Bioremediation of Cadmium Toxicity in Wheat (Triticum aestivum L.) Plants Primed with L-Proline, Bacillus subtilis and Aspergillus niger
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Culture Acquisition and Plant Growth Promoting Strains
2.2. In-Vitro Cadmium Tolerance Testing of Microbes
2.3. Preparation of the Microbial Cell Suspension for Seed Bio-Priming
2.4. Experiment Design
2.5. Plant Physiological Parameters
2.6. Photosynthetic Pigments
2.7. Plant Biochemical Attributes
2.8. Activity of Antioxidant Enzymes
2.9. Statistical Analysis
3. Results
3.1. In-Vitro Characterization of the Microbes for Metal Tolerance, Enzyme Production and PGP Traits
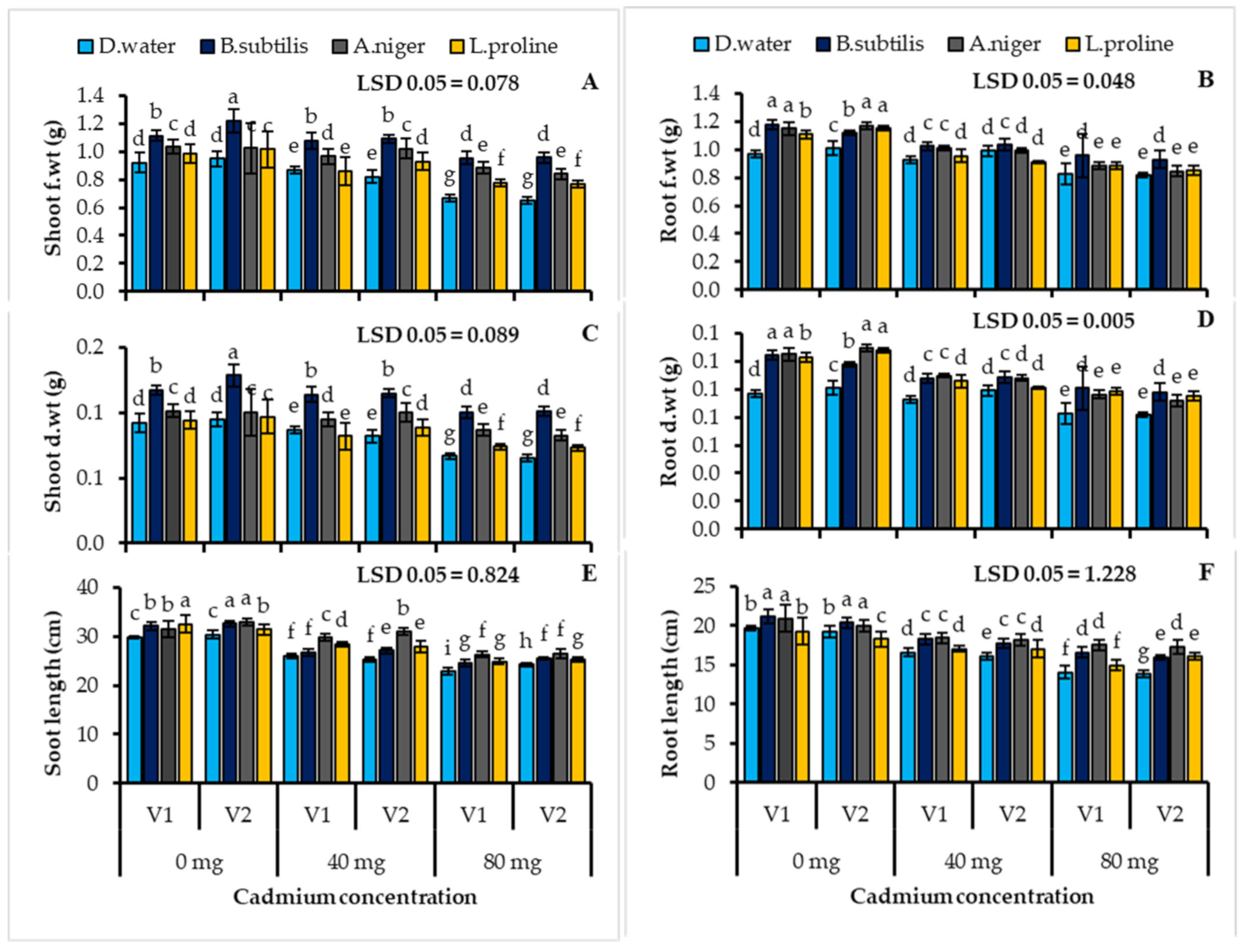
3.2. Plant Physiological Parameters Affected by the Different Treatments
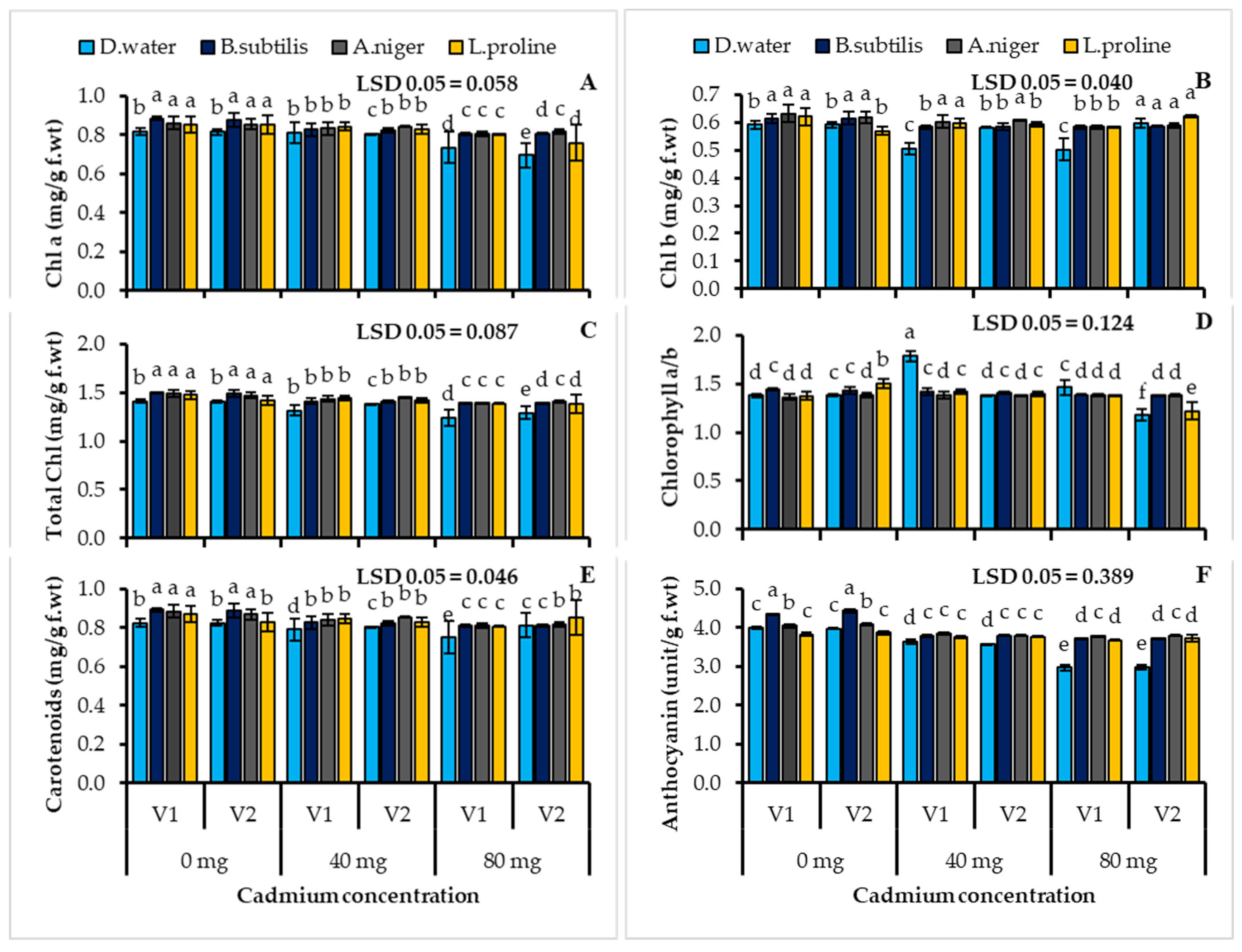
3.3. Plant Photosynthetic Pigment Contents
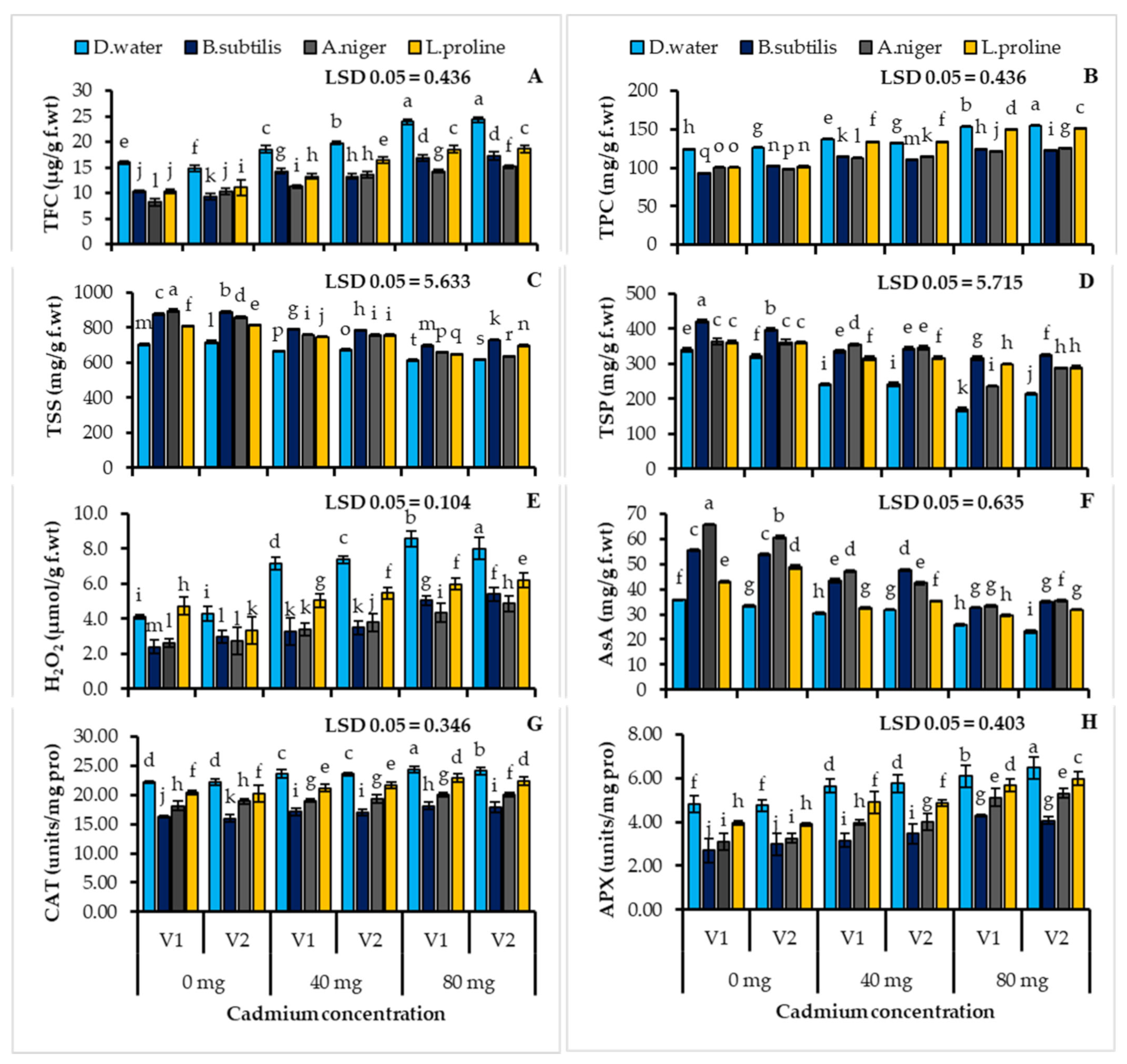
3.4. Plant Biochemical Attributes Estimation
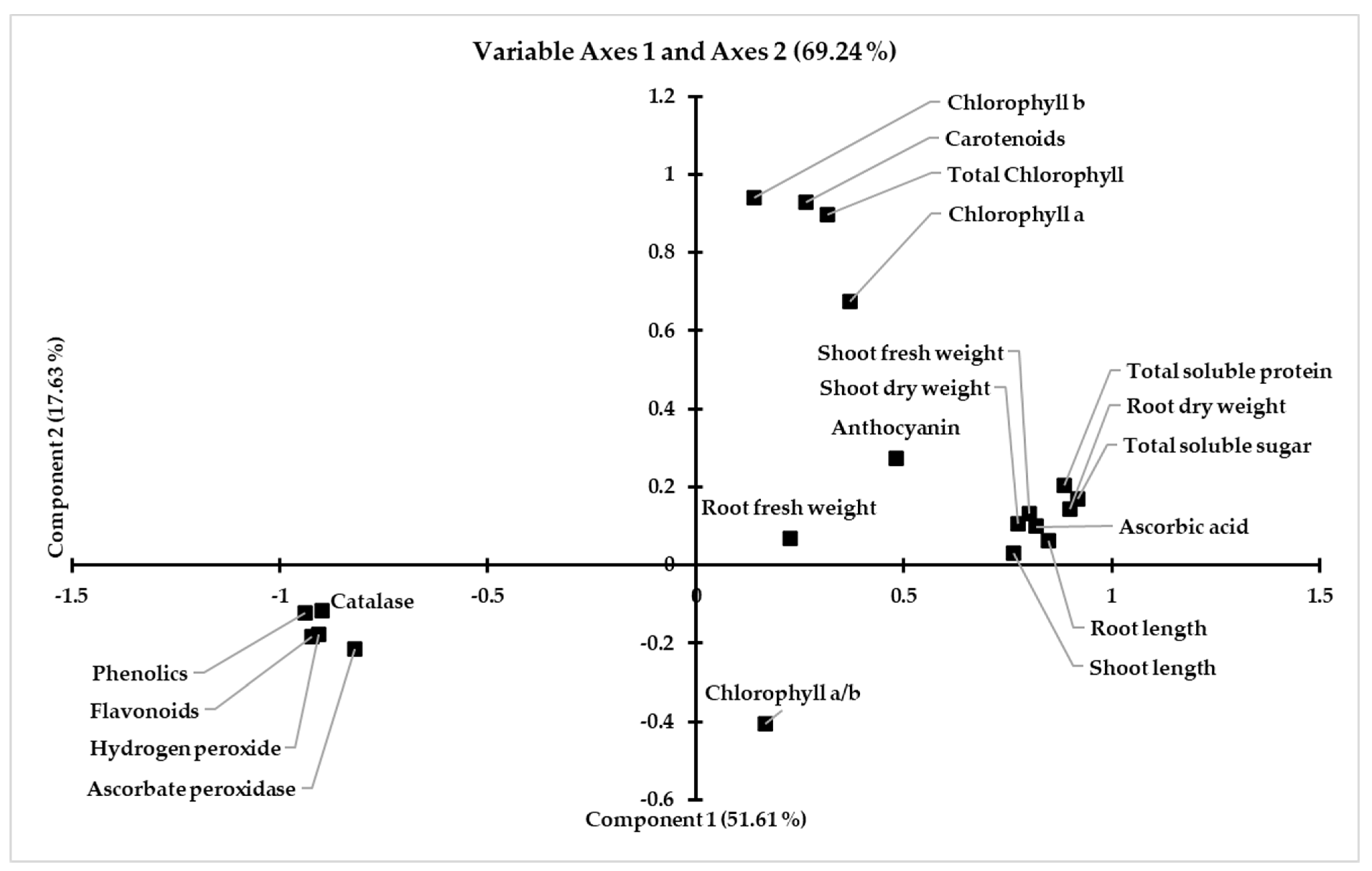
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adil, M.I.; Khan, F.; Shah, M.T.A.; Siddiqui, B.N. Cost return analysis in wheat production in federally administrated tribal area, pakistan. J. Agric. Res 2017, 55, 417–425. [Google Scholar]
- Ahmed, M.; Khan, M.S.; Iqbal, N. Wheat Production Trend in Pakistan—A Statistical Analysis. J. Agric. Res. 2017, 55, 115–124. [Google Scholar]
- Rehman, A.; Jingdong, L.; Shahzad, B.; Chandio, A.A.; Hussain, I.; Nabi, G.; Iqbal, M.S. Economic perspectives of major field crops of Pakistan: An empirical study. Pac. Sci. Rev. B Humanit. Soc. Sci. 2015, 1, 145–158. [Google Scholar] [CrossRef]
- Kang, S.-M.; Khan, A.L.; Waqas, M.; You, Y.-H.; Kim, J.-H.; Kim, J.-G.; Hamayun, M.; Lee, I.-J. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef]
- Wang, Z.; Solanki, M.K.; Pang, F.; Singh, R.K.; Yang, L.-T.; Li, Y.-R.; Li, H.-B.; Zhu, K.; Xing, Y.-X. Identification and Efficiency of a Nitrogen-fixing Endophytic Actinobacterial Strain from Sugarcane. Sugar Tech 2016, 19, 492–500. [Google Scholar] [CrossRef]
- Awan, S.; Ilyas, N.; Khan, I.; Raza, M.; Rehman, A.; Rizwan, M.; Rastogi, A.; Tariq, R.; Brestic, M. Bacillus siamensis Reduces Cadmium Accumulation and Improves Growth and Antioxidant Defense System in Two Wheat (Triticum aestivum L.) Varieties. Plants 2020, 9, 878. [Google Scholar] [CrossRef]
- Rosier, A.; Medeiros, F.H.V.; Bais, H.P. Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil 2018, 428, 35–55. [Google Scholar] [CrossRef]
- Rasheed, R.; Ashraf, M.A.; Hussain, I.; Haider, M.Z.; Kanwal, U.; Iqbal, M. Exogenous proline and glycinebetaine mitigate cadmium stress in two genetically different spring wheat (Triticum aestivum L.) cultivars. Braz. J. Bot. 2014, 37, 399–406. [Google Scholar] [CrossRef]
- Verma, N.; Sharma, R.J.R.P.O.B. Bioremediation of toxic heavy metals: A patent review. Recent Pat. Biotechnol. 2017, 11, 171–187. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, P.; Dhir, B.; Sharma, A.K.; Mehta, D. Potential of Some Fungal and Bacterial Species in Bioremediation of Heavy Metals. J. Nucl. Physics, Mater. Sci. Radiat. Appl. 2014, 1, 213–223. [Google Scholar] [CrossRef]
- Choudhary, M.; Kumar, R.; Datta, A.; Nehra, V.; Garg, N. Bioremediation of heavy metals by microbes. In Bioremediation of Salt Affected Soils: An Indian Perspective; Springer: Cham, Switzerland, 2017; pp. 233–255. [Google Scholar]
- Tarekegn, M.M.; Salilih, F.Z.; Ishetu, A.I. Microbes used as a tool for bioremediation of heavy metal from the environment. Cogent Food Agric. 2020, 6, 1783174. [Google Scholar] [CrossRef]
- Farooq, A.; Bukhari, S.A.; Akram, N.A.; Ashraf, M.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Exogenously Applied Ascorbic Acid-Mediated Changes in Osmoprotection and Oxidative Defense System Enhanced Water Stress Tolerance in Different Cultivars of Safflower (Carthamus tinctorious L.). Plants 2020, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Konotop, Y.; Kovalenko, M.; Matušíková, I.; Batsmanova, L.; Taran, N.J.P.J.B. Proline application triggers temporal redox imbalance, but alleviates cadmium stress in wheat seedlings. Pak. J. Bot 2017, 49, 2145–2151. [Google Scholar]
- Aziz, L.; Hamayun, M.; Rauf, M.; Iqbal, A.; Arif, M.; Husssin, A.; Khan, S.A. Endophytic Aspergillus niger reprograms the physicochemical traits of tomato under cadmium and chromium stress. Environ. Exp. Bot. 2021, 186, 104456. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Abbas, S.; Javed, M.T.; Shahid, M.; Hussain, I.; Haider, M.Z.; Chaudhary, H.J.; Tanwir, K.; Maqsood, A. Acinetobacter sp. SG-5 inoculation alleviates cadmium toxicity in differentially Cd tolerant maize cultivars as deciphered by improved physio-biochemical attributes, antioxidants and nutrient physiology. Plant Physiol. Biochem. 2020, 155, 815–827. [Google Scholar] [CrossRef]
- Azeem, M.; Haider, M.Z.; Javed, S.; Saleem, M.H.; Alatawi, A. Drought Stress Amelioration in Maize (Zea mays L.) by Inoculation of Bacillus spp. Strains under Sterile Soil Conditions. Agriculture 2022, 12, 50. [Google Scholar] [CrossRef]
- Mahmood, A.; Turgay, O.C.; Farooq, M.; Hayat, R. Seed biopriming with plant growth promoting rhizobacteria: A review. FEMS Microbiol. Ecol. 2016, 92, fiw112. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, J.S.; Saxena, A.K.; Singh, D.P. Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol. 2011, 14, 605–611. [Google Scholar] [CrossRef]
- Vimal, S.R.; Singh, J.S. Salt Tolerant PGPR and FYM Application in Saline Soil Paddy Agriculture Sustainability. Clim. Chang. Environ. Sustain. 2019, 7, 61. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 2003, 118, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Bilal, R.; Rasul, G.; Qureshi, J.A.; Malik, K.A. Characterization ofAzospirillum and related diazotrophs associated with roots of plants growing in saline soils. World J. Microbiol. Biotechnol. 1990, 6, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Einerich, D.W.; Sanchez-Diaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Chakraborty, U.; Chakraborty, B.N.; Chakraborty, A.P.; Dey, P.L. Water stress amelioration and plant growth promotion in wheat plants by osmotic stress tolerant bacteria. World J. Microbiol. Biotechnol. 2012, 29, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Ahmad, I.; Hilger, T.H.; Nadeem, S.M.; Akhtar, M.F.; Jamil, M.; Hussain, A.; Zahir, Z.A. Preliminary study on phosphate solubilizing Bacillus subtilis strain Q3 and Paenibacillus sp. strain Q6 for improving cotton growth under alkaline conditions. PeerJ 2018, 6, e5122. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.S.; Shahid, M.; Tariq, M.; Azeem, M.; Javed, M.T.; Saleem, S.; Riaz, S. Deciphering Staphylococcus sciuri SAT-17 Mediated Anti-oxidative Defense Mechanisms and Growth Modulations in Salt Stressed Maize (Zea mays L.). Front. Microbiol. 2016, 7, 867. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Asghar, H.N.; Arshad, M. Rhizobacteria Capable of Producing ACC-deaminase May Mitigate Salt Stress in Wheat. Soil Sci. Soc. Am. J. 2010, 74, 533–542. [Google Scholar] [CrossRef]
- Sharma, S.; Kulkarni, J.; Jha, B. Halotolerant Rhizobacteria Promote Growth and Enhance Salinity Tolerance in Peanut. Front. Microbiol. 2016, 7, 1600. [Google Scholar] [CrossRef]
- Greenway, H.; Munns, R. Mechanisms of Salt Tolerance in Nonhalophytes. Annu. Rev. Plant Physiol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Han, H.; Lee, K. Plant growth promoting rhizobacteria effect on antioxidant status, photosynthesis, mineral uptake and growth of lettuce under soil salinity. Res. J. Agric. Biol. Sci. 2005, 1, 210–215. [Google Scholar]
- Khan, M.Y.; Zahir, Z.A.; Asghar, H.N.; Waraich, E.A. Preliminary investigations on selection of synergistic halotolerant plant growth promoting rhizobacteria for inducing salinity tolerance in wheat. Pak. J. Bot 2017, 49, 1541–1551. [Google Scholar]
- Barra, P.J.; Inostroza, N.G.; Acuña, J.J.; Mora, M.L.; Crowley, D.E.; Jorquera, M.A. Formulation of bacterial consortia from avocado (Persea americana Mill.) and their effect on growth, biomass and superoxide dismutase activity of wheat seedlings under salt stress. Appl. Soil Ecol. 2016, 102, 80–91. [Google Scholar] [CrossRef]
- Hoque, M.M.; Ahmed, K.M. Declining groundwater level and aquifer dewatering in Dhaka metropolitan area, Bangladesh: Causes and quantification. Appl. Hydrogeol. 2007, 15, 1523–1534. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Gueta-Dahan, Y.; Yaniv, Z.; Zilinskas, B.A.; Ben-Hayyim, G. Salt and oxidative stress: Similar and specific responses and their relation to salt tolerance in Citrus. Planta 1997, 203, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Mittova, V.; Guy, M.; Tal, M.; Volokita, M. Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J. Exp. Bot. 2004, 55, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lim, J.-H.; Park, M.R.; Kim, Y.J.; Park, T.I.; Seo, Y.W.; Choi, K.G.; Yun, S.J. Enhanced antioxidant enzymes are associated with reduced hydrogen peroxide in barley roots under saline stress. BMB Rep. 2005, 38, 218–224. [Google Scholar] [CrossRef]
- Panuccio, M.R.S.; Jacobsen, S.-E.; Akhtar, S.S.; Muscolo, A. Effect of saline water on seed germination and early seedling growth of the halophyte quinoa. AoB PLANTS 2014, 6, plu047. [Google Scholar] [CrossRef]
- Khan, Z.; Rho, H.; Firrincieli, A.; Hung, S.H.; Luna, V.; Masciarelli, O.; Kim, S.-H.; Doty, S.L. Growth enhancement and drought tolerance of hybrid poplar upon inoculation with endophyte consortia. Curr. Plant Biol. 2016, 6, 38–47. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Kruasuwan, W.; Thamchaipenet, A. 1-Aminocyclopropane-1-carboxylate (ACC) Deaminase-Producing Endophytic Diazotrophic Enterobacter sp. EN-21 Modulates Salt–Stress Response in Sugarcane. J. Plant Growth Regul. 2018, 37, 849–858. [Google Scholar] [CrossRef]
| Parameters | Details |
|---|---|
| Treatments | |
| Control (T0) | Sterilized distilled water |
| Bacterial (T1) | Bacillus subtilis NA2 |
| Fungal (T2) | Aspergillus niger PMI-118 |
| Chemical (T3) | L-proline |
| Wheat cultivars | |
| Wheat (V1) | Akbar |
| Wheat (V2) | Dilkash |
| Cadmium stress levels | |
| Cadmium CdCl2 (S0) | 0 mg/kg of soil |
| Cadmium CdCl2 (S1) | 40 mg/kg of soil |
| Cadmium CdCl2 (S2) | 80 mg/kg of soil |
| Growth conditions | |
| Temperature (Day) | 31 °C |
| Temperature (Night) | 24 °C |
| Soil (Per Pod) | 350 g |
| Characteristics | B. subtilis | A. niger |
|---|---|---|
| IAA Synthesis | ||
| IAA (without tryptophan) | - | - |
| IAA (with tryptophan) | 23.46 (μg/mL) | 56.41 (μg/mL) |
| IAA (CdCl2 800 mg/L + tryptophan) | 14.49 (μg/mL) | 36.21 (μg/mL) |
| Nitrogen Fixation | ||
| N-free semi-solid media | + | + |
| N-free malate agar media | +++ | +++ |
| Ammonia Production | ||
| Nessler’s reagent | +++ | +++ |
| Ammonia Production (μmol/mL) | 6.89 | 5.34 |
| Phosphate solubilization | ||
| NBRIP media | +++ | - |
| Pikovaskkaya’s agar media | + | ++ |
| Phosphate solubilization (ppm) | 42.2 | 38.1 |
| Enzyme Production | ||
| Amylase | +++ | +++ |
| Proteases (Zone mm) | 18 ± 1 | 12 ± 1 |
| CdCl2 Tolerance | ||
| 0 mg/L | +++ | +++ |
| 200 mg/L | +++ | +++ |
| 400 mg/L | +++ | +++ |
| 600 mg/L | +++ | ++ |
| 700 mg/L | ++ | ++ |
| 800 mg/L | ++ | + |
| 900 mg/L | ++ | - |
| 1000 mg/L | + | - |
| 1100 mg/L | - | - |
| 1200 mg/L | - | - |
| Source | df | SFW | SDW | RFW | RDW | Root L |
| Variety (V) | 1 | 0.281 ns | 0.296 ns | 0.029 ns | 0.039 ns | 0.787 ns |
| Stress (S) | 2 | 21.69 *** | 22.22 *** | 47.78 *** | 49.58 *** | 30.08 *** |
| Treatment (T) | 3 | 15.34 *** | 26.20 *** | 6.693 *** | 17.33 *** | 5.461 *** |
| V × S | 2 | 0.339 ns | 0.354 ns | 0.439 | 0.454 ns | 0.306 ns |
| V × T | 3 | 0.189 ns | 0.203 ns | 0.359 ns | 0.342 ns | 0.109 ns |
| S × T | 6 | 0.271 ns | 0.286 ns | 1.527 ns | 1.800 ns | 0.624 ns |
| V × S × T | 6 | 0.209 ns | 0.219 ns | 0.360 ns | 0.373 ns | 0.095 ns |
| Source | df | Shoot L | Chl a | Chl b | Total Chl | Chl a/b |
| Variety (V) | 1 | 1.368 ns | 0.199 ns | 0.889 ns | 0.017 ns | 2.067 ns |
| Stress (S) | 2 | 132.6 *** | 4.401 * | 1.406 ns | 3.524 ns | 1.790 ns |
| Treatment (T) | 3 | 15.72 *** | 1.697 ns | 1.875 ns | 2.209 * | 0.282 ns |
| V × S | 2 | 0.297 ns | 0.057 ns | 1.194 ns | 0.150 ns | 1.198 ns |
| V × T | 3 | 0.715 ns | 0.065 ns | 1.061 ns | 0.196 ns | 1.633 ns |
| S × T | 6 | 1.736 ns | 0.267 ns | 0.298 ns | 0.162 ns | 0.887 ns |
| V × S × T | 6 | 0.462 ns | 0.050 ns | 0.269 ns | 0.021 ns | 0.528 ns |
| Source | df | Carotenoids | Anthocyanin | Flavonoids | Phenolics | TSS |
| Variety (V) | 1 | 0.105 ns | 0.003 ns | 18.07 *** | 7.809 ** | 6.668 * |
| Stress (S) | 2 | 3.288 * | 4.961 * | 760.3 *** | 5317 *** | 2112 *** |
| Treatment (T) | 3 | 1.603 ns | 2.033 ns | 441.9 *** | 2617 *** | 773.7 *** |
| V × S | 2 | 0.605 ns | 0.015 ns | 5.884 ** | 27.48 *** | 6.047 ** |
| V × T | 3 | 0.178 ns | 0.008 ns | 12.54 *** | 3.295 * | 19.39 *** |
| S × T | 6 | 0.252 ns | 0.824 ns | 7.946 *** | 224.1 *** | 69.66 *** |
| V × S × T | 6 | 0.127 ns | 0.002 ns | 4.275 ** | 22.18 *** | 6.147 *** |
| Source | df | TSP | H2O2 | AsA | CAT | APX |
| Variety (V) | 1 | 5.152 * | 7.621 ** | 2.373 ns | 0.008 ns | 0.177 ns |
| Stress (S) | 2 | 808.3 *** | 1756 *** | 2358 *** | 128.7 *** | 39.32 *** |
| Treatment (T) | 3 | 458.4 *** | 1429 *** | 1231 *** | 98.81 *** | 12.33 *** |
| V × S | 2 | 26.57 *** | 12.40 *** | 6.416 ** | 0.384 ns | 0.441 ns |
| V × T | 3 | 4.412 ** | 17.37 *** | 39.60 *** | 0.418 ns | 0.054 ns |
| S × T | 6 | 42.26 *** | 98.01 *** | 131.3 *** | 6.476 *** | 0.251 ns |
| V × S × T | 6 | 8.001 *** | 21.75 *** | 15.84 *** | 0.444 ns | 0.333 ns |
| SFW | SDW | RFW | RDW | SL | RL | Chla | Chlb | TChl | Chlab | Caro | Antho | Flavo | Pheno | TSS | TSP | H2O2 | AsA | CAT | APX | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SFW | 1 | |||||||||||||||||||
| SDW | 0.981 | 1 | ||||||||||||||||||
| RFW | 0.324 | 0.279 | 1 | |||||||||||||||||
| RDW | 0.526 | 0.476 | 0.242 | 1 | ||||||||||||||||
| SL | 0.511 | 0.490 | 0.121 | 0.628 | 1 | |||||||||||||||
| RL | 0.616 | 0.543 | 0.201 | 0.728 | 0.698 | 1 | ||||||||||||||
| Chl a | 0.413 | 0.383 | 0.092 | 0.324 | 0.290 | 0.329 | 1 | |||||||||||||
| Chl b | 0.217 | 0.190 | 0.099 | 0.233 | 0.165 | 0.205 | 0.508 | 1 | ||||||||||||
| TChl | 0.381 | 0.348 | 0.109 | 0.329 | 0.274 | 0.319 | 0.914 | 0.812 | 1 | |||||||||||
| Chlab | 0.115 | 0.114 | −0.009 | 0.049 | 0.067 | 0.064 | 0.310 | −0.616 | −0.079 | 1 | ||||||||||
| Caro | 0.335 | 0.303 | 0.130 | 0.320 | 0.222 | 0.284 | 0.731 | 0.886 | 0.910 | −0.245 | 1 | |||||||||
| Antho | 0.403 | 0.390 | 0.133 | 0.411 | 0.431 | 0.395 | 0.212 | 0.333 | 0.300 | −0.098 | 0.389 | 1 | ||||||||
| Flavo | −0.688 | −0.631 | −0.154 | −0.793 | −0.695 | −0.812 | −0.469 | −0.304 | −0.460 | −0.085 | −0.404 | −0.459 | 1 | |||||||
| Pheno | −0.725 | −0.710 | −0.161 | −0.787 | −0.699 | −0.775 | −0.436 | −0.251 | −0.413 | −0.100 | −0.354 | −0.426 | 0.903 | 1 | ||||||
| TSS | 0.7109 | 0.688 | 0.188 | 0.805 | 0.663 | 0.785 | 0.429 | 0.294 | 0.428 | 0.063 | 0.420 | 0.436 | −0.866 | −0.861 | 1 | |||||
| TSP | 0.703 | 0.683 | 0.102 | 0.732 | 0.656 | 0.756 | 0.412 | 0.340 | 0.438 | −0.008 | 0.402 | 0.500 | −0.852 | −0.817 | 0.866 | 1 | ||||
| H2O2 | −0.707 | −0.686 | −0.152 | −0.714 | −0.703 | −0.742 | −0.406 | −0.324 | −0.426 | 0.011 | −0.371 | −0.477 | 0.913 | 0.877 | −0.820 | −0.875 | 1 | |||
| AsA | 0.653 | 0.628 | 0.224 | 0.788 | 0.668 | 0.744 | 0.402 | 0.273 | 0.400 | 0.062 | 0.385 | 0.396 | −0.865 | −0.885 | 0.921 | 0.786 | −0.847 | 1 | ||
| CAT | −0.756 | −0.763 | −0.095 | −0.682 | −0.640 | −0.733 | −0.358 | −0.265 | −0.367 | −0.008 | −0.335 | −0.458 | 0.831 | 0.900 | −0.824 | −0.835 | 0.857 | −0.808 | 1 | |
| APX | −0.632 | −0.605 | −0.221 | −0.679 | −0.590 | −0.699 | −0.448 | −0.294 | −0.441 | −0.060 | −0.431 | −0.416 | 0.793 | 0.796 | −0.796 | −0.761 | 0.771 | −0.764 | 0.692 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashir, S.; Javed, S.; Al-Anazi, K.M.; Farah, M.A.; Ali, S. Bioremediation of Cadmium Toxicity in Wheat (Triticum aestivum L.) Plants Primed with L-Proline, Bacillus subtilis and Aspergillus niger. Int. J. Environ. Res. Public Health 2022, 19, 12683. https://doi.org/10.3390/ijerph191912683
Bashir S, Javed S, Al-Anazi KM, Farah MA, Ali S. Bioremediation of Cadmium Toxicity in Wheat (Triticum aestivum L.) Plants Primed with L-Proline, Bacillus subtilis and Aspergillus niger. International Journal of Environmental Research and Public Health. 2022; 19(19):12683. https://doi.org/10.3390/ijerph191912683
Chicago/Turabian StyleBashir, Sarmad, Sadia Javed, Khalid Mashay Al-Anazi, Mohammad Abul Farah, and Sajad Ali. 2022. "Bioremediation of Cadmium Toxicity in Wheat (Triticum aestivum L.) Plants Primed with L-Proline, Bacillus subtilis and Aspergillus niger" International Journal of Environmental Research and Public Health 19, no. 19: 12683. https://doi.org/10.3390/ijerph191912683
APA StyleBashir, S., Javed, S., Al-Anazi, K. M., Farah, M. A., & Ali, S. (2022). Bioremediation of Cadmium Toxicity in Wheat (Triticum aestivum L.) Plants Primed with L-Proline, Bacillus subtilis and Aspergillus niger. International Journal of Environmental Research and Public Health, 19(19), 12683. https://doi.org/10.3390/ijerph191912683





