Rapid Analysis of Residues of 186 Pesticides in Hawk Tea Using Modified QuEChERS Coupled with Gas Chromatography Tandem Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Equipment and Experimental Conditions
2.3. QuEChERS Extraction Procedures
3. Results and Discussions
3.1. Optimization of GC/MS/MS Conditions
3.2. Optimization of QuEChERS Procedure
3.2.1. The Application of EMR-Lipid
3.2.2. Selection of the Salt Composition
3.2.3. Pretreatment Effects of Different Combinations of Adsorbents
3.2.4. The Addition of Toluene
3.2.5. The Exchange of Solvent
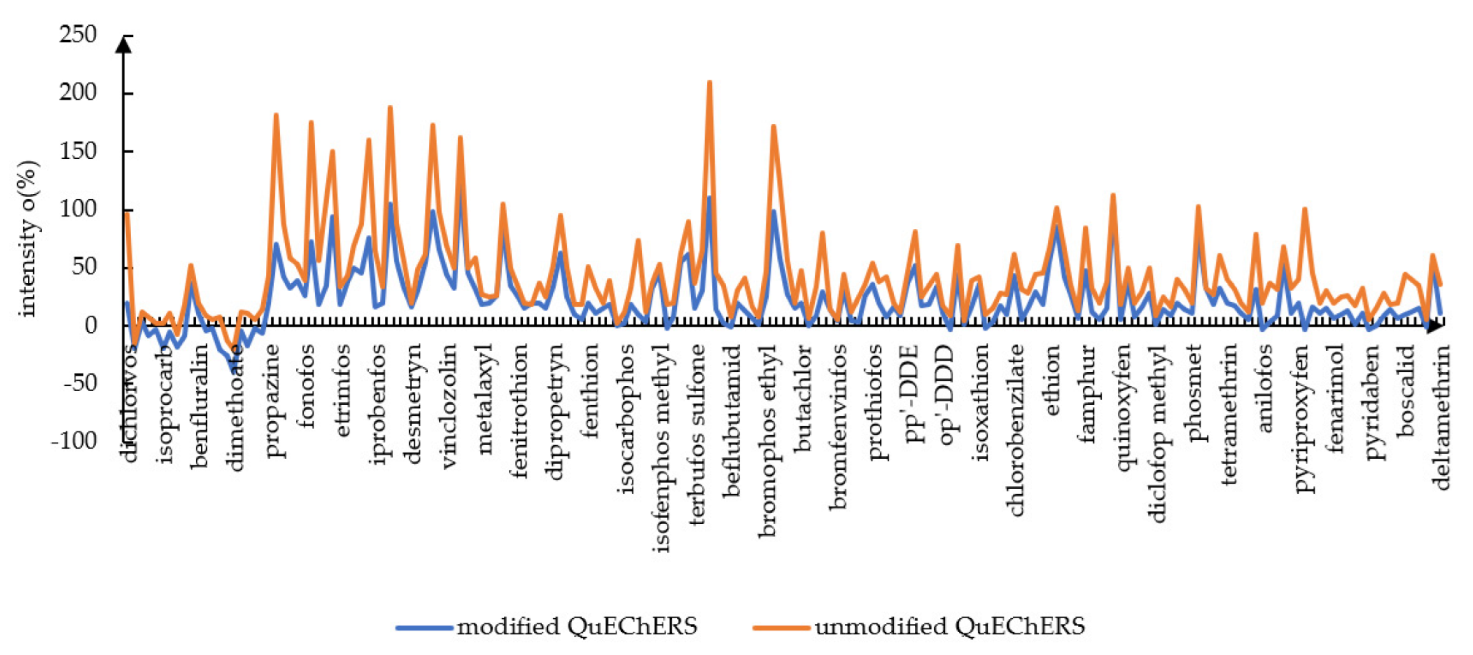
3.3. Matrix Effects Study
3.4. Recoveries and RSDs
3.5. Linearity
3.6. Limits of Detection and Limits of Quantitation
3.7. Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, X.; Yuan, M. Hawk Tea, a Traditional and Healthy Natural Beverage in South China. Non-Alcohol. Beverages 2019, 6, 107–128. [Google Scholar]
- Yu, J.; Gu, L. The chemical constituents of Laoying Tea from Guizhou. J. Plant Res. Environ. 2001, 10, 61–62. [Google Scholar]
- Yu, B.; Zhang, D.; Yan, X.W.; Wang, J.W.; Yao, L.; Tan, L.H.; Zhao, S.P.; Li, N.; Cao, W.G. Comparative Evaluation of the Chemical Composition, Antioxidant and Antimicrobial Activities of the Volatile Oils of Hawk Tea from Six Botanical Origins. Chem. Biodivers. 2016, 13, 1573–1583. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, T.; Huang, C.; Zhang, L.; Zhong, J.; Han, J.; Hu, T.; Li, J. Efficiency of transcellular transport and efflux of flavonoids with different glycosidic units from flavonoids of Litsea coreana L. in a MDCK epithelial cell monolayer model. Eur. J. Pharm. Sci. 2014, 53, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Dong, L.; Yang, Y.; Yuan, S.; Zhang, Z.; Yuan, M. Preliminary structural characterization and antioxidant activities of polysaccharides extracted from Hawk tea (Litsea coreana var. lanuginosa). Carbohydr. Polym. 2013, 95, 195–199. [Google Scholar] [CrossRef]
- Xu, Q.L.; Sun, X.T.; Li, M.J.; Zhou, X.S. Study on chemical constituents of hawk-tea and sandy-tea. Guizhou Sci. 2000, 18, 191–195. [Google Scholar]
- Ye, M.; Liu, D.; Zhang, R.; Yang, L.; Wang, J. Effect of hawk tea (Litsea coreana L.) on the numbers of lactic acid bacteria and flavour compounds of yoghurt. Int. Dairy J. 2012, 23, 68–71. [Google Scholar] [CrossRef]
- Zhao, X. Hawk tea (Litsea coreana Levl. var. lanuginose) attenuates CCl(4)-induced hepatic damage in Sprague-Dawley rats. Exp. Ther. Med. 2013, 5, 555–560. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, L.; Hu, H.; Yang, Y.; Zhang, X.; Peng, Y.; Xiao, P. DPPH Radical Scavenging and Postprandial Hyperglycemia Inhibition Activities and Flavonoid Composition Analysis of Hawk Tea by UPLC-DAD and UPLC-Q/TOF MS(E). Molecules 2017, 22, 1622. [Google Scholar] [CrossRef]
- Wu, Y.; An, Q.; Li, D.; Kang, L.; Zhou, C.; Zhang, J.; Pan, C. Multi-residue analytical method development and risk assessment of 56 pesticides and their metabolites in tea by chromatography tandem mass spectroscopy. Food Chem. 2022, 375, 131819. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction”for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Lehotay, S.J. Determination of Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning withMagnesium Sulfate: Collaborative Study. J. AOAC Int. 2007, 90, 485–520. [Google Scholar] [CrossRef] [PubMed]
- Paya, P.; Anastassiades, M.; Mack, D.; Sigalova, I.; Tasdelen, B.; Oliva, J.; Barba, A. Analysis of pesticide residues using the Quick Easy Cheap Effective Rugged and Safe (QuEChERS) pesticide multiresidue method in combination with gas and liquid chromatography and tandem mass spectrometric detection. Anal. Bioanal. Chem. 2007, 389, 1697–1714. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Qi, P.; Wang, J.; Wang, Z.; Di, S.; Xu, H.; Zhao, H.; Zhao, C.; Wang, X. Simultaneous determination of 114 pesticides in complex Chinese herbal medicine Fritillaria using ordered mesoporous carbon CMK-3 as a reversed-dispersive solid phase extraction sorbent. RSC Adv. 2021, 11, 4129–4137. [Google Scholar] [CrossRef]
- Zhu, Y.; Du, P.; Yang, J.; Yin, Q.; Yang, Y. Screening of multiclass pesticide residues in maca and Moringa oleifera by a modified QuEChERS sample preparation procedure and UPLC-ESI-MS/MS analysis. RSC Adv. 2020, 10, 36906–36919. [Google Scholar] [CrossRef]
- Lijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar]
- Zhu, B.; Xu, X.; Luo, J.; Jin, S.; Chen, W.; Liu, Z.; Tian, C. Simultaneous determination of 131 pesticides in tea by on-line GPC-GC-MS/MS using graphitized multi-walled carbon nanotubes as dispersive solid phase extraction sorbent. Food Chem. 2019, 276, 202–208. [Google Scholar] [CrossRef]
- Deng, X.; Guo, Q.; Chen, X.; Xue, T.; Wang, H.; Yao, P. Rapid and effective sample clean-up based on magnetic multiwalled carbon nanotubes for the determination of pesticide residues in tea by gas chromatography-mass spectrometry. Food Chem. 2014, 145, 853–858. [Google Scholar] [CrossRef]
- Alcantara-Duran, J.; Moreno-Gonzalez, D.; Garcia-Reyes, J.F.; Molina-Diaz, A. Use of a modified QuEChERS method for the determination of mycotoxin residues in edible nuts by nano flow liquid chromatography high resolution mass spectrometry. Food Chem. 2019, 279, 144–149. [Google Scholar] [CrossRef]
- Sun, D.; Jin, Y.; Zhao, Q.; Tang, C.; Li, Y.; Wang, H.; Qin, Y.; Zhang, J. Modified EMR-lipid method combined with HPLC-MS/MS to determine folates in egg yolks from laying hens supplemented with different amounts of folic acid. Food Chem. 2021, 337, 127767. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Q.; Yin, Z.; Hu, J.; Chen, G.; Zheng, L.; Ma, A. Determination of acetylgestagens in animal-derived matrix samples using enhanced matrix removal lipid clean-up in combination with ultra performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2021, 1649, 462227. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Matarrita, J.; Sapozhnikova, Y.; Lehotay, S.J. Lehotay, Evaluation of a recent product to remove lipids and other matrix co-extractives in the analysis of pesticide residues and environmental contaminants in foods. J. Chromatogr. A 2016, 1449, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Girame, R.; Shabeer, T.P.A.; Ghosh, B.; Hingmire, S.; Natarajan, R.; Dubey, P.N. Multi-residue method validation and safety evaluation of pesticide residues in seed spices cumin (Cuminum cyminum) and coriander (Coriandrum sativum) by gas chromatography tandem mass spectrometry (GC–MS/MS). Food Chem. 2022, 374, 131782. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X.; Li, M.; Li, X.; Zhang, Q.; Li, H. Emulsification/demulsification method coupled to GC–MS/MS for analysis of multiclass pesticide residues in edible oils. Food Chem. 2022, 379, 132098. [Google Scholar] [CrossRef]
- Song, N.E.; Lee, J.Y.; Mansur, A.R.; Jang, H.W.; Lim, M.C.; Lee, Y.; Yoo, M.; Nam, T.G. Determination of 60 pesticides in hen eggs using the QuEChERS procedure followed by LC-MS/MS and GC-MS/MS. Food Chem. 2019, 298, 125050. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Chen, C.; Xin, W.; Zhang, W. Simultaneous determination of 74 pesticide residues in Panax notoginseng by QuEChERS coupled with gas chromatography tandem mass spectrometry. Food Sci. Hum. Well 2021, 10, 241–250. [Google Scholar] [CrossRef]
- Manjusha, R.S.; Aggarwal, M.L.; Chacko, K.M. Development and validation of modified QuEChERS based GC-MS/MS method for determination of residual pesticides in herbal tea. Int. J. Curr. Res. Chem. Pharm. Sci. 2019, 6, 1–9. [Google Scholar]
- Yu, C.; Hao, D.; Chu, Q.; Wang, T.; Liu, S.; Lan, T.; Wang, F.; Pan, C. A one adsorbent QuEChERS method coupled with LC-MS/MS for simultaneous determination of 10 organophosphorus pesticide residues in tea. Food Chem. 2020, 321, 126657. [Google Scholar] [CrossRef]
- Ly, T.K.; Ho, T.D.; Behra, P.; Nhu-Trang, T.T. Determination of 400 pesticide residues in green tea leaves by UPLC-MS/MS and GC-MS/MS combined with QuEChERS extraction and mixed-mode SPE clean-up method. Food Chem. 2020, 326, 126928. [Google Scholar] [CrossRef]
- Wu, C.C. Multiresidue method for the determination of pesticides in Oolong tea using QuEChERS by gas chromatography-triple quadrupole tandem mass spectrometry. Food Chem. 2017, 229, 580–587. [Google Scholar] [CrossRef]
- González-Curbelo, M.Á.; Socas-Rodríguez, B.; Herrera-Herrera, A.V.; González-Sálamo, J.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Evolution and applications of the QuEChERS method. Trends Analyt. Chem. 2015, 71, 169–185. [Google Scholar] [CrossRef]
- Xian, Y.; Wu, Y.; Dong, H.; Chen, L.; Zhang, C.; Hou, X.; Zeng, X.; Bai, W.; Guo, X. Modified QuEChERS purification and Fe3O4 nanoparticle decoloration for robust analysis of 14 heterocyclic aromatic amines and acrylamide in coffee products using UHPLC-MS/MS. Food Chem. 2019, 285, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Kanrar, B.; Mandal, S.; Bhattacharyya, A. Validation and uncertainty analysis of a multiresidue method for 42 pesticides in made tea, tea infusion and spent leaves using ethyl acetate extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Tang, H.; Dong, X.; Liu, S.; Xu, W.; Wang, H.; Zong, Q. Determination of 23 Organophosphorus Pesticide Residues in Tea by QuEChERS Extraction with Multi-Walled Carbon Nanotubes (MWCNTs) Coupled to Gas Chromatography. Food Sci. 2018, 39, 7. [Google Scholar]
- He, Z.; Wang, L.; Peng, Y.; Luo, M.; Wang, W.; Liu, X. Multiresidue analysis of over 200 pesticides in cereals using a QuEChERS and gas chromatography-tandem mass spectrometry-based method. Food Chem. 2015, 169, 372–380. [Google Scholar] [CrossRef]
- Koesukwiwat, U.; Lehotay, S.J.; Miao, S.; Leepipatpiboon, N. High throughput analysis of 150 pesticides in fruits and vegetables using QuEChERS and low-pressure gas chromatography-time-of-flight mass spectrometry. J. Chromatogr. A 2010, 1217, 6692–6703. [Google Scholar] [CrossRef]
- Kittlaus, S.; Schimanke, J.; Kempe, G.; Speer, K. Assessment of sample cleanup and matrix effects in the pesticide residue analysis of foods using postcolumn infusion in liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2012, 1218, 8399–8410. [Google Scholar] [CrossRef]
- Tankiewicz, M.; Berg, A. Improvement of the QuEChERS method coupled with GC–MS/MS for the determination of pesticide residues in fresh fruit and vegetables. Microchem. J. 2022, 181, 107794. [Google Scholar] [CrossRef]
- Hou, X.; Lei, S.; Guo, L.; Qiu, S. Optimization of a multi-residue method for 101 pesticides in green tea leaves using gas chromatography tandem mass spectrometry. Rev. Bras. Farmacogn. 2016, 26, 401–407. [Google Scholar] [CrossRef]
- Huo, F.; Tang, H.; Wu, X.; Chen, D.; Zhao, T.; Liu, P.; Li, L. Utilizing a novel sorbent in the solid phase extraction for simultaneous determination of 15 pesticide residues in green tea by GC/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1023, 44–54. [Google Scholar] [CrossRef]
- Han, C.; Hu, B.; Chen, S.; Wang, N.; Hou, J.; Jin, N.; Shen, Y. Determination of Xinjunan pesticide residue in foodstuffs of plant origin by a modified QuEChERS method and ultra performance liquid chromatography-tandem mass spectrometry. LWT-Food Sci. Technol. 2021, 151, 112101. [Google Scholar] [CrossRef]
- Cajka, T.; Sandy, C.; Bachanova, V.; Drabova, L.; Kalachova, K.; Pulkrabova, J.; Hajslova, J. Streamlining sample preparation and gas chromatography-tandem mass spectrometry analysis of multiple pesticide residues in tea. Anal. Chim. Acta 2012, 743, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Cao, P.; Liu, R. A multi-residue method for fast determination of pesticides in tea by ultra performance liquid chromatography–electrospray tandem mass spectrometry combined with modified QuEChERS sample preparation procedure. Food Chem. 2011, 125, 1406–1411. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, G.; Liu, L.; Lin, T. Rapid determination of multi-antibiotic residues in honey based on modified QuEChERS method coupled with UPLC-MS/MS. Food Chem. 2022, 374, 131733. [Google Scholar] [CrossRef] [PubMed]

| No. | Pesticides | RT/min | Qualitative Ion Pairs (m/z) | CE/ | No. | Pesticides | RT/min | Qualitative Ion Pairs (m/z) | CE/ |
|---|---|---|---|---|---|---|---|---|---|
| eV | eV | ||||||||
| 1 | dichlorvos | 5.972 | 109.00 > 79.00 * | 8 | 94 | op’-DDE | 14.811 | 246.00 > 176.00 * | 30 |
| 185.00 > 93.00 | 14 | 248.00 > 176.00 | 28 | ||||||
| 2 | dichlorobenzonitrile | 6.935 | 170.90 > 136.00 * | 14 | 95 | paclobutrazol | 14.893 | 236.10 > 125.00 * | 14 |
| 170.90 > 100.00 | 24 | 236.10 > 167.00 | 10 | ||||||
| 3 | biphenyl | 7.389 | 154.10 > 128.10 * | 22 | 96 | butachlor | 14.92 | 176.10 > 147.10* | 14 |
| 154.10 > 115.10 | 24 | 188.10 > 160.10 | 12 | ||||||
| 4 | etridiazole | 8.034 | 210.90 > 182.90 * | 10 | 97 | fenothiocarb | 14.947 | 160.10 > 72.00 * | 10 |
| 182.90 > 139.90 | 18 | 160.10 > 106.10 | 12 | ||||||
| 5 | propoxur | 8.181 | 152.00/110.10 * | 18 | 98 | ditalimfos | 15.046 | 130.00 > 102.10 * | 10 |
| 110.00/82.00 | 8 | 148.00 > 130.10 | 10 | ||||||
| 6 | isoprocarb | 8.946 | 136.00 > 121.00 * | 10 | 99 | butamifos | 15.065 | 286.10 > 202.10 * | 14 |
| 121.00 > 77.00 | 22 | 200.10 > 65.00 | 22 | ||||||
| 7 | tecnazene | 9.553 | 260.90 > 202.90 * | 14 | 100 | napropamide | 15.155 | 128.00 > 72.10 * | 6 |
| 202.90 > 142.90 | 22 | 100.00 > 72.00 | 8 | ||||||
| 8 | diphenylamine | 9.941 | 169.10 > 66.00 * | 24 | 101 | bromfenvinfos | 15.164 | 267.00 > 159.00 * | 15 |
| 167.10 > 139.10 | 28 | 269.00 > 161.00 | 21 | ||||||
| 9 | ethoprophos | 10.016 | 200.00 > 158.00 * | 6 | 102 | fluorodifen | 15.182 | 190.00 > 126.00 * | 12 |
| 158.00 > 97.00 | 18 | 190.00 > 75.00 | 21 | ||||||
| 10 | chlorpropham | 10.263 | 127.10 > 65.00 * | 22 | 103 | flutolanil | 15.227 | 173.00 > 145.00 * | 14 |
| 213.10 > 171.10 | 6 | 173.00 > 95.00 | 26 | ||||||
| 11 | benfluralin | 10.382 | 292.10 > 264.00 * | 8 | 104 | chlorfenson | 15.236 | 175.00 > 111.00 * | 12 |
| 292.10 > 160.00 | 22 | 175.00 > 75.00 | 28 | ||||||
| 12 | sulfotep | 10.414 | 322.00 > 202.00 * | 10 | 105 | hexaconazole | 15.263 | 214.00 > 159.00 * | 20 |
| 322.00 > 174.00 | 18 | 214.00 > 172.00 | 20 | ||||||
| 13 | monocrotophos | 10.532 | 127.10 > 109.00 * | 12 | 106 | prothiofos | 15.272 | 266.90 > 238.90 * | 10 |
| 127.10 > 95.00 | 16 | 309.00 > 238.90 | 14 | ||||||
| 14 | phorate | 10.629 | 260.00 > 75.00 * | 8 | 107 | fludioxonil | 15.29 | 248.00 > 127.00 * | 26 |
| 231.00 > 129.00 | 24 | 248.00 > 154.00 | 20 | ||||||
| 15 | alpha BHC | 10.735 | 180.90 > 144.90 * | 16 | 108 | pretilachlor | 15.318 | 262.10 > 202.10 * | 10 |
| 218.90 > 182.90 | 8 | 238.10 > 162.10 | 10 | ||||||
| 16 | dimethoate | 11.008 | 125.00 > 47.00 * | 14 | 109 | isoprothiolane | 15.318 | 231.10 > 189.00 * | 10 |
| 125.00 > 79.00 | 8 | 290.10 > 118.00 | 14 | ||||||
| 17 | simazine | 11.166 | 201.10 > 173.10 * | 6 | 110 | profenofos | 15.363 | 338.90 > 268.90 * | 18 |
| 201.10 > 186.10 | 6 | 336.90 > 266.90 | 14 | ||||||
| 18 | atrazine | 11.25 | 215.10 > 58.00 * | 14 | 111 | pp’-DDE | 15.435 | 246.00 > 176.00 * | 30 |
| 215.10 > 173.10 | 6 | 317.90 > 248.00 | 24 | ||||||
| 19 | beta BHC | 11.261 | 180.90 > 144.90 * | 16 | 112 | oxadiazon | 15.453 | 258.00 > 175.00 * | 8 |
| 218.90 > 182.90 | 8 | 302.00 > 175.00 | 14 | ||||||
| 20 | clomazone | 11.292 | 204.10 > 107.00 * | 20 | 113 | DEF | 15.489 | 202.00 > 147.00 * | 6 |
| 204.10 > 78.00 | 26 | 202.00 > 113.00 | 20 | ||||||
| 21 | propazine | 11.313 | 229.10 > 58.00 * | 14 | 114 | dieldrin | 15.525 | 276.90 > 241.00 * | 8 |
| 229.10 > 187.10 | 6 | 262.90 > 193.00 | 34 | ||||||
| 22 | gamma-BHC | 11.408 | 180.90 > 144.90 * | 16 | 115 | myclobutanil | 15.534 | 179.10 > 125.00 * | 14 |
| 218.90 > 182.90 | 8 | 179.10 > 152.00 | 8 | ||||||
| 23 | profluralin | 11.429 | 318.00 > 199.10 * | 18 | 116 | op’-DDD | 15.562 | 235.00 > 165.00 * | 24 |
| 318.00 > 55.10 | 18 | 237.00 > 165.00 | 28 | ||||||
| 24 | terbuthylazine | 11.492 | 229.10 > 173.10 * | 6 | 117 | oxyfluorfen | 15.571 | 252.00 > 196.00 * | 22 |
| 214.10 > 71.00 | 16 | 361.00 > 300.00 | 14 | ||||||
| 25 | terbufos | 11.502 | 231.00 > 128.90 * | 26 | 118 | bupirimate | 15.579 | 273.10 > 108.10 * | 16 |
| 231.00 > 174.90 | 14 | 273.10 > 193.10 | 8 | ||||||
| 26 | fonofos | 11.576 | 137.10 > 109.10 * | 8 | 119 | kresoxim methyl | 15.605 | 116.00 > 89.00 * | 15 |
| 246.00 > 137.10 | 6 | 116.00 > 63.00 | 30 | ||||||
| 27 | pronamide | 11.586 | 172.90 > 144.90 * | 16 | 120 | cyflufenamid | 15.761 | 118.10 > 90.00* | 10 |
| 172.90 > 109.00 | 26 | 118.10 > 89.00 | 25 | ||||||
| 28 | diazinon | 11.639 | 304.10 > 179.10 * | 10 | 121 | isoxathion | 15.813 | 177.10 > 130.10 * | 10 |
| 179.10 > 137.10 | 18 | 177.10 > 116.10 | 12 | ||||||
| 29 | pyrimethanil | 11.723 | 198.10 > 183.10 * | 14 | 122 | cyproconazole 1 | 15.822 | 139.10 > 111.10 * | 16 |
| 198.10 > 118.10 | 28 | 222.10 > 125.10 | 24 | ||||||
| 30 | isazofos | 11.886 | 257.00 > 162.00 * | 8 | 123 | fluazifop butyl | 15.9 | 282.00 > 91.10 * | 18 |
| 257.00 > 119.00 | 18 | 282.00 > 238.10 | 18 | ||||||
| 31 | etrimfos | 11.967 | 181.10 > 153.10 * | 10 | 124 | nitrofen | 15.909 | 202.00 > 139.00 * | 24 |
| 292.10 > 181.10 | 8 | 282.90 > 253.00 | 12 | ||||||
| 32 | delta-BHC | 11.987 | 180.90 > 144.90 * | 16 | 125 | endrin | 15.917 | 262.90 > 191.00 * | 30 |
| 218.90 > 182.90 | 8 | 244.90 > 173.00 | 32 | ||||||
| 33 | triallate | 11.998 | 268.10 > 184.00 * | 20 | 126 | chlorobenzilate | 16.064 | 139.00 > 111.00 * | 16 |
| 270.10 > 186.00 | 20 | 251.00 > 139.00 | 14 | ||||||
| 34 | tebupirimfos | 12.099 | 261.10 > 137.10 * | 18 | 127 | fensulfothion | 16.108 | 293.00 > 125.00 * | 14 |
| 318.10 > 152.10 | 14 | 293.00 > 153.00 | 8 | ||||||
| 35 | pirimicarb | 12.13 | 238.10 > 166.10 * | 12 | 128 | diniconazole | 16.151 | 268.00 > 232.00 * | 12 |
| 166.10 > 55.00 | 20 | 270.00 > 234.00 | 10 | ||||||
| 36 | iprobenfos | 12.15 | 204.00 > 91.00 * | 8 | 129 | oxadixyl | 16.212 | 163.10 > 132.10 * | 8 |
| 204.00 > 122.00 | 12 | 132.10 > 117.10 | 18 | ||||||
| 37 | formothion | 12.282 | 170.00 > 93.00 * | 8 | 130 | pp’-DDD | 16.229 | 235.00 > 165.00 * | 24 |
| 224.00 > 125.00 | 18 | 237.00 > 165.00 | 28 | ||||||
| 38 | pentachloroaniline | 12.303 | 262.80 > 191.90 * | 21 | 131 | ethion | 16.229 | 153.00 > 97.00 * | 14 |
| 264.80 > 193.90 | 21 | 230.90 > 129.00 | 24 | ||||||
| 39 | phosphamidon | 12.425 | 127.10 > 109.10 * | 12 | 132 | op’-DDT | 16.264 | 235.00 > 165.00 * | 24 |
| 127.10 > 95.10 | 18 | 237.00 > 165.00 | 28 | ||||||
| 40 | dichlofenthion | 12.445 | 279.00 > 222.90 * | 14 | 133 | chlorthiophos | 16.264 | 324.90 > 268.90 * | 14 |
| 222.90 > 204.90 | 14 | 268.90 > 205.00 | 18 | ||||||
| 41 | desmetryn | 12.475 | 213.00 > 171.10 * | 6 | 134 | aclonifen | 16.307 | 212.00 > 182.10 * | 15 |
| 213.00 > 58.10 | 18 | 264.00 > 194.10 | 18 | ||||||
| 42 | propanil | 12.516 | 217.00 > 161.00 * | 10 | 135 | triazophos | 16.521 | 161.00 > 134.00 * | 8 |
| 160.90 > 99.00 | 24 | 161.00 > 106.00 | 14 | ||||||
| 43 | acetochlor | 12.547 | 174.10 > 146.10 * | 12 | 136 | famphur | 16.646 | 218.00 > 109.00 * | 16 |
| 223.10 > 132.10 | 22 | 218.00 > 79.00 | 24 | ||||||
| 44 | phenthoate | 12.58 | 273.9/125.0 * | 20 | 137 | benalaxyl | 16.671 | 148.10 > 105.10 * | 16 |
| 273.9/246.0 | 6 | 148.10 > 79.10 | 24 | ||||||
| 45 | malaoxon | 12.618 | 126.90 > 99.00 * | 10 | 138 | carbophenothion | 16.729 | 157.00 > 45.00* | 18 |
| 268.00 > 126.90 | 10 | 341.90 > 157.00 | 14 | ||||||
| 46 | vinclozolin | 12.658 | 212.00 > 172.00 * | 16 | 139 | trifloxystrobi | 16.762 | 116.00 > 89.00 * | 15 |
| 285.00 > 212.00 | 12 | 131.00 > 89.00 | 30 | ||||||
| 47 | parathion methyl | 12.709 | 263.00 > 109.00 * | 14 | 140 | edifenphos | 16.779 | 173.00 > 109.00 * | 10 |
| 125.00 > 47.00 | 12 | 310.00 > 173.00 | 14 | ||||||
| 48 | tolclofos methyl | 12.719 | 264.90 > 249.90 * | 14 | 141 | propiconazole | 16.829 | 173.00 > 145.00 * | 16 |
| 264.90 > 93.00 | 24 | 259.00 > 69.00 | 14 | ||||||
| 49 | alachlor | 12.73 | 188.10 > 160.10 * | 10 | 142 | quinoxyfen | 16.912 | 237.10 > 208.10 * | 28 |
| 188.10 > 132.10 | 18 | 307.10 > 237.10 | 22 | ||||||
| 50 | ametryn | 12.877 | 227.10 > 185.10 * | 6 | 143 | pp’-DDT | 16.929 | 235.00 > 165.00 * | 24 |
| 227.10 > 58.00 | 14 | 237.00 > 165.00 | 28 | ||||||
| 51 | metalaxyl | 12.877 | 249.20 > 190.10 * | 8 | 144 | hexazinone | 17.046 | 171.10 > 71.00 * | 16 |
| 206.10 > 132.10 | 20 | 171.10 > 85.00 | 16 | ||||||
| 52 | ronnel | 12.906 | 284.90 > 269.90 * | 16 | 145 | tebuconazole | 17.196 | 250.10 > 125.10 * | 22 |
| 286.90 > 271.90 | 18 | 125.10 > 89.00 | 18 | ||||||
| 53 | prometryn | 12.936 | 226.10 > 184.10 * | 10 | 146 | diclofop methyl | 17.212 | 340.00 > 253.00 * | 14 |
| 241.20 > 184.10 | 12 | 253.00 > 162.00 | 22 | ||||||
| 54 | pirimiphos methyl | 13.141 | 290.10 > 125.00 * | 22 | 147 | piperonylbutoxide | 17.334 | 176.10 > 131.10 * | 12 |
| 290.10 > 233.10 | 12 | 176.10 > 117.10 | 20 | ||||||
| 55 | terbutryn | 13.18 | 241.20 > 185.10 * | 6 | 148 | epoxiconazol | 17.454 | 192.00 > 138.00 * | 14 |
| 241.20 > 170.10 | 14 | 192.00 > 111.00 | 26 | ||||||
| 56 | fenitrothion | 13.2 | 277.00 > 260.00 * | 6 | 149 | pyridaphenthion | 17.654 | 340.00 > 199.10 * | 8 |
| 277.00 > 109.10 | 14 | 199.10 > 92.00 | 16 | ||||||
| 57 | ethofumesate | 13.239 | 207.10 > 161.10 * | 8 | 150 | iprodione | 17.678 | 187.00 > 124.00 * | 25 |
| 207.10 > 137.10 | 12 | 243.90 > 187.00 | 5 | ||||||
| 58 | bromacil | 13.307 | 204.90 > 187.90 * | 14 | 151 | phosmet | 17.798 | 160.00 > 77.00 * | 24 |
| 206.90 > 189.90 | 16 | 160.00 > 133.00 | 14 | ||||||
| 59 | phorate sulfoxide | 13.366 | 153.00 > 97.00 * | 12 | 152 | bifenthrin | 17.822 | 181.10 > 166.10 * | 12 |
| 199.00 > 171.10 | 6 | 181.10 > 179.10 | 12 | ||||||
| 60 | malathion | 13.376 | 173.10 > 99.00 * | 14 | 153 | EPN | 17.846 | 156.90 > 77.00 * | 24 |
| 173.10 > 127.00 | 6 | 169.10 > 77.00 | 22 | ||||||
| 61 | dipropetryn | 13.444 | 255.00 > 222.20 * | 9 | 154 | bromopropylate | 17.869 | 340.90 > 182.90 * | 18 |
| 255.00 > 180.20 | 18 | 340.90 > 184.90 | 20 | ||||||
| 62 | metolachlor | 13.464 | 162.10 > 133.10 * | 16 | 155 | piperophos | 17.877 | 320.10 > 122.10 * | 14 |
| 238.10 > 162.10 | 12 | 140.10 > 98.00 | 12 | ||||||
| 63 | phoratesulfone | 13.493 | 153.00 > 97.00 * | 12 | 156 | tetramethrin | 17.893 | 164.10 > 107.10 * | 14 |
| 153.00 > 125.00 | 6 | 164.10 > 77.00 | 22 | ||||||
| 64 | chlorpyrifos | 13.503 | 196.90 > 168.90 * | 14 | 157 | methoxychlor | 17.965 | 227.10 > 169.10 * | 24 |
| 313.90 > 257.90 | 14 | 227.10 > 212.10 | 14 | ||||||
| 65 | thiobencarb | 13.532 | 100.00 > 72.00 * | 5 | 158 | etoxazole | 17.973 | 141.00 > 113.00* | 15 |
| 125.00 > 89.00 | 18 | 141.00 > 63.10 | 30 | ||||||
| 66 | fenthion | 13.591 | 278.00 > 109.00 * | 20 | 159 | fenamidone | 18.037 | 238.00 > 237.20 * | 10 |
| 278.00 > 169.00 | 14 | 268.10 > 180.10 | 16 | ||||||
| 67 | parathion | 13.659 | 109.00 > 91.00 * | 6 | 160 | tebufenpyrad | 18.1 | 333.10 > 171.10 * | 20 |
| 148.90 > 119.00 | 5 | 333.10 > 276.10 | 8 | ||||||
| 68 | isofenphos oxon | 13.689 | 229.10 > 201.00 * | 10 | 161 | anilofos | 18.131 | 226.10 > 157.00 * | 14 |
| 201.00 > 121.00 | 20 | 226.10 > 184.00 | 6 | ||||||
| 69 | triadimefon | 13.718 | 208.10 > 181.00 * | 10 | 162 | bifenox | 18.162 | 340.90 > 309.90 * | 10 |
| 208.10 > 111.00 | 22 | 340.90 > 188.90 | 20 | ||||||
| 70 | buprofezin | 13.726 | 175.10/132.10 * | 14 | 163 | tetradifon | 18.354 | 226.90 > 199.00 * | 16 |
| 175.10/117.10 | 12 | 355.90 > 159.00 | 18 | ||||||
| 71 | isocarbophos | 13.737 | 289.10 > 136.00 * | 14 | 164 | phosalone | 18.462 | 182.00 > 111.00* | 14 |
| 230.00 > 212.00 | 10 | 182.00 > 138.00 | 8 | ||||||
| 72 | dicofol | 13.803 | 139.00 > 111.00 * | 16 | 165 | leptopho | 18.47 | 376.90 > 361.90 * | 24 |
| 139.00 > 75.00 | 28 | 374.90 > 359.90 | 24 | ||||||
| 73 | trichloronat | 13.84 | 297.00 > 269.00 * | 15 | 166 | pyriproxyfen | 18.631 | 136.10 > 78.00 * | 20 |
| 299.00 > 271.00 | 15 | 136.10 > 96.00 | 14 | ||||||
| 74 | pirimiphos ethyl | 13.915 | 304.00 > 168.00 * | 10 | 167 | iambda cyhalothrin | 18.631 | 208.00 > 181.00 * | 8 |
| 318.00 > 166.00 | 15 | 197.00 > 141.00 | 12 | ||||||
| 75 | bromophos | 13.925 | 330.90 > 315.90 * | 14 | 168 | mefenacet | 18.708 | 192.00 > 136.00 * | 14 |
| 328.90 > 313.90 | 18 | 192.00 > 109.00 | 24 | ||||||
| 76 | isofenphos methyl | 14.019 | 199.00 > 121.00 * | 14 | 169 | acrinathrin | 18.77 | 289.10 > 93.00 * | 14 |
| 241.10 > 121.10 | 22 | 289.10 > 77.00 | 26 | ||||||
| 77 | fosthiazate | 14.019 | 195.00 > 103.00 * | 10 | 170 | pyrazophos | 18.981 | 221.10 > 193.10 * | 12 |
| 195.00 > 60.00 | 22 | 221.10 > 149.10 | 14 | ||||||
| 78 | pendimethalin | 14.141 | 252.10 > 162.10 * | 10 | 171 | fenarimol | 19.003 | 251.00 > 139.00 * | 14 |
| 252.10 > 191.10 | 8 | 330.00 > 139.00 | 8 | ||||||
| 79 | chlorfenvinphos | 14.15 | 323.00 > 267.00 * | 16 | 172 | azinphos ethyl | 19.13 | 160.10 > 132.10 * | 4 |
| 267.00 > 159.00 | 18 | 132.10 > 77.00 | 14 | ||||||
| 80 | cyprodinil | 14.169 | 224.10 > 208.10 * | 16 | 173 | permethrin 1 | 19.598 | 183.10 > 153.10 * | 14 |
| 224.10 > 197.10 | 22 | 183.10 > 168.10 | 14 | ||||||
| 81 | terbufos sulfone | 14.235 | 153.00 > 97.00 * | 21 | 174 | coumaphos | 19.712 | 362.00 > 109.00 * | 16 |
| 199.00 > 97.00 | 21 | 362.00 > 226.00 | 14 | ||||||
| 82 | fipronil | 14.244 | 366.90 > 212.90 * | 30 | 175 | fluquinconazole | 19.734 | 340.00 > 298.00 * | 20 |
| 368.90 > 214.90 | 30 | 340.00 > 313.00 | 14 | ||||||
| 83 | penconazole | 14.272 | 248.10 > 157.10 * | 26 | 176 | pyridaben | 19.756 | 147.10 > 117.10 * | 22 |
| 159.10 > 123.10 | 22 | 147.10 > 132.10 | 14 | ||||||
| 84 | phosfolan | 14.301 | 255.00 > 227.00 * | 6 | 177 | dioxathion | 19.77 | 152.90 > 96.90 * | 10 |
| 255.00 > 140.00 | 22 | 185.00 > 129.00 | 12 | ||||||
| 85 | isofenphos | 14.338 | 213.00 > 121.00 * | 15 | 178 | fenbuconazole | 20.121 | 198.10 > 129.10 * | 10 |
| 213.00 > 185.00 | 6 | 129.10 > 102.10 | 18 | ||||||
| 86 | beflubutamid | 14.46 | 176.00 > 91.10 * | 15 | 179 | cyfluthrin | 20.136 | 226.10 > 206.10 * | 14 |
| 221.00 > 193.00 | 12 | 198.90 > 170.10 | 25 | ||||||
| 87 | quinalphos | 14.47 | 146.10 > 118.00 * | 10 | 180 | cypermethri | 20.46 | 163.10 > 127.10 * | 6 |
| 146.10 > 91.00 | 24 | 163.10 > 91.00 | 14 | ||||||
| 88 | mephosfolan | 14.498 | 196.00 > 140.00 * | 12 | 181 | boscalid | 20.522 | 140.10 > 112.10 * | 12 |
| 196.00 > 168.00 | 6 | 140.10 > 76.00 | 24 | ||||||
| 89 | procymidone | 14.535 | 283.00 > 96.00 * | 10 | 182 | flucythrinate | 20.626 | 199.10 > 157.10 * | 10 |
| 285.00 > 96.00 | 10 | 157.10 > 107.10 | 12 | ||||||
| 90 | triadimenol | 14.545 | 168.10 > 70.00 * | 10 | 183 | fenvalerate | 21.338 | 225.10 > 119.10 * | 20 |
| 128.10 > 65.00 | 22 | 225.10 > 147.10 | 10 | ||||||
| 91 | bromophos ethyl | 14.721 | 358.90 > 302.90 * | 16 | 184 | fluvalinate | 21.452 | 250.10 > 55.00 * | 20 |
| 302.90 > 284.90 | 18 | 250.10 > 200.00 | 20 | ||||||
| 92 | methidathion | 14.739 | 145.00 > 85.00 * | 8 | 185 | difenoconazole | 21.793 | 323.00 > 265.00 * | 14 |
| 145.00 > 58.00 | 14 | 265.00 > 202.00 | 20 | ||||||
| 93 | chlordane trans | 14.757 | 374.80 > 265.90 * | 26 | 186 | deltamethrin | 22.109 | 180.90 > 151.90 * | 22 |
| 372.80 > 263.90 | 28 | 252.90 > 93.00 | 20 |
| No. | 4 g NaCl | 4 g MgSO4 + 1 g NaCl |
|---|---|---|
| Co-Extracts (g) | Co-Extracts (g) | |
| replicate 1 | 0.10805 | 0.12837 |
| replicate 2 | 0.10925 | 0.13428 |
| replicate 3 | 0.11052 | 0.13125 |
| replicate 4 | 0.10892 | 0.13172 |
| replicate 5 | 0.10977 | 0.12953 |
| average | 0.10930 | 0.13103 |
| No. | Pesticides | R2 | Spiked 0.02 mg/kg | Spiked 0.05 mg/kg | Spiked 0.1 mg/kg | LODs mg/kg | LOQs mg/kg | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Recovery (%) | RSD | Recovery (%) | RSD | Recovery (%) | RSD | |||||
| 1 | dichlorvos | 0.9985 | 81.9 | 6.1 | 88.7 | 5.2 | 106.3 | 4.2 | 0.01 | 0.02 |
| 2 | dichlorobenzonitrile | 0.9901 | 64.8 | 11.6 | 78.6 | 3 | 94 | 5.7 | 0.01 | 0.02 |
| 3 | biphenyl | 0.9906 | 100.3 | 21.5 | 87 | 5 | 89.3 | 3.5 | 0.005 | 0.01 |
| 4 | etridiazole | 0.9973 | 118.7 | 3.3 | 87.6 | 1.8 | 101.2 | 7.2 | 0.005 | 0.01 |
| 5 | propoxur | 0.9965 | 56.2 | 5.2 | 68.8 | 8.4 | 73.6 | 8.4 | 0.02 | 0.05 |
| 6 | isoprocarb | 0.9976 | 85 | 6.4 | 71.8 | 9.4 | 96 | 6.7 | 0.005 | 0.01 |
| 7 | tecnazene | 0.9942 | 61.9 | 6.4 | 64.9 | 11 | 104.9 | 11.3 | 0.01 | 0.02 |
| 8 | diphenylamine | 0.9962 | 103.8 | 15.5 | 95.6 | 10.5 | 103.5 | 5.9 | 0.005 | 0.01 |
| 9 | ethoprophos | 0.9981 | 97.8 | 12.6 | 98.7 | 16.7 | 96.1 | 14.4 | 0.005 | 0.01 |
| 10 | chlorpropham | 0.9976 | 66.6 | 4.8 | 70.4 | 13.1 | 81.6 | 5.4 | 0.01 | 0.02 |
| 11 | benfluralin | 0.9992 | 107.7 | 16.6 | 95.5 | 5.3 | 111.9 | 5.6 | 0.01 | 0.02 |
| 12 | sulfotep | 0.999 | 85.7 | 20.2 | 81.2 | 21.3 | 92.3 | 6.8 | 0.01 | 0.02 |
| 13 | monocrotophos | 0.9993 | 76.1 | 12.2 | 70.2 | 4.2 | 110.4 | 2.1 | 0.005 | 0.01 |
| 14 | phorate | 0.9896 | 56.5 | 10.3 | 62.2 | 4.2 | 81.4 | 0.9 | 0.02 | 0.05 |
| 15 | alpha-BHC | 0.9986 | 79.1 | 9.3 | 85.4 | 13.1 | 104.1 | 9.1 | 0.005 | 0.01 |
| 16 | dimethoate | 0.9922 | 82.1 | 20.8 | 79.4 | 6.5 | 94.6 | 8.1 | 0.005 | 0.01 |
| 17 | simazine | 0.9984 | 60.7 | 6.1 | 71.4 | 9.4 | 84.2 | 4.5 | 0.01 | 0.02 |
| 18 | atrazine | 0.9907 | 85.4 | 12.8 | 77.8 | 5.7 | 91.4 | 1.2 | 0.01 | 0.02 |
| 19 | beta-BHC | 0.9987 | 90.8 | 13.5 | 88.5 | 8.2 | 108.8 | 2.1 | 0.005 | 0.01 |
| 20 | clomazone | 0.9992 | 61.9 | 4.3 | 59.8 | 9.7 | 97 | 7.5 | 0.01 | 0.02 |
| 21 | propazine | 0.9946 | 96.9 | 6.7 | 72 | 14.4 | 95.4 | 13 | 0.01 | 0.02 |
| 22 | gamma- BHC | 0.9991 | 106.6 | 3 | 105.3 | 8.8 | 119.4 | 2.3 | 0.005 | 0.01 |
| 23 | profluralin | 0.9985 | 54.1 | 2 | 49.4 | 14.7 | 93.5 | 2.4 | 0.01 | 0.02 |
| 24 | terbuthylazine | 0.9532 | 50 | 26 | 48.7 | 16 | 91.2 | 5.5 | 0.02 | 0.05 |
| 25 | terbufos | 0.998 | 115.4 | 7.2 | 133.8 | 6.1 | 116.2 | 8.1 | 0.01 | 0.02 |
| 26 | fonofos | 0.9986 | 101.7 | 9.9 | 101.3 | 10.9 | 105.9 | 10.5 | 0.01 | 0.02 |
| 27 | pronamide | 0.9967 | 104.8 | 6.1 | 94.7 | 10.3 | 103.8 | 1.8 | 0.005 | 0.01 |
| 28 | diazinon | 0.9935 | 88.3 | 3.7 | 80.9 | 6.7 | 105.1 | 1.9 | 0.005 | 0.01 |
| 29 | pyrimethanil | 0.9955 | 54.9 | 7.4 | 56.7 | 11 | 76 | 6.5 | 0.02 | 0.05 |
| 30 | isazofos | 0.9991 | 58.8 | 7.8 | 65 | 11.4 | 104.3 | 7.4 | 0.01 | 0.02 |
| 31 | etrimfos | 0.9963 | 93.2 | 3 | 100.3 | 2.5 | 114.7 | 6.6 | 0.005 | 0.01 |
| 32 | delta- BHC | 0.9921 | 76 | 6.9 | 78.4 | 5.5 | 105.6 | 7.9 | 0.005 | 0.01 |
| 33 | triallate | 0.9974 | 81.5 | 14.5 | 110.2 | 6.2 | 99.1 | 9.7 | 0.005 | 0.01 |
| 34 | tebupirimfos | 0.9952 | 82.2 | 8.6 | 83.7 | 5.1 | 92.9 | 4.6 | 0.01 | 0.02 |
| 35 | pirimicarb | 0.9998 | 85.4 | 8.4 | 82.6 | 8.7 | 92.7 | 9.1 | 0.005 | 0.01 |
| 36 | iprobenfos | 0.9962 | 114.3 | 8.8 | 99.1 | 9.1 | 103.5 | 5.8 | 0.005 | 0.01 |
| 37 | formothion | 0.9989 | 70.2 | 5.2 | 72.3 | 2.2 | 78 | 1 | 0.005 | 0.01 |
| 38 | pentachloroaniline | 0.9979 | 94.4 | 11.1 | 89.3 | 5.1 | 100.3 | 1.1 | 0.005 | 0.01 |
| 39 | phosphamidon | 0.998 | 102.3 | 7.3 | 86.5 | 6.9 | 96.5 | 5.2 | 0.005 | 0.01 |
| 40 | dichlofenthion | 0.997 | 66.2 | 3.9 | 70.9 | 10.8 | 103 | 2.3 | 0.01 | 0.02 |
| 41 | desmetryn | 0.9851 | 65.7 | 9 | 65.5 | 3 | 80.9 | 4.4 | 0.01 | 0.02 |
| 42 | propanil | 0.9995 | 98.5 | 14.4 | 84.6 | 1.7 | 94 | 10.4 | 0.005 | 0.01 |
| 43 | acetochlor | 0.9923 | 76 | 10.7 | 90.6 | 5.1 | 111.7 | 7 | 0.01 | 0.02 |
| 44 | phenthoate | 0.9959 | 65.3 | 5.6 | 70.3 | 2.1 | 75.5 | 1.9 | 0.005 | 0.01 |
| 45 | malaoxon | 0.9992 | 62.5 | 11.1 | 70.9 | 14.5 | 70.6 | 1.2 | 0.01 | 0.02 |
| 46 | vinclozolin | 0.99 | 76.6 | 3.3 | 78.4 | 14.8 | 97.5 | 9 | 0.01 | 0.02 |
| 47 | parathion methyl | 0.9989 | 70.8 | 4.4 | 76.6 | 11.3 | 85.4 | 8.9 | 0.005 | 0.01 |
| 48 | tolclofos methyl | 0.9971 | 74.8 | 6.4 | 90.8 | 2.4 | 104.1 | 2.6 | 0.01 | 0.02 |
| 49 | alachlor | 0.9989 | 117.1 | 3.4 | 108.8 | 10.3 | 118.9 | 5.2 | 0.005 | 0.01 |
| 50 | ametryn | 0.9921 | 64.2 | 14.8 | 68.5 | 6.3 | 82.5 | 3.6 | 0.01 | 0.02 |
| 51 | metalaxyl | 0.9987 | 81.3 | 13.1 | 80.6 | 6.1 | 86.1 | 7 | 0.005 | 0.01 |
| 52 | ronnel | 0.9994 | 65.8 | 11.9 | 65.7 | 3.8 | 75.5 | 0.3 | 0.01 | 0.02 |
| 53 | prometryn | 0.9908 | 66.1 | 7.5 | 71.1 | 11.4 | 78.4 | 1.4 | 0.01 | 0.02 |
| 54 | pirimiphos methyl | 0.9927 | 67.2 | 6.6 | 82 | 8.7 | 100 | 2.3 | 0.01 | 0.02 |
| 55 | terbutryn | 0.9975 | 79.4 | 6 | 82.4 | 4.2 | 97.9 | 4.2 | 0.005 | 0.01 |
| 56 | fenitrothion | 0.9959 | 91.1 | 6.4 | 99.6 | 0.1 | 118.5 | 5.8 | 0.005 | 0.01 |
| 57 | ethofumesate | 0.9962 | 95.5 | 6.5 | 100.5 | 4.3 | 107.4 | 3.6 | 0.005 | 0.01 |
| 58 | bromacil | 0.9989 | 77 | 8.2 | 73.6 | 10.7 | 88.6 | 4.6 | 0.005 | 0.01 |
| 59 | phorate sulfoxide | 0.9964 | 78 | 7 | 93.3 | 4.9 | 117 | 5.6 | 0.01 | 0.02 |
| 60 | malathion | 0.9969 | 88.7 | 5.9 | 95.3 | 1.6 | 111.5 | 2 | 0.005 | 0.01 |
| 61 | dipropetryn | 0.9902 | 72 | 6.6 | 76.8 | 4.9 | 91.3 | 2.9 | 0.005 | 0.01 |
| 62 | metolachlor | 0.9921 | 82.5 | 6 | 85.9 | 1.3 | 97.3 | 2.4 | 0.005 | 0.01 |
| 63 | phoratesulfone | 0.9971 | 50 | 9 | 41.1 | 7.5 | 96.4 | 3.8 | 0.02 | 0.05 |
| 64 | chlorpyrifos | 0.9989 | 94.7 | 5.3 | 88.1 | 10.5 | 98.7 | 11.2 | 0.005 | 0.01 |
| 65 | thiobencarb | 0.9921 | 70.7 | 5.7 | 69.9 | 10.3 | 95.3 | 4.2 | 0.01 | 0.02 |
| 66 | fenthion | 0.9987 | 73 | 3.8 | 90.6 | 3.1 | 94.2 | 3.7 | 0.005 | 0.01 |
| 67 | parathion | 0.9914 | 126.5 | 5.3 | 104.5 | 2.1 | 114.2 | 2 | 0.005 | 0.01 |
| 68 | isofenphos oxon | 0.9988 | 86.9 | 3.5 | 84.8 | 1.4 | 100.1 | 2.7 | 0.005 | 0.01 |
| 69 | triadimefon | 0.9987 | 77.8 | 4 | 92.5 | 5.2 | 107.9 | 7.6 | 0.005 | 0.01 |
| 70 | buprofezin | 0.9979 | 65.2 | 1.3 | 70.8 | 3.3 | 73.2 | 8.7 | 0.01 | 0.02 |
| 71 | isocarbophos | 0.9959 | 91 | 3 | 87.8 | 10.5 | 99.1 | 3.2 | 0.005 | 0.01 |
| 72 | dicofol | 0.9962 | 76.9 | 9.2 | 73.5 | 6 | 93.3 | 1.4 | 0.005 | 0.01 |
| 73 | trichloronat | 0.9991 | 77.2 | 9.2 | 86.2 | 4.1 | 93.2 | 2.7 | 0.005 | 0.01 |
| 74 | pirimiphos ethyl | 0.9963 | 59.5 | 6.2 | 67.4 | 2 | 75.4 | 2.7 | 0.02 | 0.05 |
| 75 | bromophos | 0.9921 | 84.9 | 5.7 | 88.3 | 4.3 | 97.1 | 0.7 | 0.01 | 0.02 |
| 76 | isofenphos methyl | 0.9974 | 74.7 | 5.3 | 87.6 | 2.1 | 99.9 | 0.8 | 0.005 | 0.01 |
| 77 | fosthiazate | 0.9952 | 68.7 | 13.2 | 67.8 | 4.7 | 95 | 2.9 | 0.01 | 0.02 |
| 78 | pendimethalin | 0.9992 | 76.4 | 9.5 | 91.5 | 2.3 | 102.5 | 4.9 | 0.005 | 0.01 |
| 79 | chlorfenvinphos | 0.9962 | 79.2 | 2.9 | 86 | 2.4 | 102.7 | 3.1 | 0.005 | 0.01 |
| 80 | cyprodinil | 0.9989 | 61.8 | 6.6 | 65.4 | 7.3 | 79.7 | 2.9 | 0.01 | 0.02 |
| 81 | terbufos sulfone | 0.9979 | 84.1 | 1.8 | 93.7 | 8.3 | 107.8 | 2.9 | 0.005 | 0.01 |
| 82 | fipronil | 0.9911 | 75.1 | 9.7 | 80.9 | 2.7 | 100.8 | 5.2 | 0.005 | 0.01 |
| 83 | penconazole | 0.9963 | 65.2 | 4.8 | 76.8 | 10.5 | 88.2 | 3.5 | 0.01 | 0.02 |
| 84 | phosfolan | 0.9921 | 53.4 | 11.2 | 45.7 | 14.2 | 58.6 | 6.4 | 0.02 | 0.05 |
| 85 | isofenphos | 0.9974 | 88.9 | 4.1 | 90.7 | 5.5 | 101.4 | 2.3 | 0.005 | 0.01 |
| 86 | beflubutamid | 0.9952 | 80.2 | 5.2 | 91.9 | 1.9 | 100.7 | 0.5 | 0.005 | 0.01 |
| 87 | quinalphos | 0.9905 | 81.8 | 3.3 | 92.7 | 2.2 | 87.2 | 1.4 | 0.005 | 0.01 |
| 88 | mephosfolan | 0.9962 | 67.7 | 4.5 | 71.3 | 6.8 | 79.5 | 4.1 | 0.01 | 0.02 |
| 89 | procymidone | 0.9989 | 82.8 | 4.3 | 89.5 | 7.5 | 99 | 5 | 0.005 | 0.01 |
| 90 | triadimenol | 0.9979 | 122.8 | 2.8 | 116.4 | 11.7 | 98.4 | 3.8 | 0.01 | 0.02 |
| 91 | bromophos ethyl | 0.9901 | 88.8 | 4.5 | 95.2 | 4.3 | 103.6 | 2.6 | 0.005 | 0.01 |
| 92 | methidathion | 0.9963 | 89.2 | 9.2 | 96.2 | 1.7 | 106.3 | 0.3 | 0.005 | 0.01 |
| 93 | chlordane trans | 0.9921 | 79.8 | 0.5 | 86.2 | 14 | 90.4 | 2.9 | 0.01 | 0.02 |
| 94 | op’-DDE | 0.9974 | 73 | 4.3 | 78.2 | 1.6 | 85.7 | 0.6 | 0.005 | 0.01 |
| 95 | paclobutrazol | 0.9952 | 90.8 | 6.2 | 94.5 | 0.9 | 110.1 | 2.7 | 0.005 | 0.01 |
| 96 | butachlor | 0.9908 | 112.9 | 2.4 | 106 | 2.2 | 108.3 | 0.9 | 0.005 | 0.01 |
| 97 | fenothiocarb | 0.9962 | 81.3 | 3.1 | 80.9 | 2.7 | 90.9 | 1.2 | 0.005 | 0.01 |
| 98 | ditalimfos | 0.9989 | 57.8 | 4.5 | 59.9 | 5.1 | 79.8 | 1.7 | 0.01 | 0.02 |
| 99 | butamifos | 0.9979 | 73 | 5.8 | 75.7 | 2.9 | 86.3 | 2.2 | 0.01 | 0.02 |
| 100 | napropamide | 0.9901 | 82.3 | 3.5 | 89.9 | 4.7 | 96.1 | 5.2 | 0.01 | 0.02 |
| 101 | bromfenvinfos | 0.9963 | 87.7 | 5.6 | 87.3 | 3.1 | 97.4 | 0.5 | 0.01 | 0.02 |
| 102 | fluorodifen | 0.9921 | 104.8 | 4.7 | 108.5 | 6.1 | 123.6 | 4.8 | 0.005 | 0.01 |
| 103 | flutolanil | 0.9974 | 87.6 | 3.4 | 86.1 | 1.8 | 98.3 | 0.4 | 0.005 | 0.01 |
| 104 | chlorfenson | 0.9952 | 95.9 | 7.2 | 90.3 | 2.7 | 102 | 0.4 | 0.01 | 0.02 |
| 105 | hexaconazole | 0.9988 | 79.6 | 5.3 | 86.9 | 8.6 | 98.4 | 7.5 | 0.01 | 0.02 |
| 106 | prothiofos | 0.9962 | 57.8 | 5.9 | 64.5 | 1.8 | 70.6 | 2.8 | 0.01 | 0.02 |
| 107 | fludioxonil | 0.9989 | 78.9 | 1.8 | 89.2 | 7.8 | 97.3 | 1.6 | 0.01 | 0.02 |
| 108 | pretilachlor | 0.9979 | 78.6 | 6.7 | 86.6 | 1.7 | 98.9 | 5 | 0.005 | 0.01 |
| 109 | isoprothiolane | 0.9991 | 68.9 | 11.7 | 74.1 | 4.8 | 83.2 | 3 | 0.01 | 0.02 |
| 110 | profenofos | 0.9963 | 76 | 5.3 | 83.6 | 1.6 | 95 | 2.8 | 0.005 | 0.01 |
| 111 | pp’-DDE | 0.9921 | 68.4 | 12.7 | 72.7 | 2.6 | 83.3 | 2.1 | 0.005 | 0.01 |
| 112 | oxadiazon | 0.9974 | 73.9 | 7.1 | 83.6 | 5.6 | 88.8 | 4.2 | 0.005 | 0.01 |
| 113 | DEF | 0.9952 | 75.6 | 1.3 | 85 | 6.5 | 92.2 | 3.3 | 0.005 | 0.01 |
| 114 | dieldrin | 0.9908 | 66.9 | 3.9 | 67.5 | 7 | 72.8 | 2.7 | 0.005 | 0.01 |
| 115 | myclobutanil | 0.9962 | 86.3 | 6 | 88.1 | 3.2 | 102.9 | 1.6 | 0.01 | 0.02 |
| 116 | op’-DDD | 0.9989 | 69.7 | 7.3 | 78.2 | 3.9 | 90.1 | 1.3 | 0.005 | 0.01 |
| 117 | oxyfluorfen | 0.9979 | 119.3 | 5.4 | 106.7 | 10.1 | 119.7 | 1.6 | 0.005 | 0.01 |
| 118 | bupirimate | 0.9911 | 52.2 | 4.3 | 52.9 | 1.3 | 71.4 | 1.2 | 0.02 | 0.05 |
| 119 | kresoxim methyl | 0.9963 | 92.1 | 7.9 | 89.9 | 3.7 | 97.2 | 1.2 | 0.005 | 0.01 |
| 120 | cyflufenamid | 0.9921 | 82.6 | 3.8 | 83.9 | 8.4 | 92.1 | 4.6 | 0.005 | 0.01 |
| 121 | isoxathion | 0.9974 | 79.8 | 3.8 | 95.4 | 7.5 | 102.9 | 5.4 | 0.01 | 0.02 |
| 122 | cyproconazole 1 | 0.9952 | 88.1 | 6.1 | 73.2 | 22.3 | 99.5 | 3.2 | 0.01 | 0.02 |
| 123 | fluazifop butyl | 0.9922 | 92.5 | 5.9 | 86.4 | 3.4 | 104.3 | 3.2 | 0.01 | 0.02 |
| 124 | nitrofen | 0.9962 | 86.1 | 9.1 | 86.1 | 5.7 | 98.7 | 5.3 | 0.005 | 0.01 |
| 125 | endrin | 0.9989 | 77.8 | 2.8 | 83.4 | 5.8 | 116.2 | 11.1 | 0.01 | 0.02 |
| 126 | chlorobenzilate | 0.9979 | 103.4 | 3.8 | 99.2 | 2.2 | 95.9 | 2.2 | 0.005 | 0.01 |
| 127 | fensulfothion | 0.9991 | 86.1 | 2.3 | 97.3 | 5.4 | 103.3 | 5.7 | 0.01 | 0.025 |
| 128 | diniconazole | 0.9963 | 71.2 | 2.7 | 70.5 | 1.3 | 87.8 | 2.7 | 0.01 | 0.02 |
| 129 | oxadixyl | 0.9921 | 93 | 4.6 | 91.1 | 1.5 | 102.7 | 2.5 | 0.01 | 0.02 |
| 130 | pp’-DDD | 0.9974 | 71.9 | 2.9 | 77.7 | 3.1 | 90.5 | 1.5 | 0.005 | 0.01 |
| 131 | ethion | 0.9952 | 86.3 | 3.5 | 94.5 | 3.2 | 109.9 | 2 | 0.005 | 0.01 |
| 132 | op’-DDT | 0.9998 | 73.9 | 4.2 | 77.7 | 3.1 | 90.5 | 1.5 | 0.005 | 0.01 |
| 133 | chlorthiophos | 0.9962 | 90.8 | 2.9 | 88 | 7.6 | 98 | 0.5 | 0.005 | 0.01 |
| 134 | aclonifen | 0.9989 | 108.3 | 5.5 | 94.5 | 8.8 | 103.4 | 7.2 | 0.005 | 0.01 |
| 135 | triazophos | 0.9979 | 122.5 | 5.5 | 101.5 | 3.5 | 108.7 | 3 | 0.005 | 0.01 |
| 136 | famphur | 0.9991 | 68.9 | 2.5 | 74 | 3 | 78.9 | 0.8 | 0.01 | 0.02 |
| 137 | benalaxyl | 0.992 3 | 81 | 6.5 | 91.1 | 1.5 | 104.1 | 0.6 | 0.005 | 0.01 |
| 138 | carbophenothion | 0.992 1 | 90.8 | 8.9 | 88.1 | 2.2 | 100.9 | 2.6 | 0.005 | 0.01 |
| 139 | trifloxystrobi | 0.997 4 | 85.6 | 4.8 | 86.4 | 2.9 | 99.5 | 2.2 | 0.005 | 0.01 |
| 140 | edifenphos | 0.995 2 | 84.8 | 0.6 | 86.9 | 3.9 | 101.5 | 1.2 | 0.005 | 0.01 |
| 141 | quinoxyfen | 0.9991 | 52.1 | 13.3 | 60.5 | 3.8 | 65.1 | 3.1 | 0.02 | 0.05 |
| 142 | propiconazole | 0.9962 | 85.5 | 4.1 | 88.2 | 10.4 | 97.9 | 7 | 0.005 | 0.01 |
| 143 | pp’-DDT | 0.9989 | 66.7 | 5.7 | 78.5 | 0.9 | 85.2 | 1.1 | 0.01 | 0.02 |
| 144 | hexazinone | 0.9929 | 66.7 | 5.3 | 72.1 | 2.2 | 79 | 1.1 | 0.01 | 0.02 |
| 145 | tebuconazole | 0.9991 | 83.4 | 3.4 | 85.7 | 3.2 | 92.6 | 3.9 | 0.005 | 0.01 |
| 146 | diclofop methyl | 0.9963 | 82.1 | 3.9 | 90.3 | 5.3 | 100.1 | 0.4 | 0.005 | 0.01 |
| 147 | piperonylbutoxide | 0.9921 | 71.6 | 5.2 | 81.4 | 1 | 89.7 | 1.3 | 0.01 | 0.02 |
| 148 | epoxiconazol | 0.9904 | 75.6 | 4.9 | 78.5 | 3.4 | 90.7 | 4.7 | 0.01 | 0.02 |
| 149 | pyridaphenthion | 0.9952 | 68.8 | 1.9 | 70.9 | 5.5 | 77.1 | 1 | 0.01 | 0.02 |
| 150 | iprodione | 0.9808 | 123 | 6.7 | 113.1 | 7.7 | 106.8 | 3.4 | 0.005 | 0.01 |
| 151 | phosmet | 0.9962 | 69.2 | 8.4 | 68.3 | 1.8 | 76.4 | 2.9 | 0.01 | 0.02 |
| 152 | bifenthrin | 0.9902 | 63.4 | 4.1 | 77.2 | 0.4 | 87 | 1.6 | 0.001 | 0.005 |
| 153 | EPN | 0.997 | 99.2 | 10.2 | 97.6 | 6 | 107.5 | 3.6 | 0.005 | 0.01 |
| 154 | bromopropylate | 0.9921 | 70.6 | 4.1 | 81.9 | 3.1 | 93.7 | 0.4 | 0.01 | 0.02 |
| 155 | piperophos | 0.9913 | 79.7 | 1.2 | 82.8 | 2 | 93.6 | 2.1 | 0.01 | 0.02 |
| 156 | tetramethrin | 0.9921 | 74.4 | 5.4 | 82.1 | 1.2 | 92.1 | 0.9 | 0.01 | 0.02 |
| 157 | methoxychlor | 0.9904 | 71.7 | 5.4 | 86.3 | 4.3 | 95.5 | 1.4 | 0.01 | 0.02 |
| 158 | etoxazole | 0.9952 | 76.7 | 8.5 | 95.4 | 10.4 | 102.6 | 1.7 | 0.01 | 0.02 |
| 159 | fenamidone | 0.9908 | 79.5 | 5.3 | 88.4 | 2.7 | 99 | 1.6 | 0.01 | 0.02 |
| 160 | tebufenpyrad | 0.9962 | 81.7 | 9.2 | 84 | 2.8 | 90.5 | 0.7 | 0.005 | 0.01 |
| 161 | anilofos | 0.9989 | 80.5 | 6.5 | 93.3 | 3.4 | 102.8 | 3.7 | 0.005 | 0.01 |
| 162 | bifenox | 0.9979 | 80.3 | 1.2 | 90.4 | 10.5 | 104 | 3 | 0.005 | 0.01 |
| 163 | tetradifon | 0.9901 | 78.7 | 6.6 | 82.5 | 3.2 | 93.3 | 0.5 | 0.01 | 0.02 |
| 164 | phosalone | 0.9963 | 63.3 | 8.3 | 67.7 | 2.3 | 78.7 | 1.9 | 0.01 | 0.02 |
| 165 | leptopho | 0.9921 | 61 | 6.5 | 65.8 | 3.9 | 74.9 | 1.4 | 0.01 | 0.02 |
| 166 | pyriproxyfen | 0.9974 | 80.5 | 5.8 | 82.5 | 1.6 | 91.3 | 1.9 | 0.005 | 0.01 |
| 167 | iambda cyhalothrin | 0.9952 | 81.3 | 6.3 | 87.9 | 6.2 | 98.1 | 3.5 | 0.005 | 0.01 |
| 168 | mefenacet | 0.9998 | 83 | 3.8 | 88.3 | 1 | 98.4 | 0.8 | 0.005 | 0.01 |
| 169 | acrinathrin | 0.9962 | 73 | 8.3 | 80.7 | 2 | 93.6 | 4 | 0.01 | 0.02 |
| 170 | pyrazophos | 0.9982 | 76.8 | 5.3 | 73.1 | 1.4 | 79.8 | 2 | 0.01 | 0.02 |
| 171 | fenarimol | 0.9979 | 73.7 | 7.2 | 80.3 | 3.4 | 94 | 6.5 | 0.01 | 0.02 |
| 172 | azinphos ethyl | 0.9951 | 84.3 | 9 | 84.2 | 7.9 | 80.9 | 1.7 | 0.01 | 0.02 |
| 173 | permethrin | 0.9963 | 85.5 | 7.9 | 93.1 | 7.4 | 89.1 | 6.1 | 0.005 | 0.01 |
| 174 | coumaphos | 0.9921 | 80.2 | 8.1 | 89.3 | 2.1 | 100.7 | 2.3 | 0.005 | 0.01 |
| 175 | fluquinconazole | 0.9904 | 87.4 | 7.3 | 91.4 | 4.5 | 94.9 | 2.1 | 0.005 | 0.01 |
| 176 | pyridaben | 0.9992 | 73.7 | 7.3 | 76.1 | 1.7 | 87 | 1.5 | 0.01 | 0.02 |
| 177 | dioxathion | 0.9998 | 72.3 | 7.1 | 78 | 9.5 | 91.7 | 5.3 | 0.01 | 0.02 |
| 178 | fenbuconazole | 0.9952 | 81.2 | 4.8 | 89.1 | 1.2 | 103.5 | 4.5 | 0.005 | 0.01 |
| 179 | cyfluthrin | 0.9989 | 74.7 | 7 | 82 | 7.2 | 96.4 | 2 | 0.01 | 0.02 |
| 180 | cypermethri | 0.991 | 76.3 | 7.8 | 78.4 | 8.2 | 89.1 | 1.2 | 0.01 | 0.02 |
| 181 | boscalid | 0.9921 | 69.4 | 1.2 | 76.7 | 1.4 | 83.5 | 1 | 0.01 | 0.02 |
| 182 | flucythrinate | 0.9974 | 76.7 | 4.4 | 80.4 | 1.3 | 94.9 | 0.7 | 0.01 | 0.02 |
| 183 | fenvalerate | 0.9902 | 74.5 | 4.7 | 75.5 | 2.1 | 94.8 | 1.1 | 0.01 | 0.02 |
| 184 | fluvalinate | 0.9926 | 84.4 | 9.6 | 83 | 2.9 | 96.2 | 2.2 | 0.005 | 0.01 |
| 185 | difenoconazole | 0.9822 | 92.1 | 2.6 | 100.9 | 5 | 105.7 | 4.8 | 0.005 | 0.01 |
| 186 | deltamethrin | 0.9902 | 78.8 | 4.1 | 87.5 | 6.2 | 88.8 | 2.2 | 0.01 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, X.; Chu, N.; Zhang, X.; Yang, X.; Meng, X.; Yang, J.; Wang, N. Rapid Analysis of Residues of 186 Pesticides in Hawk Tea Using Modified QuEChERS Coupled with Gas Chromatography Tandem Mass Spectrometry. Int. J. Environ. Res. Public Health 2022, 19, 12639. https://doi.org/10.3390/ijerph191912639
Shu X, Chu N, Zhang X, Yang X, Meng X, Yang J, Wang N. Rapid Analysis of Residues of 186 Pesticides in Hawk Tea Using Modified QuEChERS Coupled with Gas Chromatography Tandem Mass Spectrometry. International Journal of Environmental Research and Public Health. 2022; 19(19):12639. https://doi.org/10.3390/ijerph191912639
Chicago/Turabian StyleShu, Xiao, Nengming Chu, Xuemei Zhang, Xiaoxia Yang, Xia Meng, Junying Yang, and Na Wang. 2022. "Rapid Analysis of Residues of 186 Pesticides in Hawk Tea Using Modified QuEChERS Coupled with Gas Chromatography Tandem Mass Spectrometry" International Journal of Environmental Research and Public Health 19, no. 19: 12639. https://doi.org/10.3390/ijerph191912639
APA StyleShu, X., Chu, N., Zhang, X., Yang, X., Meng, X., Yang, J., & Wang, N. (2022). Rapid Analysis of Residues of 186 Pesticides in Hawk Tea Using Modified QuEChERS Coupled with Gas Chromatography Tandem Mass Spectrometry. International Journal of Environmental Research and Public Health, 19(19), 12639. https://doi.org/10.3390/ijerph191912639










