 ± 0.08 | ||
| Xavarido | 0 mg L−1 | 0.12 ± 0.03 | 0.65 ± 0.01 | 0.52 ± 0.05 | |
| 0.3 mg L−1 | 0.13 ± 0.05 | 0.63 ± 0.02 | 0.54 ± 0.05 | ||
| 1 mg L−1 | 0.09 ± 0.02 | 0.64 ± 0.01 | 0.49 ± 0.07 | ||
| 3 mg L−1 | 0.12 ± 0.03 | 0.62 ± 0.02 | 0.50 ± 0.01 | ||
| 10 mg L−1 | 0.09 ± 0.01 | 0.58 ± 0.02 | 0.53 ± 0.05 | ||
| 30 mg L−1 | 0.12 ± 0.07 | 0.53 ± 0.04 | 0.44 ± 0.03 | ||
| Yeff | Eguas | 0 mg L−1 | 0.17 ± 0.06 | 0.54 ± 0.06 | 0.58 ± 0.01 |
| 0.3 mg L−1 | 0.04 ± 0.01 | 0.55 ± 0.04 | 0.39 ± 0.18 | ||
| 1 mg L−1 | 0.10 ± 0.07 | 0.53 ± 0.04 | 0.59 ± 0.01 | ||
| 3 mg L−1 | 0.06 ± 0.02 | 0.54 ± 0.03 | 0.50 ± 0.05 | ||
| 10 mg L−1 | - | 0.43 ± 0.03 | 0.51 ± 0.02 | ||
| 30 mg L−1 | 0.03 ± 0.01 | 0.39 ± 0.05 | 0.55 ± 0.02 | ||
| Xavarido | 0 mg L−1 | 0.06 ± 0.06 | 0.48 ± 0.07 | 0.49 ± 0.03 | |
| 0.3 mg L−1 | 0.12 ± 0.00 | 0.51 ± 0.02 | 0.46 ± 0.08 | ||
| 1 mg L−1 | 0.08 ± 0.09 | 0.57 ± 0.04 | 0.39 ± 0.08 | ||
| 3 mg L−1 | 0.03 ± 0.05 | 0.53 ± 0.05 | 0.43 ± 0.11 | ||
| 10 mg L−1 | 0.04 ± 0.01 | 0.47 ± 0.02 | 0.46 ± 0.03 | ||
| 30 mg L−1 | 0.16 ± 0.17 | 0.47 ± 0.15 | 0.39 ± 0.01 | ||
| qP | Eguas | 0 mg L−1 | 0.62 ± 0.48 | 0.90 ± 0.03 | 0.94 ± 0.01 |
| 0.3 mg L−1 | 0.32 ± 0.23 | 0.94 ± 0.04 | 0.86 ± 0.12 | ||
| 1 mg L−1 | −1.31 ± 3.63 | 0.88 ± 0.04 | 0.96 ± 0.05 | ||
| 3 mg L−1 | 0.07 ± 0.64 | 0.92 ± 0.09 | 0.93 ± 0.03 | ||
| 10 mg L−1 | −0.74 ± 0.77 | 0.88 ± 0.04 | 0.92 ± 0.04 | ||
| 30 mg L−1 | −0.05 ± 0.64 | 0.99 ± 0.07 | 0.91 ± 0.06 | ||
| Xavarido | 0 mg L−1 | 0.82 ± 0.98 | 0.84 ± 0.07 | 0.85 ± 0.02 | |
| 0.3 mg L−1 | 6.56 ± 9.56 | 0.86 ± 0.10 | 0.78 ± 0.14 | ||
| 1 mg L−1 | 0.83 ± 0.61 | 0.89 ± 0.06 | 0.75 ± 0.11 | ||
| 3 mg L−1 | 3.75 ± 2.17 | 0.87 ± 0.06 | 0.81 ± 0.15 | ||
| 10 mg L−1 | 0.69 ± 0.27 | 0.80 ± 0.05 | 0.85 ± 0.01 | ||
| 30 mg L−1 | 3.40 ± 3.38 | 0.91 ± 0.09 | 0.80 ± 0.01 |
| Extracellular As | Intracellular As | ||
|---|---|---|---|
| Eguas | Control | 0.230 ± 0.015 | 0.080 ± 0.007 |
| 3 mg L−1 | 0.400 ± 0.049 | 0.083 ± 0.033 | |
| 30 mg L−1 | 6.630 ± 1.669 | 0.325 ± 0.249 | |
| Xavarido | Control | 0.182 ± 0.018 | 0.093 ± 0.008 |
| 3 mg L−1 | 1.571 ± 0.197 | 0.218 ± 0.048 | |
| 30 mg L−1 | 2.097 ± 0.527 | 0.096 ± 0.031 |
| As | Carbohydrates (mg g−1) | As EPS (µg g−1) | As/Carbohydrates | |
|---|---|---|---|---|
| Eguas | 0.3 mg L−1 | 0.62 ± 0.11 | 0.30 ± 0.06 | 0.5 |
| 1 mg L−1 | 0.37 ± 0.13 | 0.20 ± 0.10 | 0.5 | |
| 10 mg L−1 | 0.38 ± 0.28 | 0.34 ± 0.32 | 0.9 | |
| Xavarido | 0.3 mg L−1 | 0.24 ± 0.03 | 0.09 ± 0.03 | 0.4 |
| 1 mg L−1 | 0.60 ± 0.14 | 0.62 ± 0.07 | 1.0 | |
| 10 mg L−1 | 0.76 ± 0.16 | 1.36 ± 0.09 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barral, M.T.; Rodríguez-Iglesias, D.; Martiñá-Prieto, D.; Paradelo, R. Assessment of the Chronic Toxicity and Interactions between Arsenic and Riverbed Biofilms. Int. J. Environ. Res. Public Health 2022, 19, 12689. https://doi.org/10.3390/ijerph191912689
Barral MT, Rodríguez-Iglesias D, Martiñá-Prieto D, Paradelo R. Assessment of the Chronic Toxicity and Interactions between Arsenic and Riverbed Biofilms. International Journal of Environmental Research and Public Health. 2022; 19(19):12689. https://doi.org/10.3390/ijerph191912689
Chicago/Turabian StyleBarral, María Teresa, Diego Rodríguez-Iglesias, Diego Martiñá-Prieto, and Remigio Paradelo. 2022. "Assessment of the Chronic Toxicity and Interactions between Arsenic and Riverbed Biofilms" International Journal of Environmental Research and Public Health 19, no. 19: 12689. https://doi.org/10.3390/ijerph191912689
APA StyleBarral, M. T., Rodríguez-Iglesias, D., Martiñá-Prieto, D., & Paradelo, R. (2022). Assessment of the Chronic Toxicity and Interactions between Arsenic and Riverbed Biofilms. International Journal of Environmental Research and Public Health, 19(19), 12689. https://doi.org/10.3390/ijerph191912689








