Great Facilitation of Thirty Years of Reforestation with Mixed Species to Ecosystem Nitrogen Accumulation in Dry-Hot Valley in the Jinsha River
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Sampling and Analysis
2.3. Statistical Analyses
3. Results
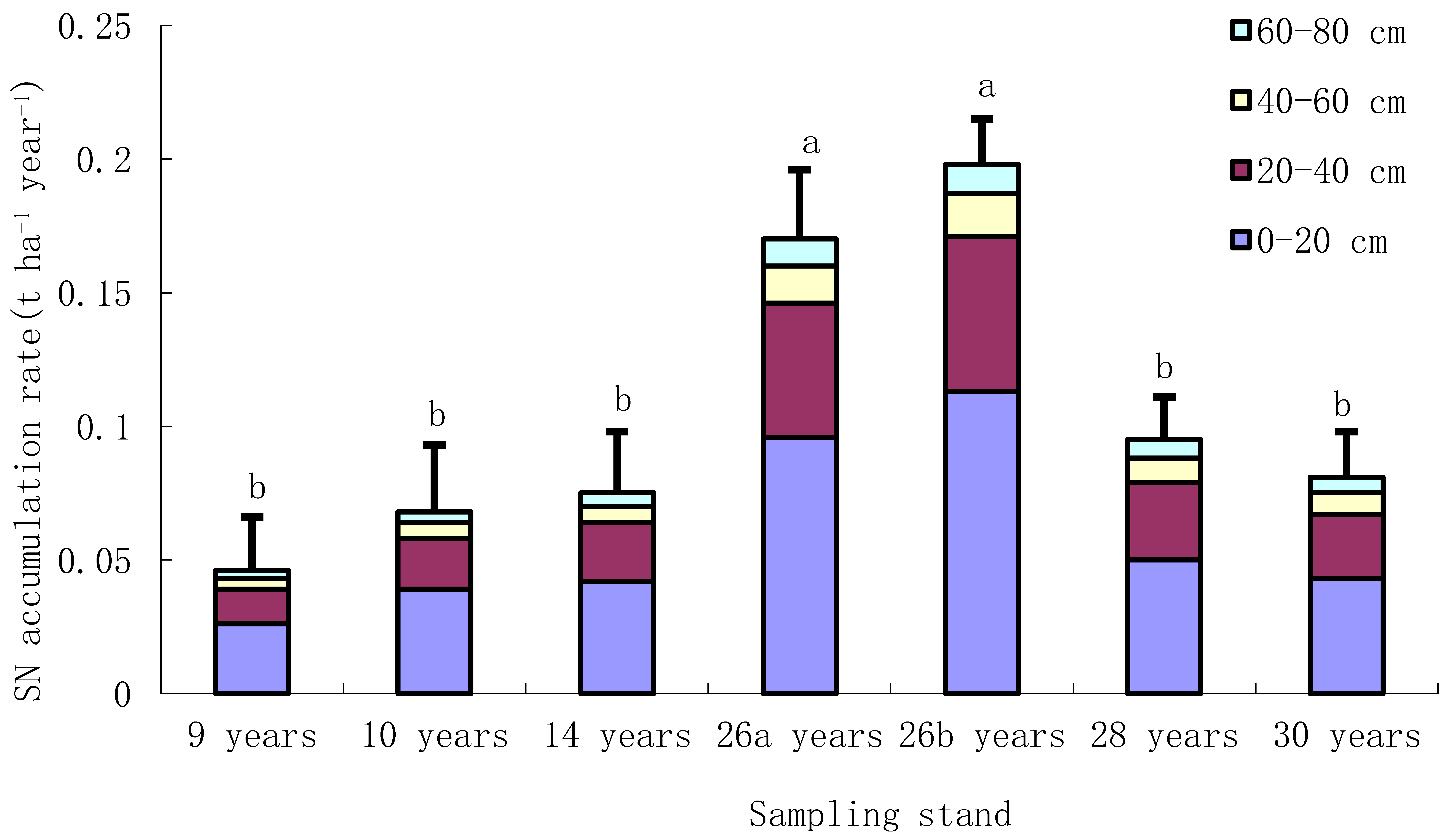
3.1. Soil Nitrogen Accumulation in 0–80 cm Soil Profile
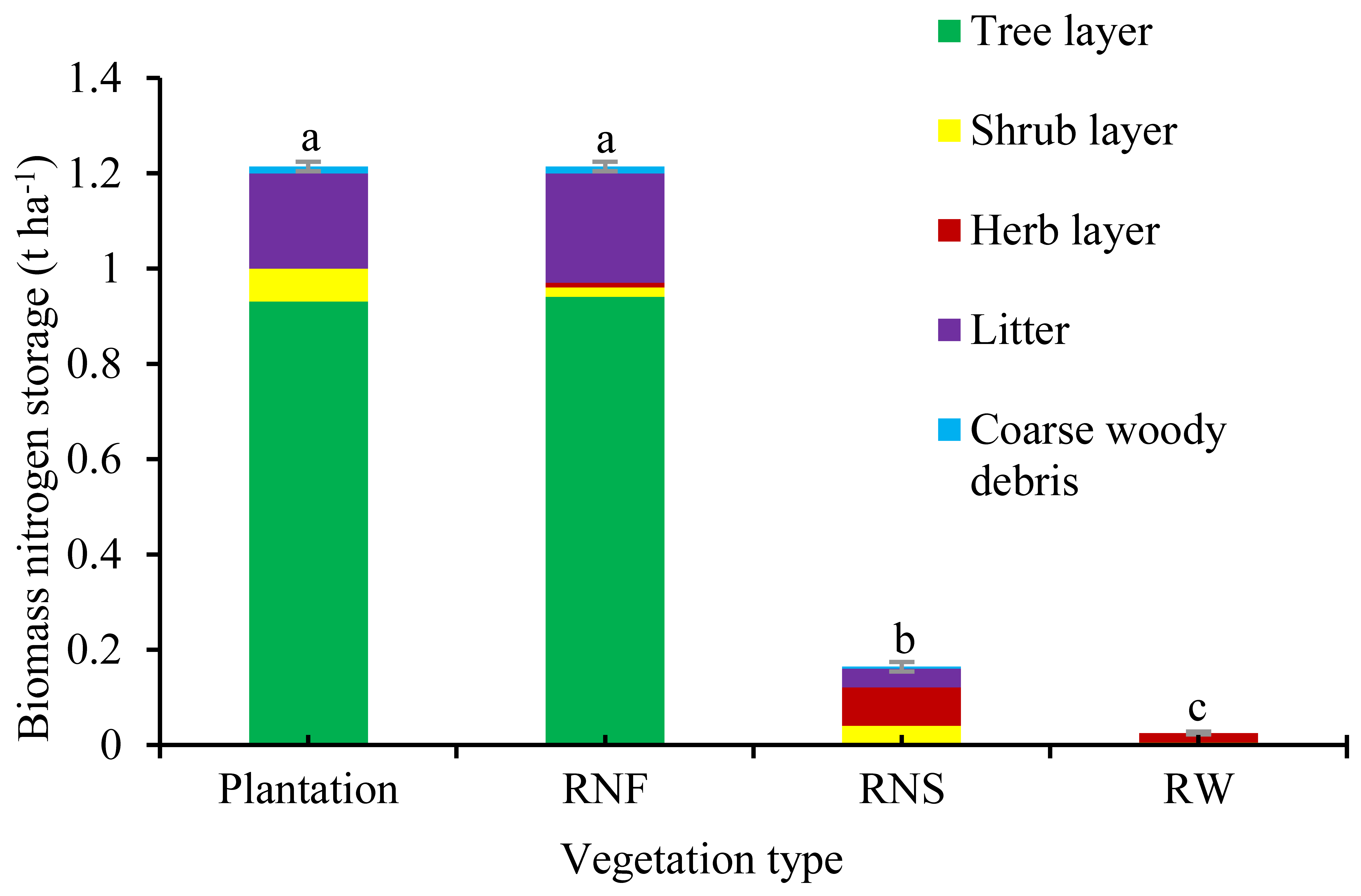
3.2. Biomass Nitrogen Storage
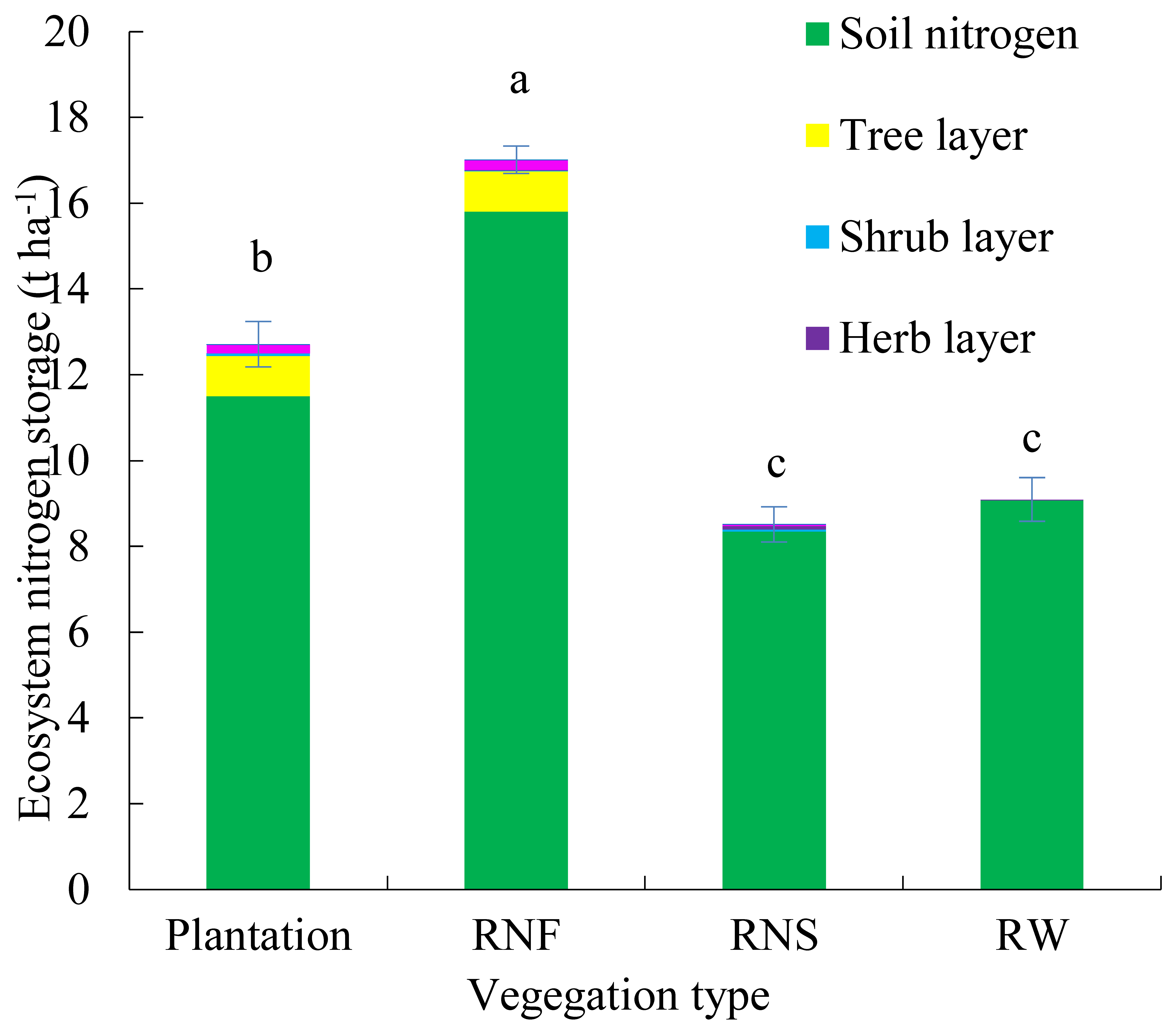
3.3. Total Ecosystem Nitrogen Storage
4. Discussion
4.1. Nitrogen Accumulation in the Soil Profile after 30 Years of Reforestation
4.2. Nitrogen Accumulation in Deep Soil
4.3. Biomass Nitrogen Accumulation
4.4. Nitrogen Accumulation in 30-Year-Old Plantation Ecosystem
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yu, G.R.; Jia, Y.L.; He, N.P.; Zhu, J.X.; Chen, Z.; Wang, Q.F. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci. 2019, 12, 424–429. [Google Scholar] [CrossRef]
- Zhao, H.; He, N.P.; Xu, L.; Zhang, X.M.; Wang, Q.F.; Wang, B.; Yu, G.R. Variation in the nitrogen concentration of the leaf, branch, trunk, and root in vegetation in China. Ecol. Indic. 2019, 96, 496–504. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, L.; Zhu, T.B. Rapid recovery of nitrogen retention capacity in a subtropical acidic soil following afforestation. Soil Biol. Biochem. 2018, 120, 171–180. [Google Scholar] [CrossRef]
- Liu, X.; Yang, T.; Wang, Q. Dynamics of soil carbon and nitrogen stocks after afforestation in arid and semi-arid regions: A meta-analysis. Sci. Total Environ. 2018, 618, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.Y.; Li, K.; Sun, Y. Dynamics and stabilization of soil organic carbon after nineteen years of afforestation in valley-type savannah in southwest China. Soil Use Manag. 2013, 29, 48–56. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Ai, J.J.; Sun, Q.W.; Li, Z.C.; Hou, L.Y.; Song, L.G.; Tang, G.Y.; Li, L.; Shao, G.D. Soil organic carbon and total nitrogen stocks as affected by vegetation types and altitude across the mountainous regions in the Yunnan Province, south western China. Catena 2021, 196, 104872. [Google Scholar] [CrossRef]
- Zeng, D.H.; Hu, Y.L.; Chang, S.X. Land cover change effects on soil chemical and biological properties after planting Mongolian pine (Pinus sylvestris var. mongolica) in sandy lands in Keerqin, northeastern China. Plant Soil. 2009, 317, 121–133. [Google Scholar] [CrossRef]
- Laughlin, D.C. Nitrification is linked to dominant leaf traits rather than functional diversity. J. Ecol. 2011, 99, 1091–1099. [Google Scholar] [CrossRef]
- Tuo, D.F.; Gao, G.Y.; Chang, R.Y.; Li, Z.S.; Ma, Y.; Wang, S.; Wang, C.; Fu, B.J. Effects of revegetation and precipitation gradient on soil carbon and nitrogen variations in deep profiles on the Loess Plateau of China. Sci. Total Environ. 2018, 626, 399–411. [Google Scholar] [CrossRef]
- Li, C.Z.; Li, C.J.; Zhao, L.H.; Ma, Y.D.; Tong, X.G.; Deng, J.; Ren, C.J.; Han, X.H.; Yang, G.H. Dynamics of storage and relative availability of soil inorganic nitrogen along revegetation chronosequence in the loess hilly region of China. Soil Tillage Res. 2019, 187, 11–20. [Google Scholar] [CrossRef]
- Xu, L.; He, N.P. Nitrogen storage and allocation in China’s forest ecosystems. Sci. China Earth Sci. 2020, 63, 1475–1484. [Google Scholar] [CrossRef]
- Hu, P.L.; Liu, S.J.; Ye, Y.Y. Soil carbon and nitrogen accumulation following agricultural abandonment in a subtropical karst region. Appl. Soil Ecol. 2018, 132, 169–178. [Google Scholar] [CrossRef]
- Xu, L.; He, N.P.; Yu, G.R. Nitrogen storage in China’s terrestrial ecosystems. Sci. Total Environ. 2020, 709, 136201–136211. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.A.; Jackson, R.B. Global patterns of root turnover for terrestrial ecosystems. New Phytol. 2020, 147, 13–31. [Google Scholar] [CrossRef]
- Zhou, T.; Shi, P.J.; Jia, G.S.; Li, X.J.; Luo, Y.Q. Spatial patterns of ecosystem carbon residence time in Chinese forests. Sci. China Earth Sci. 2010, 53, 1229–1240. [Google Scholar] [CrossRef]
- Lin, M.Y.; Chen, A.M.; Yan, S.W.; Rafay, L.; Du, K.; Wang, D.J.; Ge, Y.G.; Li, J. Available soil nutrients and water content affect leaf nutrient concentrations and stoichiometry at different ages of Leucaena leucocephala forests in dry-hot valley. J. Soils Sediments 2019, 19, 511–521. [Google Scholar] [CrossRef]
- Wang, X.M.; Yan, B.G.; Shi, L.T.; Liu, G.C. Different responses of biomass alllocation and leaf traits of Dodonaea viscosa to concentrations of nitrogen and phosphorus. Chin. J. Plant Ecol. 2020, 44, 1247–1261. [Google Scholar] [CrossRef]
- Yue, J.W.; Guan, J.H.; Deng, L.; Zhang, J.G.; Du, S. Dynamics and allocation patterns of carbon and nitrogen storage in Picea asperata plantations in subalpine areas of Gansu Province. Acta Ecol. Sin. 2018, 38, 7790–7800. [Google Scholar]
- Ai, Z.M.; Chen, Y.M.; Cao, Y. Storage and allocation of carbon and nitrogen in Robinia pseudoacacia plantation at different ages in the loess hilly region, China. Chin. J. Appl. Ecol. 2014, 25, 333–341. [Google Scholar]
- Li, J.Q.; Chen, Q.B.; Li, Z.; Peng, B.X.; Zhang, J.L.; Xing, X.X.; Zhao, B.Y.; Song, D.H. Distribution and altitudinal patterns of carbon and nitrogen storage in various forest ecosystems in the central Yunnan Plateau, China. Sci. Rep. 2021, 11, 6269. [Google Scholar] [CrossRef]
- Peng, S.L.; Chen, A.Q.; Fang, H.D. Effects of vegetation restoration types on soil quality in Yuanmou dry-hot valley, China. Soil Sci. Plant Nutr. 2013, 59, 347–360. [Google Scholar] [CrossRef] [Green Version]
- Gong, Z.L.; Tang, Y. Impacts of reforestation on woody species composition; species diversity and community structure in dry-hot valley of the Jinsha River, southwestern China. J. Mt. Sci. 2016, 13, 2182–2191. [Google Scholar] [CrossRef]
- Gong, Z.L.; Tang, Y.; Xu, W.L.; Mou, Z.S. Rapid sequestration of ecosystem carbon in 30-year reforestation with mixed species in dry Hot valley of the Jinsha River. Int. J. Environ. Res. Public Health. 2019, 16, 1937. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.K. Analytical Methods of Soil Agrochemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Guo, L.H.; Wang, D.J.; Zhang, Y.H.; Jiao, Z.; Chen, D. Dynamics and vertical distribution patterns of fine root weights of different aged leucaena leucocephala stand in debris flow source area. Sci. Soil Water Conserv. 2010, 8, 41–46. [Google Scholar]
- Madsen, E.L. Microorganisms and their roles in fundamental biogeochemical cycles. Curr. Opin. Biotechnol. 2011, 22, 456–464. [Google Scholar] [CrossRef]
- Hao, J.; Chai, Y.N.; Lopes, L.D.; Ordóñez, R.A.; Wright, E.E.; Archontoulis, S.; Schachtman, D.P. The Effects of Soil Depth on the Structure of Microbial Communities in Agricultural Soils in Iowa (United States). Appl. Environ. Microbiol. 2021, 87, e02673-20. [Google Scholar] [CrossRef]
- Vincent, B.; Jourand, P.; Juillot, F.; Ducousso, M.; Galiana, A. Biological in situ nitrogen fixation by an Acacia species reaches optimal rates on extremely contrasted soils. Eur. J. Soil Biol. 2018, 86, 52–62. [Google Scholar] [CrossRef]
- Lee, C.K.; Laughlin, D.C.; Bottos, E.M. Biotic interactions are an unexpected yet critical control on the complexity of an abiotically driven polar ecosystem. Commun. Biol. 2019, 2, 62. [Google Scholar] [CrossRef]
- Coyne, K.J.; Parker, A.E.; Lee, C.K.; Sohm, J.A.; Kalmbach, A.; Gunderson, T.; Leon-Zayas, R.; Capone, D.G.; Carpenter, E.J.; Cary, S.C. The distribution and relative ecological roles of autotrophic and heterotrophic diazotrophs in the McMurdo Dry Valleys, Antarctica. FEMS Microbiol. Ecol. 2020, 96, 16. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yuan, L.; Xue, S. Artificial root exudates excite bacterial nitrogen fixation in the subsurface of mine soils. Appl. Soil Ecol. 2021, 157, 103774. [Google Scholar] [CrossRef]
- Berthrong, S.T.; Jobbagy, E.; Jackson, R.B. A global meta-analysis of soil exchangeable cations, pH, carbon, and nitrogen with afforestation. Ecol. Appl. 2009, 19, 2228–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Zhou, X.H.; Zhang, Q.F. Consequences of afforestation for soil nitrogen dynamics in central China. Agric. Ecosyst. Environ. 2014, 183, 40–46. [Google Scholar] [CrossRef]
- Wei, X.R.; Shao, M.G.; Fu, X.L. Changes in soil organic carbon and total nitrogen after 28 years grassland afforestation: Effects of tree species, slope position, and soil order. Plant Soil. 2010, 331, 165–179. [Google Scholar] [CrossRef]
- Gao, C.J.; Li, K.; Tang, G.Y.; Zhang, C.H.; Li, B. Nutrient accumulation and cycling in pure and mixed plantations of Azadirachta indica and Acacia auriculiformis in a dry-hot valley, Yunnan Province, southwest China. Chin. J. Appl. Ecol. 2014, 25, 1889–1897. [Google Scholar]

| Sites | Stand Description | Slope Aspect (°) | Slope (°) | Altitude (m) |
|---|---|---|---|---|
| Dongchuan municipality, Yunnan (N26°25′12′′; E103°04′43′′) (MAT: 22 °C; MAP: 700 mm) | 9 years old plantation established with Leucaena leucocephala, Acacia confusa, Eucalyptus camaldulensis and Dodonaea viscosa (9 years) | NE80 | 20 | 895 |
| Reference wasteland in Tuobuka (RWa) (being wasteland for over 30 years) | NE80 | 20 | 910 | |
| Ningnan county, Sichuan (N27°04′15′′; 102.43′42′′) (MAT: 20–22 °C; MAP: 700–800 mm) | 10 years old plantation established with L. leucocephala and D. viscosa (10 years) | NE63 | 18 | 860 |
| 14 years old plantation established with L. leucocephala and D. viscosa (14 years) | NE66 | 19 | 800 | |
| 26 years old plantation established with L. leucocephala, E. camaldulensis and Cajanus cajan (26a years) | NE75 | 20 | 1273 | |
| 26 years old plantation established with L. leucocephala and C. cajan (26b years) | NE76 | 19 | 1273 | |
| 28 years old plantation established with L. leucocephala and Tephrosia candida (28 years) | NE60 | 17 | 840 | |
| 30 years old plantation established with 18 woody species, including A. confusa, Bombaxceiba, E. camaldulensis, L. leucocephala, Tamarindus indica, Trema tomentosa, C. cajan and D. viscosa(30 years) | NE70 | 18 | 805 | |
| Reference wasteland near the two 26-year-old plantations (RWb) (being wasteland for over 46 years) | NE77 | 22 | 1260 | |
| Reference wasteland near 10-,14-, 28-, 30-year-old plantations (RWc) (being wasteland for over 55 years) | NE35 | 21 | 821 | |
| Reference natural recovery shrub grassland (RNS) (about 35 years old) | NE63 | 21 | 840 | |
| Reference natural forest (RNF) (about 200 years old) | NE45 | 27 | 1230 |
| Sites | Stand | pH | Carbon Stock (t ha−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–20 cm | 20–40 cm | 40–60 cm | 60–80 cm | 0–20 cm | 20–40 cm | 40–60 cm | 60–80 cm | ||
| Dongchuan municipality, Yunnan | 9 years | 7.6 | 7.5 | 7.9 | 8.0 | 32.55 | 21.64 | 14.31 | 10.76 |
| RWa | 7.9 | 7.8 | 8.0 | 8.0 | 28.37 | 19.88 | 14.00 | 10.64 | |
| Ningnan county, Sichuan | 10 years | 7.5 | 7.6 | 7.7 | 8.0 | 33.51 | 22.70 | 16.16 | 12.00 |
| 14 years | 8.0 | 7.9 | 8.0 | 8.0 | 36.95 | 24.41 | 16.68 | 12.25 | |
| 26a years | 5.9 | 5.7 | 5.6 | 5.8 | 63.39 | 37.25 | 20.23 | 14.65 | |
| 26b years | 6.8 | 6.7 | 7.0 | 7.0 | 64.89 | 38.04 | 20.11 | 14.57 | |
| 28 years | 7.6 | 7.4 | 7.3 | 7.3 | 46.45 | 29.48 | 19.08 | 13.24 | |
| 30 years | 7.9 | 7.7 | 7.7 | 7.6 | 46.43 | 29.00 | 19.02 | 13.27 | |
| RWb | 7.5 | 7.4 | 7.7 | 7.7 | 31.43 | 22.16 | 15.63 | 11.74 | |
| RWc | 8.2 | 8.1 | 8.1 | 8.2 | 28.80 | 20.66 | 15.49 | 11.45 | |
| RNS | 8.0 | 7.8 | 7.7 | / | 32.62 | 23.66 | 16.35 | / | |
| RNF | 5.6 | 5.8 | 5.5 | 5.6 | 80.31 | 52.16 | 29.64 | 21.23 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Z.; Li, Y.; Liu, L.; Deng, S. Great Facilitation of Thirty Years of Reforestation with Mixed Species to Ecosystem Nitrogen Accumulation in Dry-Hot Valley in the Jinsha River. Int. J. Environ. Res. Public Health 2022, 19, 12660. https://doi.org/10.3390/ijerph191912660
Gong Z, Li Y, Liu L, Deng S. Great Facilitation of Thirty Years of Reforestation with Mixed Species to Ecosystem Nitrogen Accumulation in Dry-Hot Valley in the Jinsha River. International Journal of Environmental Research and Public Health. 2022; 19(19):12660. https://doi.org/10.3390/ijerph191912660
Chicago/Turabian StyleGong, Zhilian, Yong Li, Luqing Liu, and Shuang Deng. 2022. "Great Facilitation of Thirty Years of Reforestation with Mixed Species to Ecosystem Nitrogen Accumulation in Dry-Hot Valley in the Jinsha River" International Journal of Environmental Research and Public Health 19, no. 19: 12660. https://doi.org/10.3390/ijerph191912660
APA StyleGong, Z., Li, Y., Liu, L., & Deng, S. (2022). Great Facilitation of Thirty Years of Reforestation with Mixed Species to Ecosystem Nitrogen Accumulation in Dry-Hot Valley in the Jinsha River. International Journal of Environmental Research and Public Health, 19(19), 12660. https://doi.org/10.3390/ijerph191912660





